Change-Point Detection of Peak Tibial Acceleration in Overground Running Retraining
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
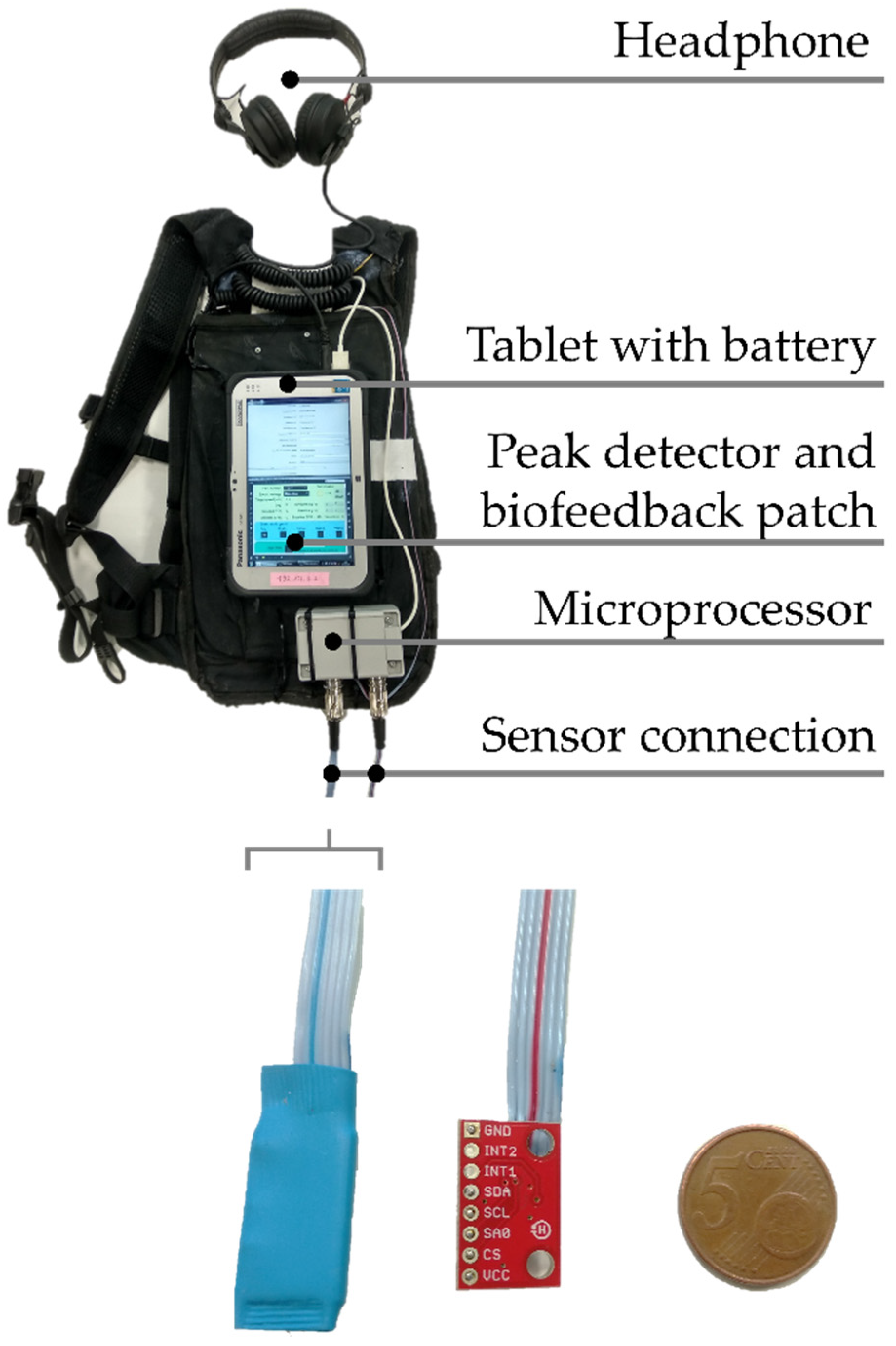
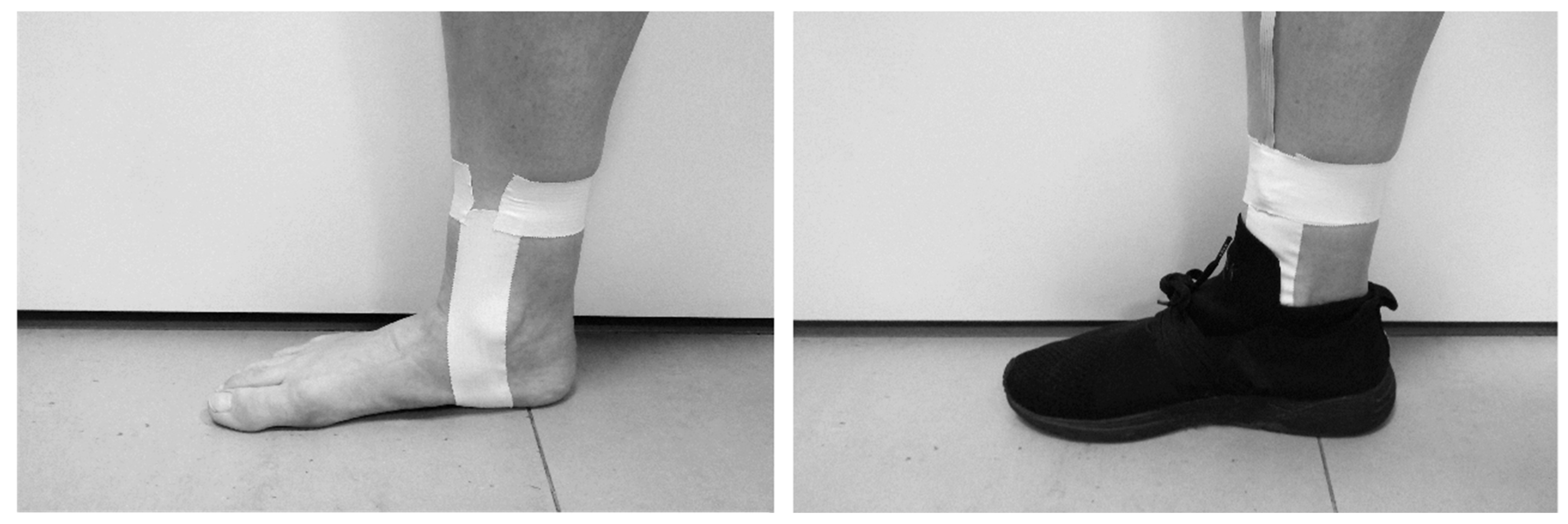
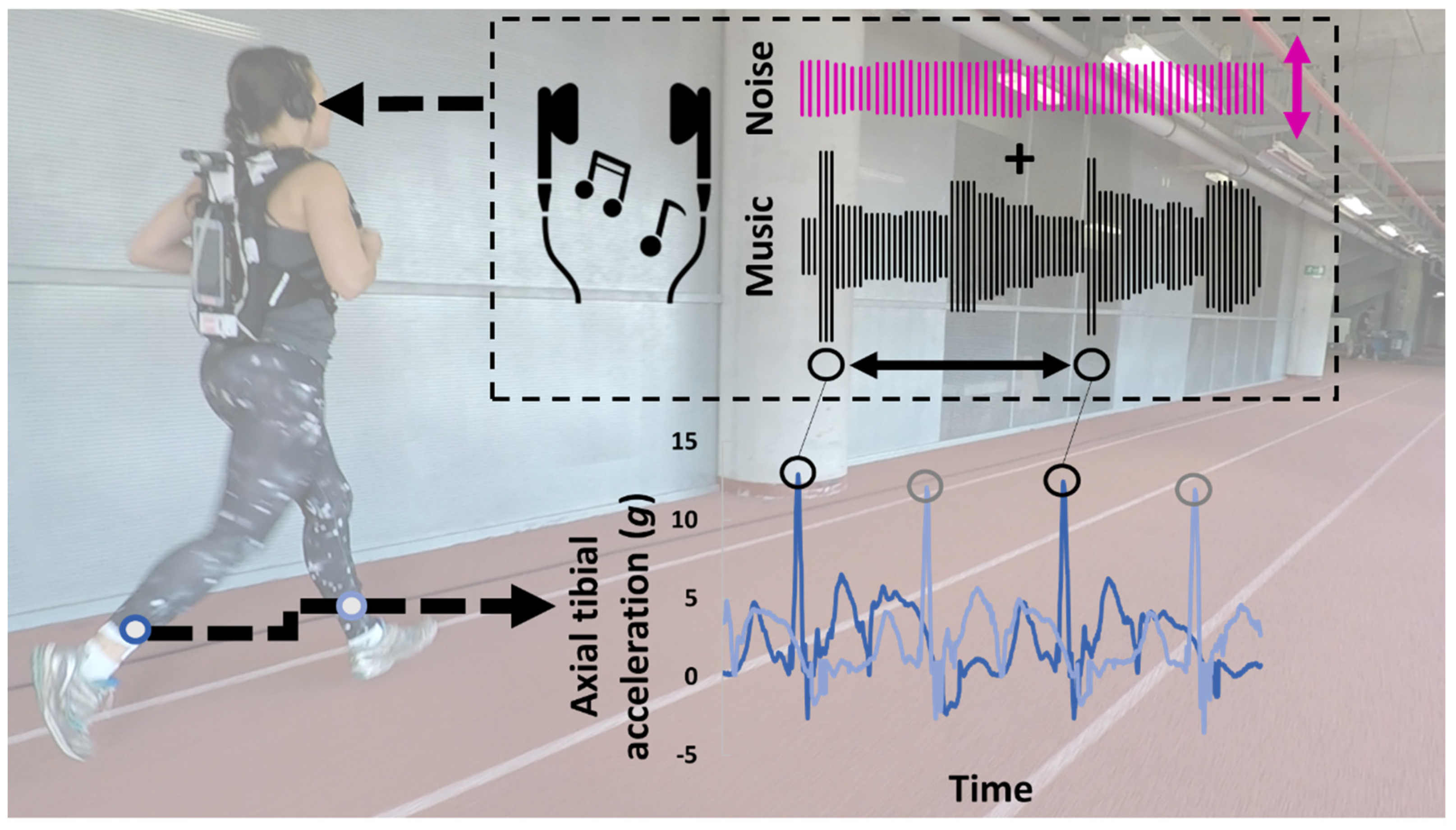
2.2. Intervention
2.3. Data Processing
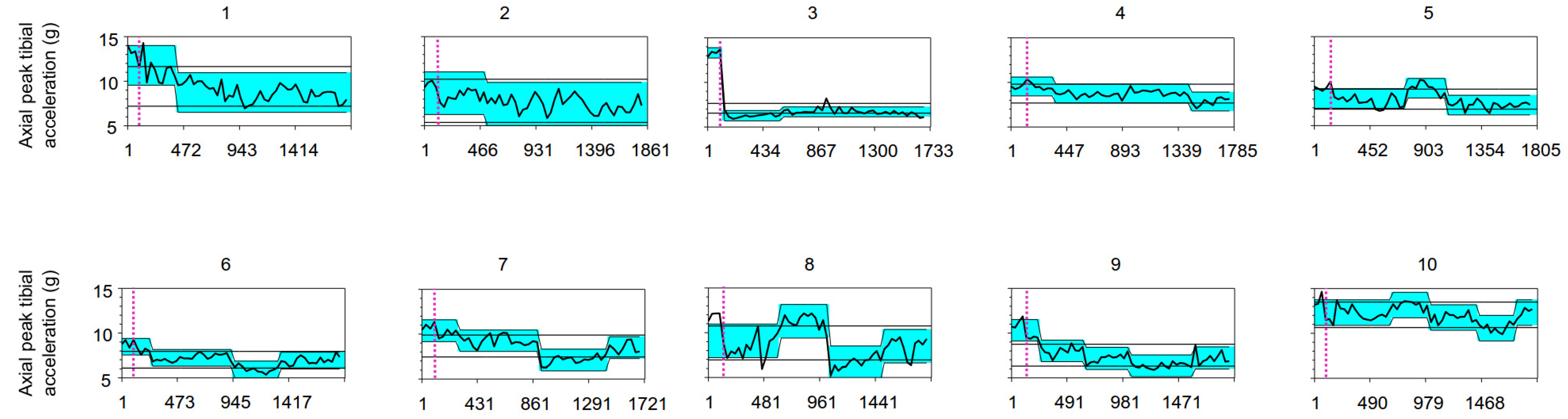
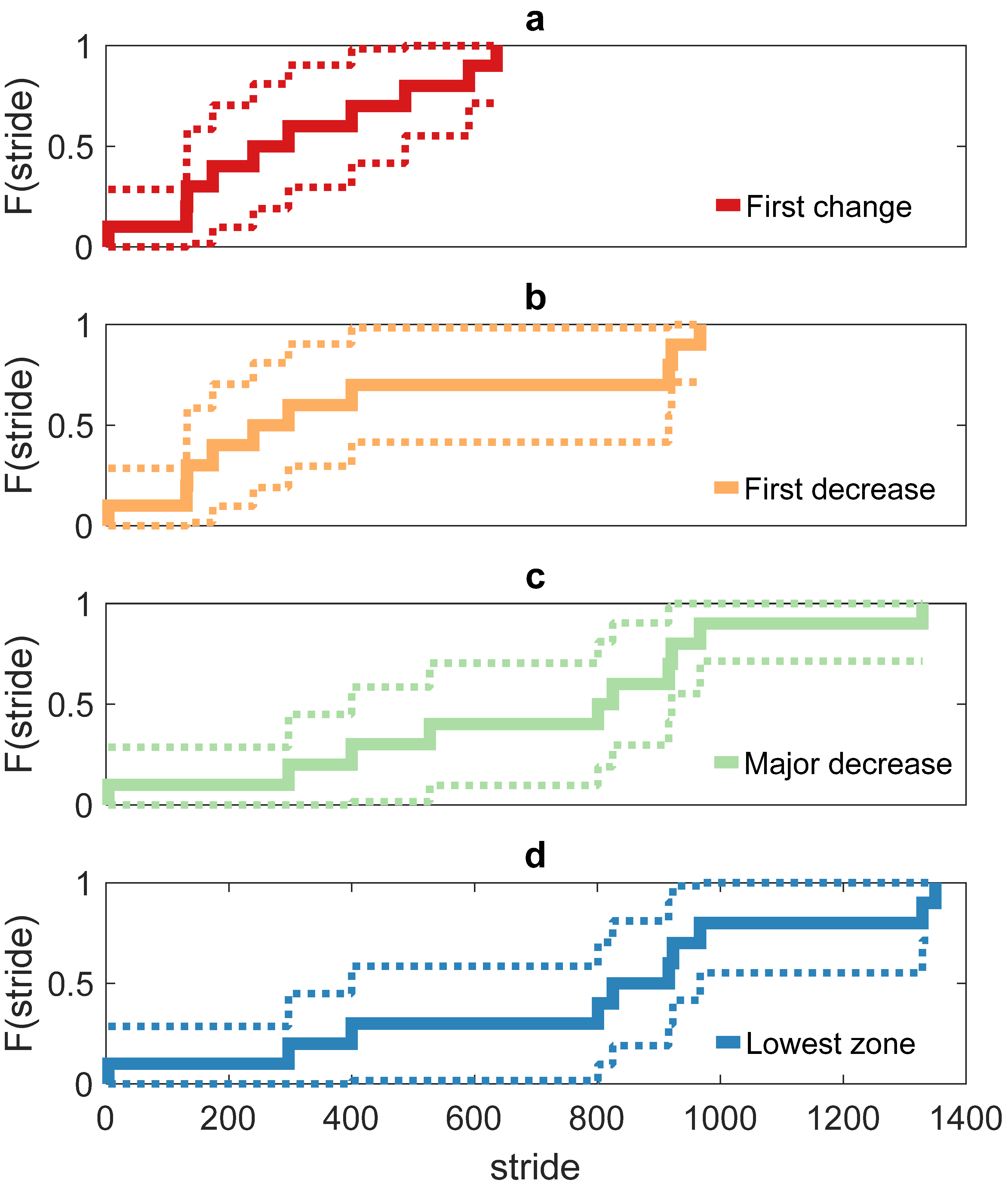
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Sheerin, K.R.; Reid, D.; Besier, T.F. The measurement of tibial acceleration in runners: A review of the factors that can affect tibial acceleration during running and evidence-based guidelines for its use. Gait Posture 2019, 67, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Provot, T.; Chiementin, X.; Oudin, E.; Bolaers, F.; Murer, S. Validation of a high sampling rate inertial measurement unit for acceleration during running. Sensors 2017, 17, 1958. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, C.; Kiesewetter, P.; Milani, T.L. The effect of the accelerometer operating range on biomechanical parameters: Stride length, velocity, and peak tibial acceleration during running. Sensors 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Kipp, K. Use of audio biofeedback to reduce tibial impact accelerations during running. J. Biomech. 2014, 47, 1739–1741. [Google Scholar] [CrossRef]
- Van den Berghe, P.; Lorenzoni, V.; Gerlo, J.; Breine, B.; Derie, R.; Six, J.; Leman, M.; De Clercq, D. Real-time music-based biofeedback to reduce impact loading during over-ground running. In Proceedings of the 42nd Annual meeting of the American Society of Biomechanics, Rochester, MN, USA, 8–11 August 2018. [Google Scholar]
- Creaby, M.W.; Franettovich Smith, M.M. Retraining running gait to reduce tibial loads with clinician or accelerometry guided feedback. J. Sci. Med. Sport 2016, 19, 288–292. [Google Scholar] [CrossRef]
- Crowell, H.P.; Milner, C.E.; Hamill, J.; Davis, I.S. Reducing Impact Loading During Running With the Use of Real-Time Visual Feedback. J. Orthop. Sports Phys. Ther. 2010, 40, 206–213. [Google Scholar] [CrossRef]
- Crowell, H.P.; Davis, I.S. Gait retraining to reduce lower extremity loading in runners. Clin. Biomech. 2011, 26, 78–83. [Google Scholar] [CrossRef]
- Clansey, A.C.; Hanlon, M.; Wallace, E.S.; Nevill, A.; Lake, M.J. Influence of Tibial shock feedback training on impact loading and running economy. Med. Sci. Sports Exerc. 2014, 46, 973–981. [Google Scholar] [CrossRef]
- Davis, I.S.; Futrell, E. Gait Retraining: Altering the Fingerprint of Gait. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 339–355. [Google Scholar] [CrossRef]
- Bowser, B.J.; Fellin, R.; Milner, C.E.; Pohl, M.B.; Davis, I.S. Reducing impact loading in runners: A one-year follow-up. Med. Sci. Sports Exerc. 2018, 50, 2500–2506. [Google Scholar] [CrossRef]
- Van den Berghe, P.; Six, J.; Gerlo, J.; Leman, M.; De Clercq, D. Validity and reliability of peak tibial accelerations as real-time measure of impact loading during over-ground rearfoot running at different speeds. J. Biomech. 2019, 86, 238–242. [Google Scholar] [CrossRef]
- Lorenzoni, V.; Van den Berghe, P.; Maes, P.-J.; De Bie, T.; De Clercq, D.; Leman, M. Design and validation of an auditory biofeedback system for modification of running parameters. J. Multimodal User Interfaces 2018, 3, 167–180. [Google Scholar] [CrossRef]
- Willy, R.W.; Buchenic, L.; Rogacki, K.; Ackerman, J.; Schmidt, A.; Willson, J.D. In-field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand. J. Med. Sci. Sports 2016, 26, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Luft, A.R.; Buitrago, M.M. Stages of motor skill learning. Mol. Neurobiol. 2005, 32, 205–216. [Google Scholar] [CrossRef]
- Buitrago, M.M.; Ringer, T.; Schulz, J.B.; Dichgans, J.; Luft, A.R. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav. Brain Res. 2004, 155, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; McClean, S.; Zhang, S.; Nugent, C. Optimal parameter exploration for online change-point detection in activity monitoring using genetic algorithms. Sensors 2016, 16, 1784. [Google Scholar] [CrossRef]
- Khan, T.; Lundgren, L.E.; Järpe, E.; Olsson, M.C.; Viberg, P. A Novel Method for Classification of Running Fatigue Using Change-Point Segmentation. Sensors 2019, 19, 4729. [Google Scholar] [CrossRef]
- Pashami, S.; Lilienthal, A.J.; Trincavelli, M. Detecting changes of a distant gas source with an array of MOX gas sensors. Sensors 2012, 12, 16404–16419. [Google Scholar] [CrossRef]
- Pashami, S.; Lilienthal, A.J.; Schaffernicht, E.; Trincavelli, M. TREFEX: Trend estimation and change detection in the response of MOX gas sensors. Sensors 2013, 13, 7323–7344. [Google Scholar] [CrossRef]
- Zuo, G.; Dou, Y.; Lei, R. Discrimination algorithm and procedure of snow depth and sea ice thickness determination using measurements of the vertical ice temperature profile by the ice-tethered buoys. Sensors 2018, 18, 4162. [Google Scholar] [CrossRef]
- Culman, C.; Aminikhanghahi, S.; J Cook, D. Easing Power Consumption of Wearable Activity Monitoring with Change Point Detection. Sensors 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, M.; Skorupa, A.; Boguszewicz, Ł.; Sokół, M.; Polański, A. Quality Control Procedure Based on Partitioning of NMR Time Series. Sensors 2018, 18, 792. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, L.M.A.; Barnes, A.; Wheat, J.S.; Heller, B.W. Characterizing the Learning Effect in Response to Biofeedback Aimed at Reducing Tibial Acceleration during Running. Proc. ISEA 2018, 2, 200. [Google Scholar] [CrossRef]
- Taylor, W.A. Change-Point Analysis: A Powerful New Tool for Detecting Changes. Available online: https://variation.com/wp-content/uploads/change-point-analyzer/change-point-analysis-a-powerful-new-tool-for-detecting-changes.pdf (accessed on 19 March 2020).
- Yamato, T.P.; Saragiotto, B.T.; Lopes, A.D. A Consensus Definition of Running-Related Injury in Recreational Runners: A Modified Delphi Approach. J. Orthop. Sports Phys. Ther. 2015, 45, 375–380. [Google Scholar] [CrossRef]
- Breine, B.; Malcolm, P.; Frederick, E.C.; De Clercq, D. Relationship between running speed and initial foot contact patterns. Med. Sci. Sports Exerc. 2014, 46, 1595–1603. [Google Scholar] [CrossRef]
- STMicroelecronics LIS331HH MEMS Digital Output Motion Sensor Ultra Low-Power High Performance Three-Axis “nano” Accelerometer. Available online: http://www.st.com/st-web-ui/static/active/en/resource/technical/document/datasheet/DM00040962.pdf (accessed on 19 March 2020).
- Gruber, A.H.; Boyer, K.A.; Derrick, T.R.; Hamill, J. Impact shock frequency components and attenuation in rearfoot and forefoot running. J. Sport Heal. Sci. 2014, 3, 113–121. [Google Scholar] [CrossRef]
- Milner, C.E.; Ferber, R.; Pollard, C.D.; Hamill, J.; Davis, I.S. Biomechanical factors associated with tibial stress fracture in female runners. Med. Sci. Sports Exerc. 2006, 38, 323–328. [Google Scholar] [CrossRef]
- Moens, B.; Leman, M. Alignment strategies for the entrainment of music and movement rhythms. Ann. N. Y. Acad. Sci. 2015, 1337, 86–93. [Google Scholar] [CrossRef]
- Van Dyck, E.; Moens, B.; Buhmann, J.; Demey, M.; Coorevits, E.; Dalla Bella, S.; Leman, M. Spontaneous Entrainment of Running Cadence to Music Tempo. Sports Med. Open 2015, 2, 15. [Google Scholar] [CrossRef]
- Hinkley, D.V. Inference about the change-point from cumulative sum tests. Biometrika 1971, 58, 509–523. [Google Scholar] [CrossRef]
- Pettitt, A.N. A simple cumulative sum type statistic for the change-point problem with zero-one observations. Biometrika 1980, 67, 79–84. [Google Scholar] [CrossRef]
- Hinkley, D.; Schechtman, E. Conditional bootstrap methods in the mean-shift model. Biometrika 1987, 74, 85–93. [Google Scholar] [CrossRef]
- Cram, P.; Fendrick, A.M.; Inadomi, J.; Cowen, M.E.; Carpenter, D.; Vijan, S. The impact of a celebrity promotional campaign on the use of colon cancer screening: The Katie Couric effect. Arch. Intern. Med. 2003, 163, 1601–1605. [Google Scholar] [CrossRef]
- Taylor, W. A Pattern Test for Distinguishing between Autoregressive and Mean-Shift Data. Available online: https://variation.com/wp-content/uploads/change-point-analyzer/a-pattern-test-for-distinguishing-between-autoregressive-and-mean-shift-data.pdf (accessed on 19 March 2020).
| Studies | Hardware for Biofeedback | Feedback Modality | Running Environment | Trials for Analysis |
|---|---|---|---|---|
| Crowell et al. (2010) | 1 × accelerometer 1 × computer 1 × monitor screen |  | Treadmill, laboratory | 20 averaged per condition |
| Clansey et al. (2014) | 1 × accelerometer 1 × computer 1 × projection screen 1 × speaker set |  + +  | Treadmill, laboratory | 6 averaged per condition |
| Wood and Kipp (2014) | 1 × accelerometer 1 × computer with speakers |  | Treadmill, laboratory | 20 averaged per condition |
| Present study | 2 × accelerometers 1 × instrumented backpack 1 × headphone |  | Overground, athletic facility | 1853 ± 88 (mean ± SD) in total |
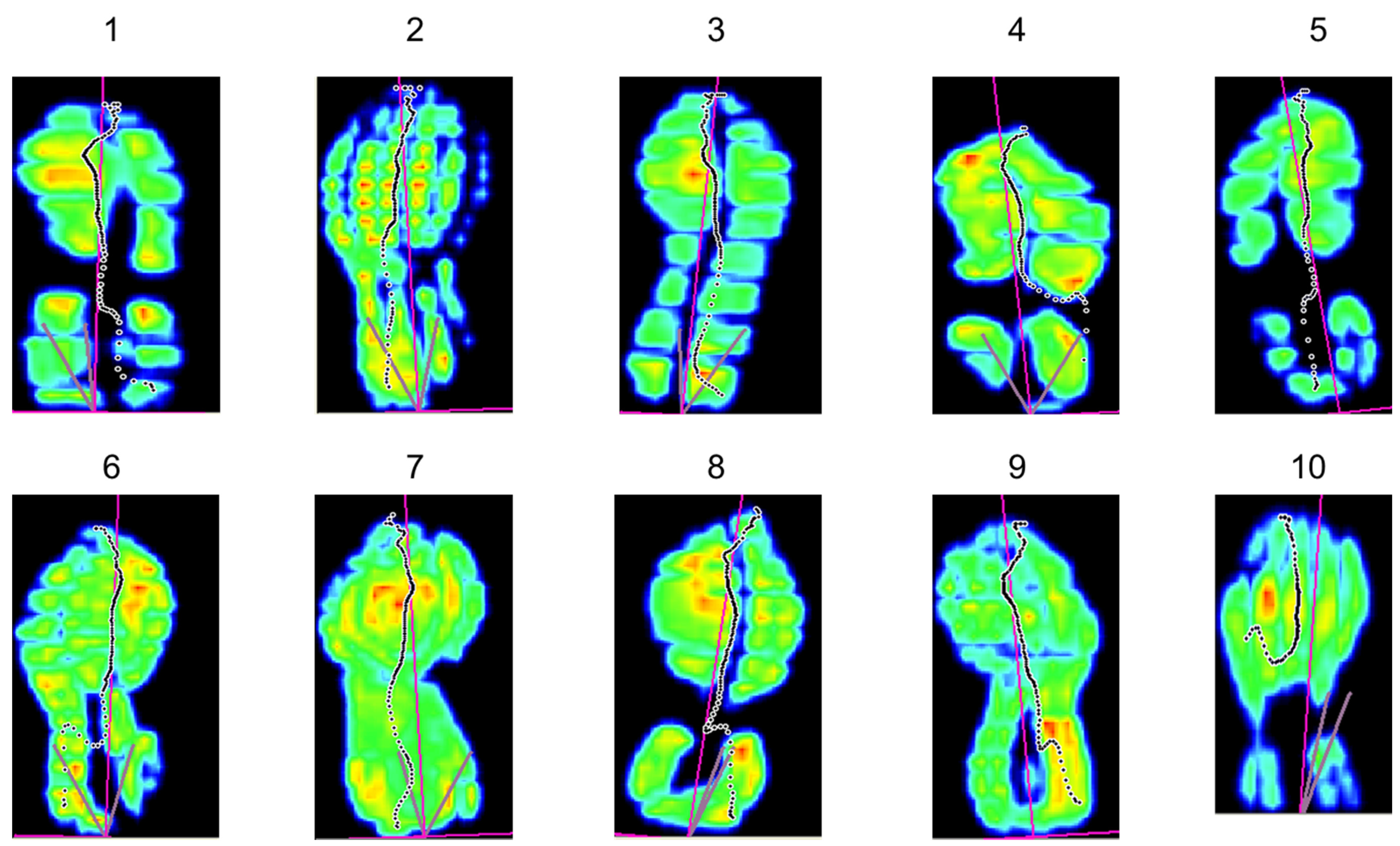
| ID | APTA (g) Baseline | Number of Change Points | Location of the Change Point | 95% Confidence Interval | Δ Change Inter-Segments in APTA (g) | Zone of Lowest APTA (g) {% vs. Baseline} | Estimated Standard Deviation |
|---|---|---|---|---|---|---|---|
| 1 | 13.21 | 1 | 297 a | 231–330 | −3.04 | 8.75 {66%} | 0.75 |
| 2 | 9.66 | 1 | 400 a | 235–631 | −1.24 | 7.44 {77%} | 0.81 |
| 3 | 13.43 | 2 | 4 a 466 | 4–4 367–1555 | −7.05 +0.42 | 6.30 {47%} | 0.19 |
| 4 | 9.40 | 2 | 240 1329 a | 240–306 1263–1362 | −0.81 −0.90 | 7.86 {84%} | 0.35 |
| 5 | 9.28 | 2 | 636 967 a | 373–703 934–967 | +1.17 −1.90 | 7.33 {79%} | 0.37 |
| 6 | 8.87 | 3 | 132 825 a 1221 | 66–165 825–858 1188–1254 | −1.24 −1.28 +0.99 | 5.96 {67%} | 0.31 |
| 7 | 10.83 | 3 | 174 801 a 1329 | 75–273 768–801 1296–1362 | −1.12 −2.14 +1.36 | 7.14 {66%} | 0.40 |
| 8 | 11.83 | 3 | 487 916 a 1378 | 190–520 916–916 1345–1477 | +2.18 −4.64 +1.86 | 6.65 {56%} | 0.63 |
| 9 | 11.03 | 4 | 131 527 a 923 1484 | 131–131 428–626 824–956 1451–1715 | −2.30 −0.85 −0.81 +0.88 | 6.37 {58%} | 0.41 |
| 10 | 13.67 | 4 | 591 921 a 1350 1680 | 129–657 888–954 1284–1383 1680–1680 | +0.86 −1.42 −1.18 +1.73 | 10.62 {78%} | 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Berghe, P.; Gosseries, M.; Gerlo, J.; Lenoir, M.; Leman, M.; De Clercq, D. Change-Point Detection of Peak Tibial Acceleration in Overground Running Retraining. Sensors 2020, 20, 1720. https://doi.org/10.3390/s20061720
Van den Berghe P, Gosseries M, Gerlo J, Lenoir M, Leman M, De Clercq D. Change-Point Detection of Peak Tibial Acceleration in Overground Running Retraining. Sensors. 2020; 20(6):1720. https://doi.org/10.3390/s20061720
Chicago/Turabian StyleVan den Berghe, Pieter, Maxim Gosseries, Joeri Gerlo, Matthieu Lenoir, Marc Leman, and Dirk De Clercq. 2020. "Change-Point Detection of Peak Tibial Acceleration in Overground Running Retraining" Sensors 20, no. 6: 1720. https://doi.org/10.3390/s20061720
APA StyleVan den Berghe, P., Gosseries, M., Gerlo, J., Lenoir, M., Leman, M., & De Clercq, D. (2020). Change-Point Detection of Peak Tibial Acceleration in Overground Running Retraining. Sensors, 20(6), 1720. https://doi.org/10.3390/s20061720





