Light-addressable Electrodes for Dynamic and Flexible Addressing of Biological Systems and Electrochemical Reactions
Abstract
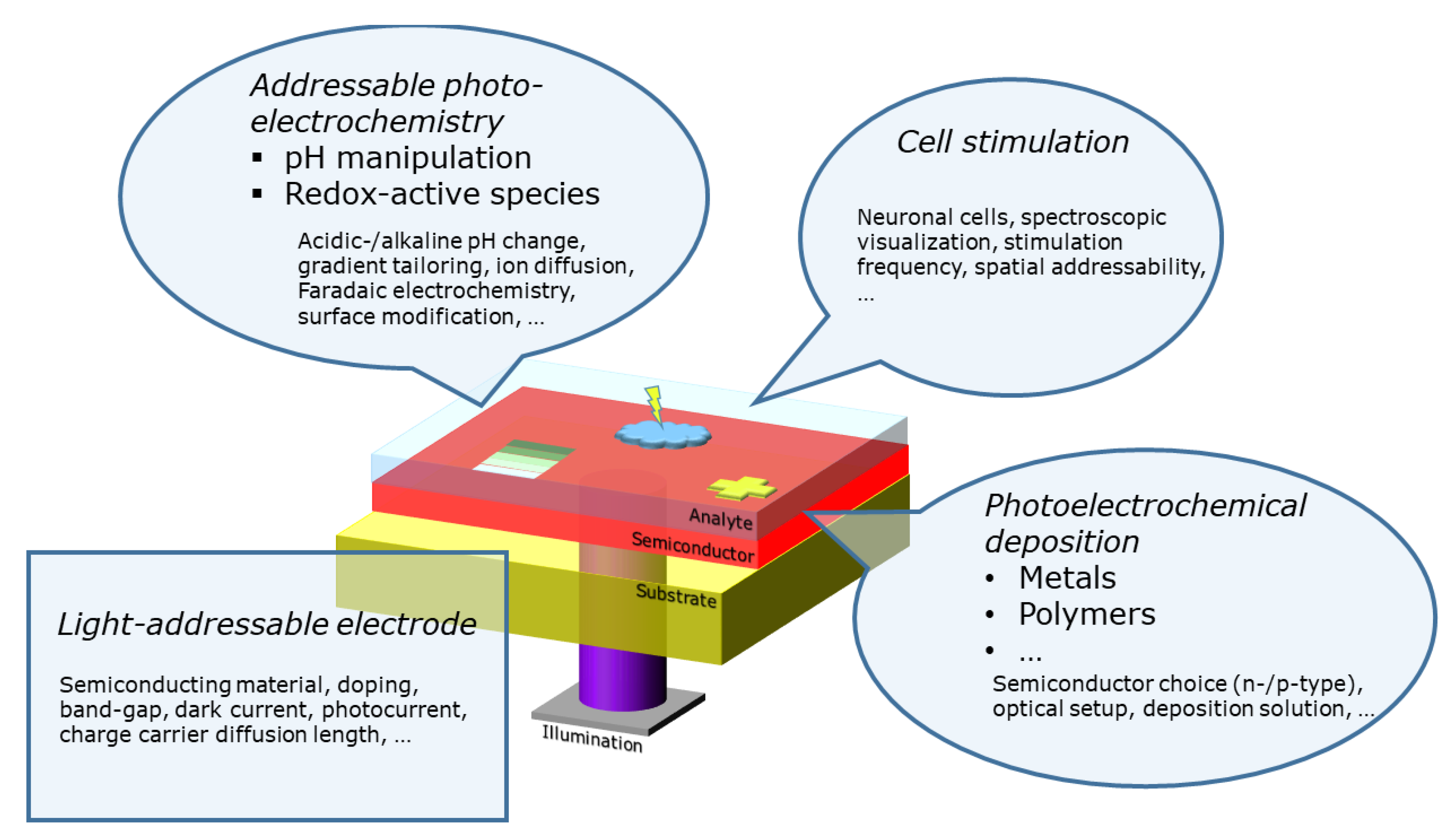
1. Introduction
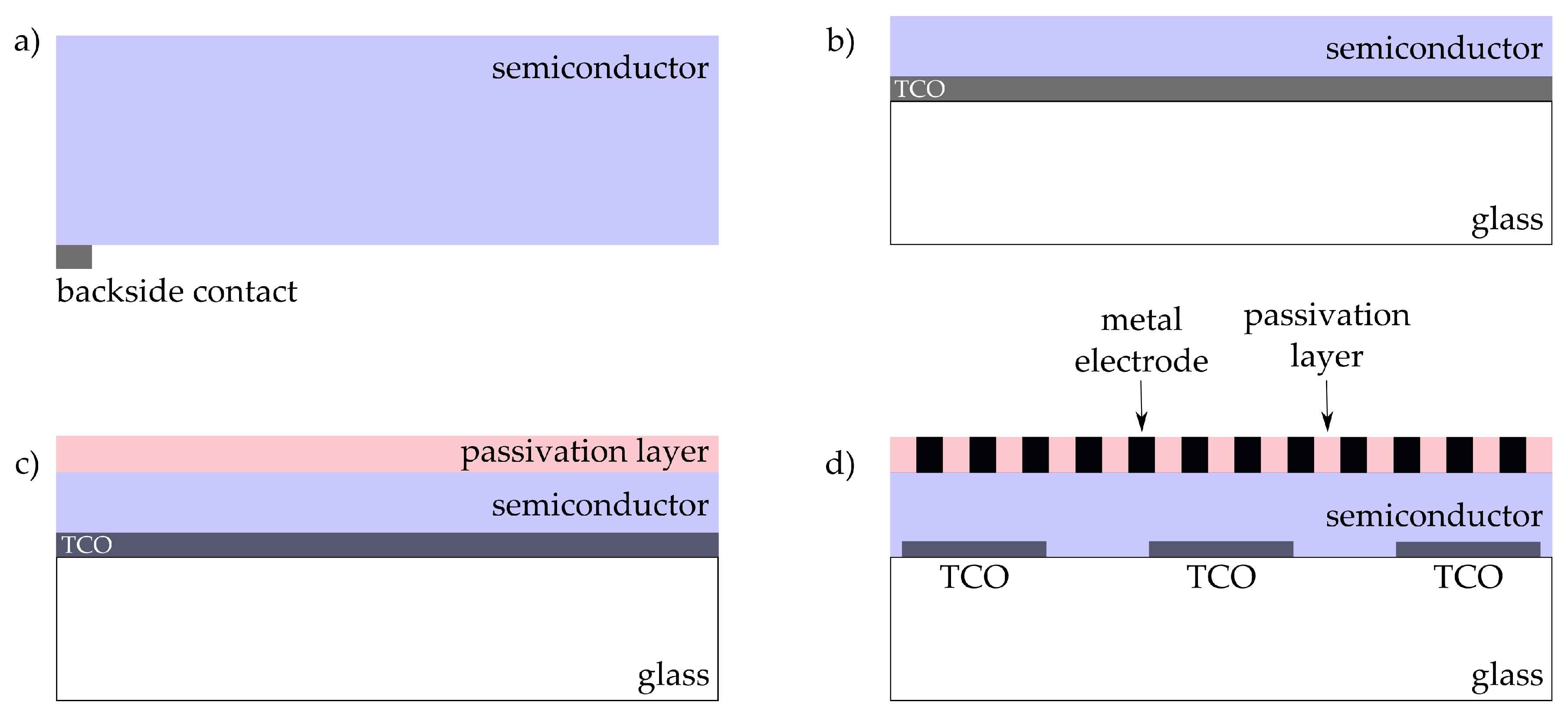
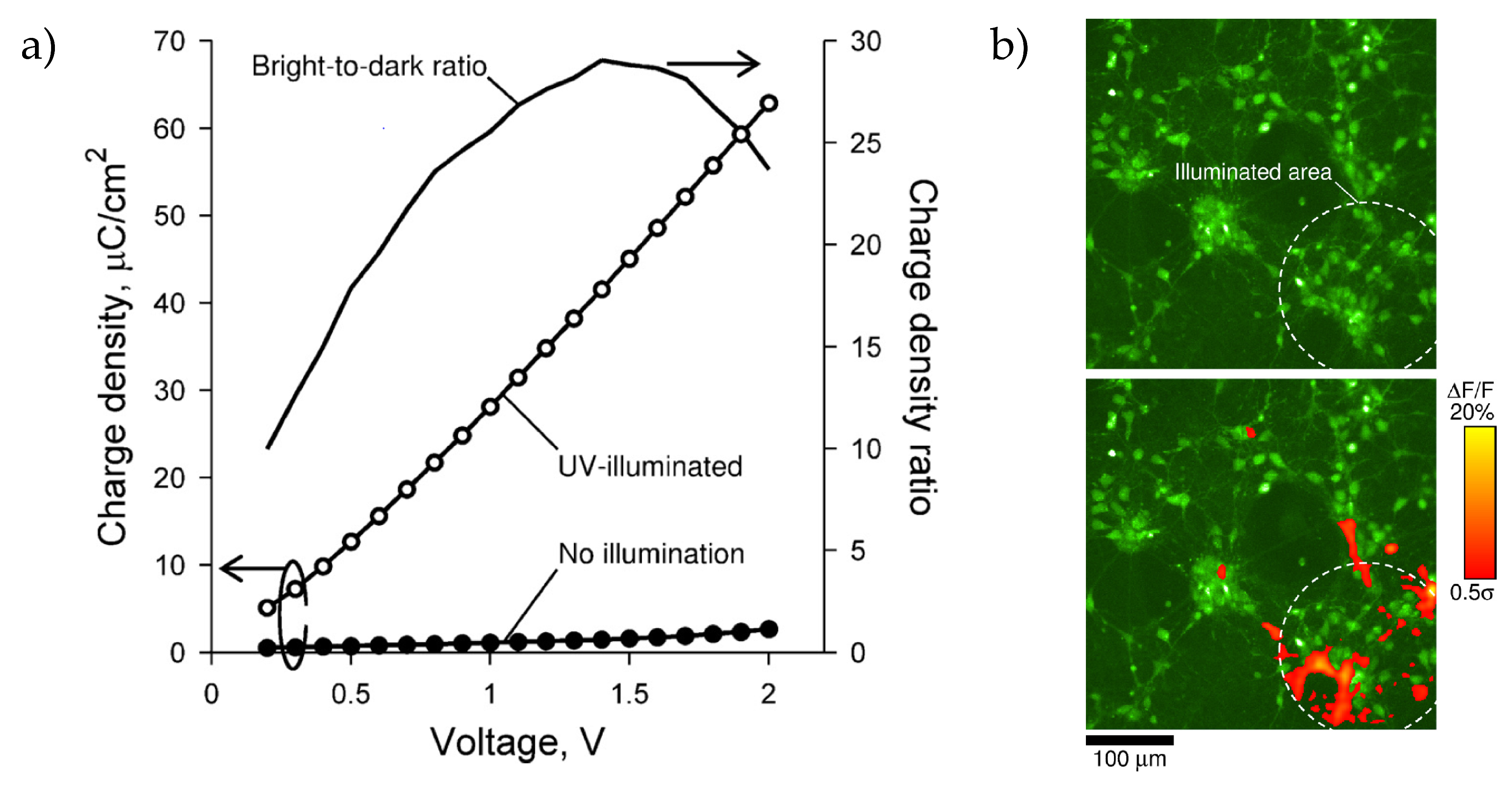
2. Stimulation of Cells
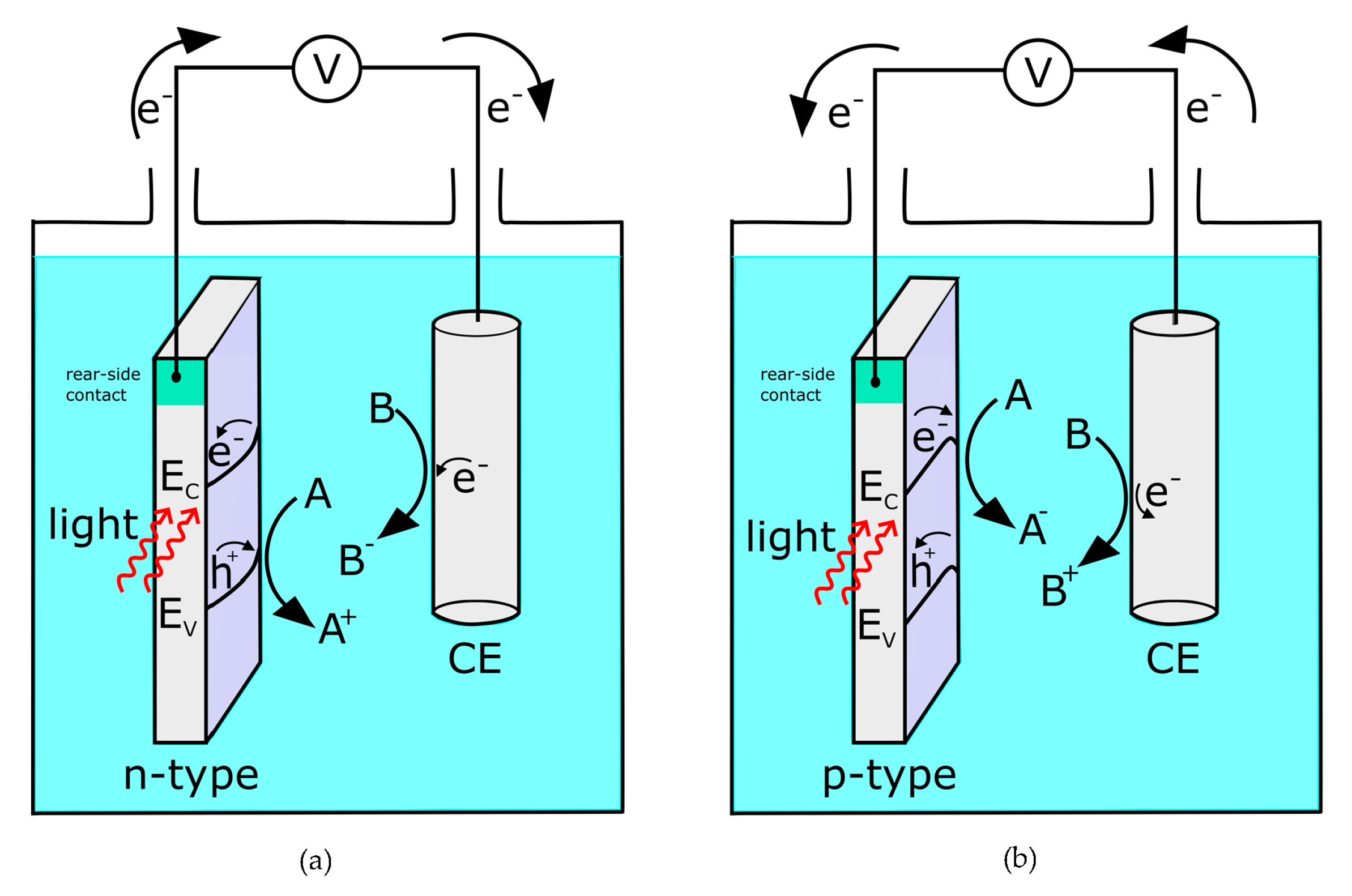
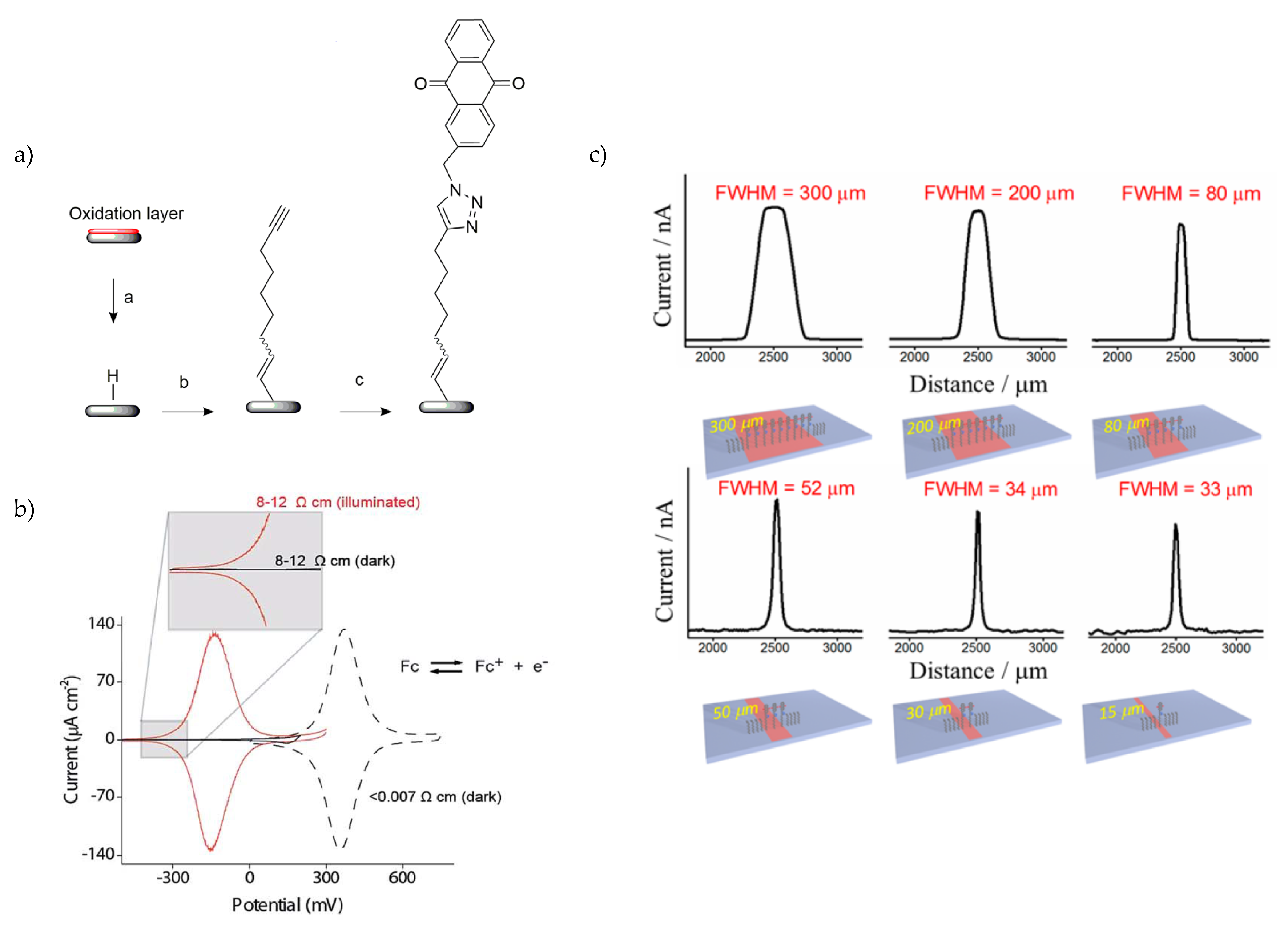
3. Addressable Photoelectrochemistry
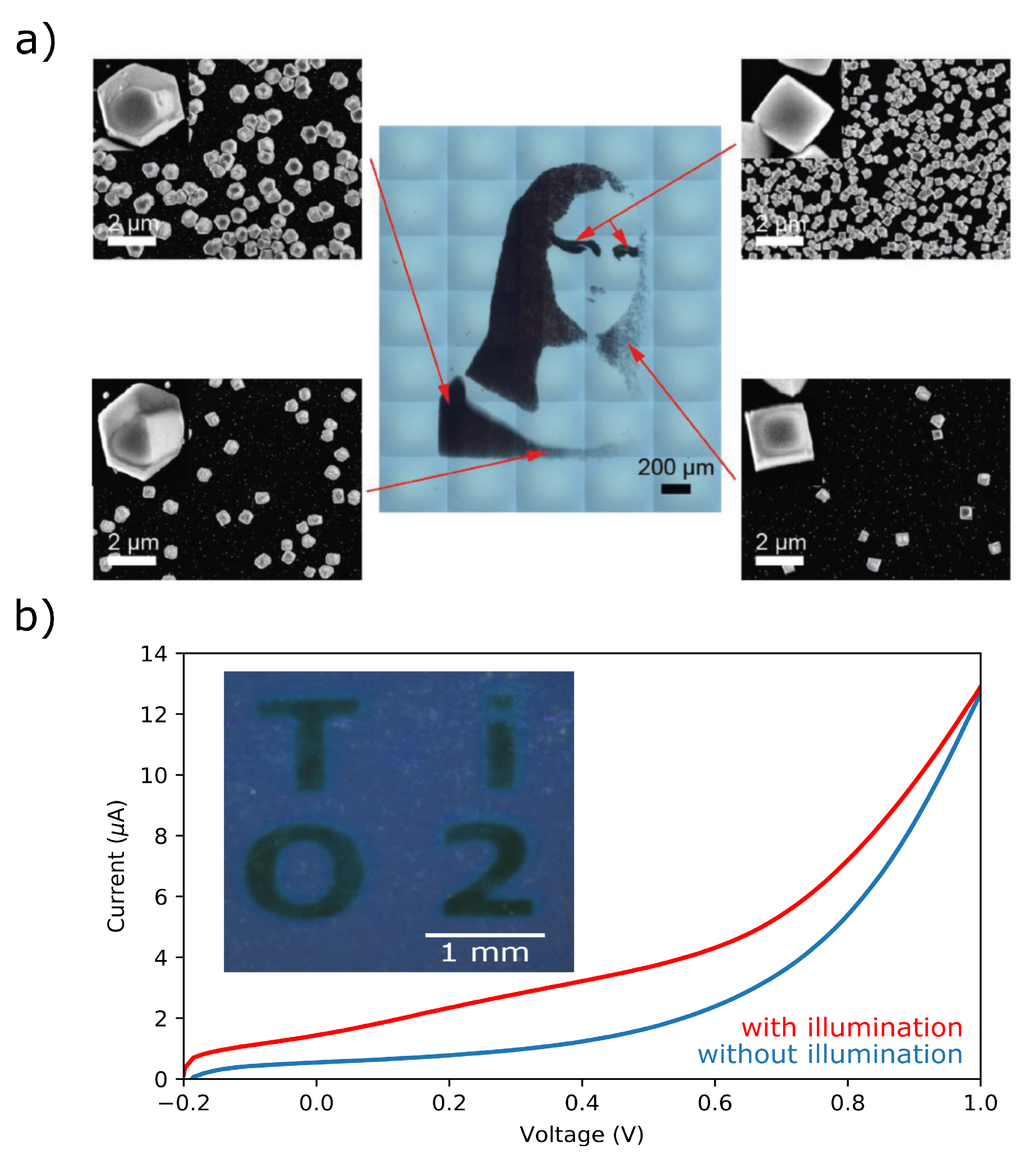
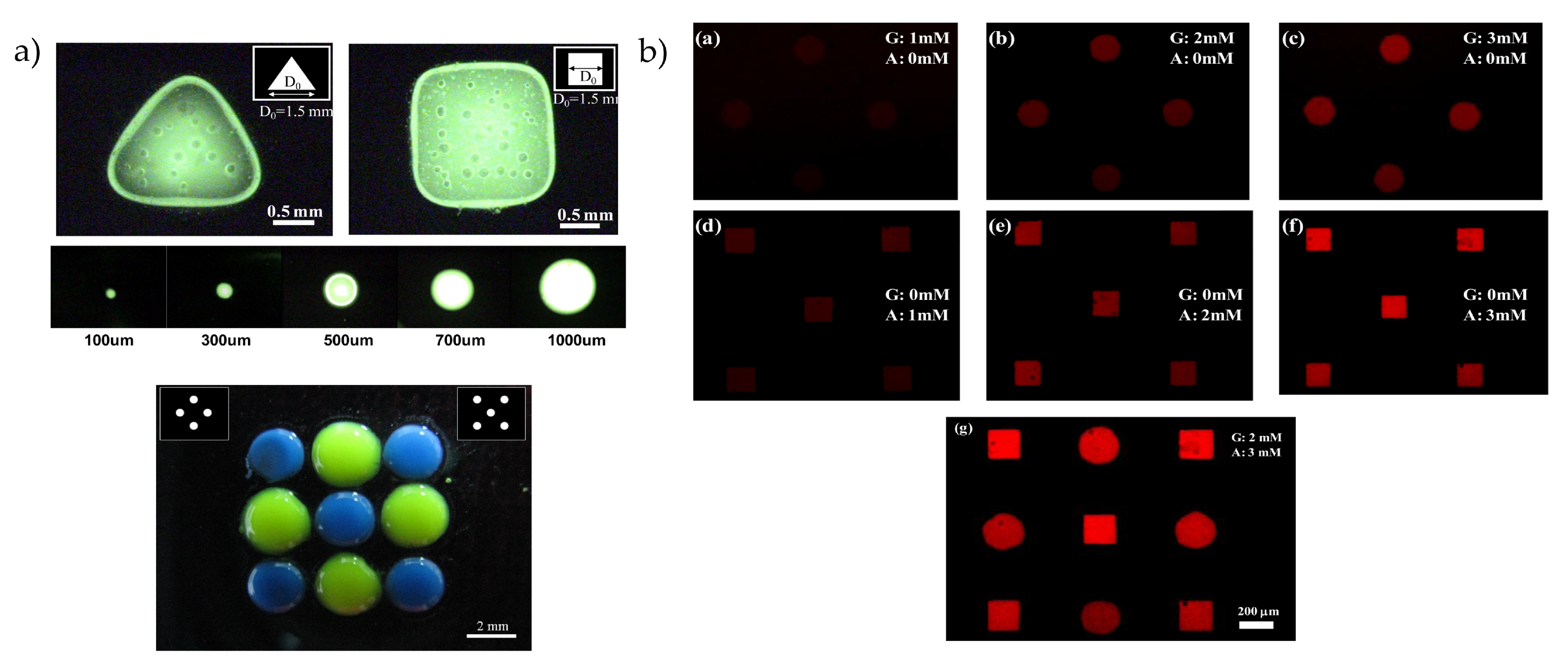
4. Photoelectrochemical Deposition
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Bruce, P.G. Solid State Electrochemistry, 5th ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Schmickler, W.; Santos, E. Interfacial Electrochemistry; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Turner, A.; Karube, I.; Wilson, G. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Pilas, J.; Selmer, T.; Keusgen, M.; Schöning, M.J. Screen-printed carbon electrodes modified with graphene oxide for the design of a reagent-free NAD+-dependent biosensor array. Anal. Chem. 2019, 91, 15293–15299. [Google Scholar] [CrossRef]
- Molinnus, D.; Poghossian, A.; Keusgen, M.; Katz, E.; Schöning, M.J. Coupling of biomolecular logic gates with electronic transducers: From single enzyme logic gates to sense/act/treat chips. Electroanalysis 2017, 29, 1840–1849. [Google Scholar] [CrossRef]
- Molinnus, D.; Hardt, G.; Käver, L.; Willenberg, H.S.; Kröger, J.C.; Poghossian, A.; Keusgen, M.; Schöning, M.J. Chip-based biosensor for the detection of low adrenaline concentrations to support adrenal venous sampling. Sens. Actuators B 2018, 272, 21–27. [Google Scholar] [CrossRef]
- Riedel, M.; Kartchemnik, J.; Schöning, M.J.; Lisdat, F. Impedimetric DNA detection–steps forward to sensorial application. Anal. Chem. 2014, 86, 7867–7874. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef]
- Forster, R.J. Microelectrodes: New dimensions in electrochemistry. Chem. Soc. Rev. 1994, 23, 289–297. [Google Scholar] [CrossRef]
- Arrigan, D.W. Nanoelectrodes, nanoelectrode arrays and their applications. Analyst 2004, 129, 1157–1165. [Google Scholar] [CrossRef]
- Rothe, J.; Frey, O.; Stettler, A.; Chen, Y.; Hierlemann, A. Fully integrated CMOS microsystem for electrochemical measurements on 32× 32 working electrodes at 90 frames per second. Anal. Chem. 2014, 86, 6425–6432. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-based electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Hahn, Y.B.; Ahmad, R.; Tripathy, N. Chemical and biological sensors based on metal oxide nanostructures. Chem. Commun. 2012, 48, 10369–10385. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Lee, S.B. Fast electrochemistry of conductive polymer nanotubes: Synthesis, mechanism, and application. Acc. Chem. Res. 2008, 41, 699–707. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Gerischer, H. Electrochemical behavior of semiconductors under illumination. J. Electrochem. Soc. 1966, 113, 1174–1182. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Neamen, D.A. Semiconductor Physics and Devices: Basic Principles; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Vogel, Y.B.; Gooding, J.J.; Ciampi, S. Light-addressable electrochemistry at semiconductor electrodes: Redox imaging, mask-free lithography and spatially resolved chemical and biological sensing. Chem. Soc. Rev. 2019, 48, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Van de Krol, R. Principles of photoelectrochemical cells. In Photoelectrochemical Hydrogen Production; Springer: Boston, MA, USA, 2012; pp. 13–67. [Google Scholar]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zang, Y.; Fan, J.; Yun, J.; Xue, H.G. Current advances in semiconductor nanomaterials-based photoelectrochemical biosensing. Chem. Eur. J. 2018, 24, 14010–14027. [Google Scholar]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical DNA biosensors. Chem. Rev. 2014, 114, 7421–7441. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical immunoassays. Anal. Chem. 2017, 90, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical enzymatic biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical detection of metal ions. Analyst 2016, 141, 4262–4271. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Werner, C.F.; Miyamoto, K.i.; Schöning, M.J.; Yoshinobu, T. Development and characterisation of a compact light-addressable potentiometric sensor (LAPS) based on the digital light processing (DLP) technology for flexible chemical imaging. Sens. Actuators B 2012, 170, 34–39. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Miyamoto, K.i.; Wagner, T.; Schöning, M.J. Recent developments of chemical imaging sensor systems based on the principle of the light-addressable potentiometric sensor. Sens. Actuators B 2015, 207, 926–932. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Miyamoto, K.i.; Werner, C.F.; Poghossian, A.; Wagner, T.; Schöning, M.J. Light-addressable potentiometric sensors for quantitative spatial imaging of chemical species. Annu. Rev. Anal. Chem. 2017, 10, 225–246. [Google Scholar] [CrossRef]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370–372. [Google Scholar] [CrossRef]
- Ohta, A.T.; Chiou, P.Y.; Han, T.H.; Liao, J.C.; Bhardwaj, U.; McCabe, E.R.; Yu, F.; Sun, R.; Wu, M.C. Dynamic cell and microparticle control via optoelectronic tweezers. J. Microelectromech. Syst. 2007, 16, 491–499. [Google Scholar] [CrossRef]
- Jamshidi, A.; Pauzauskie, P.J.; Schuck, P.J.; Ohta, A.T.; Chiou, P.Y.; Chou, J.; Yang, P.; Wu, M.C. Dynamic manipulation and separation of individual semiconducting and metallic nanowires. Nat. Photonics 2008, 2, 85–89. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef]
- Mryasov, O.; Freeman, A.J. Electronic band structure of indium tin oxide and criteria for transparent conducting behavior. Phys. Rev. B 2001, 64, 233111. [Google Scholar] [CrossRef]
- Rakhshani, A.; Makdisi, Y.; Ramazaniyan, H. Electronic and optical properties of fluorine-doped tin oxide films. J. Appl. Phys. 1998, 83, 1049–1057. [Google Scholar] [CrossRef]
- Van Gompel, M.; Conings, B.; Jiménez Monroy, K.; D ‘Haen, J.; Gilissen, K.; D ‘Olieslaeger, M.; Van Bael, M.; Wagner, P. Preparation of epitaxial films of the transparent conductive oxide Al: ZnO by reactive high-pressure sputtering in Ar/O2 mixtures. Phys. Status Solidi (a) 2013, 210, 1013–1018. [Google Scholar] [CrossRef]
- Bae, D.; Seger, B.; Vesborg, P.C.; Hansen, O.; Chorkendorff, I. Strategies for stable water splitting via protected photoelectrodes. Chem. Soc. Rev. 2017, 46, 1933–1954. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Kitayama, M. Photocatalytic deposition of light-localized polypyrrole film pattern on n-type silicon wafers. Chem. Lett. 1986, 15, 657–660. [Google Scholar] [CrossRef]
- Okano, M.; Itoh, K.; Fujishima, A.; Honda, K. Generation of organic conducting patterns on semiconductors by photoelectrochemical polymerization of pyrrole. Chem. Lett. 1986, 15, 469–472. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Kanzaki, R.; Takahashi, H. Microscale pH gradient generation by electrolysis on a light-addressable planar electrode. Sens. Actuators B 2010, 149, 205–211. [Google Scholar] [CrossRef]
- Vogel, Y.B.; Gonçales, V.R.; Gooding, J.J.; Ciampi, S. Electrochemical microscopy based on spatial light modulators: A projection system to spatially address electrochemical reactions at semiconductors. J. Electrochem. Soc. 2018, 165, H3085–H3092. [Google Scholar] [CrossRef]
- Thomas , C., Jr.; Springer, P.; Loeb, G.; Berwald-Netter, Y.; Okun, L. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Ballini, M.; Müller, J.; Livi, P.; Chen, Y.; Frey, U.; Stettler, A.; Shadmani, A.; Viswam, V.; Jones, I.L.; Jäckel, D.; et al. A 1024-channel CMOS microelectrode array with 26,400 electrodes for recording and stimulation of electrogenic cells in vitro. IEEE J. Solid-State Circuits 2014, 49, 2705–2719. [Google Scholar] [CrossRef] [PubMed]
- Colicos, M.A.; Collins, B.E.; Sailor, M.J.; Goda, Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell 2001, 107, 605–616. [Google Scholar] [CrossRef][Green Version]
- Hung, J.; Colicos, M.A. Astrocytic Ca2+ waves guide CNS growth cones to remote regions of neuronal activity. PLoS ONE 2008, 3, e3692. [Google Scholar] [CrossRef]
- Gutiérrez, R.C.; Flynn, R.; Hung, J.; Kertesz, A.C.; Sullivan, A.; Zamponi, G.W.; El-Husseini, A.; Colicos, M.A. Activity-driven mobilization of post-synaptic proteins. Eur. J. Neurosci. 2009, 30, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Singh, D.; Hollett, G.; Dravid, S.M.; Sailor, M.J.; Arikkath, J. Spatially selective photoconductive stimulation of live neurons. Front. Cell. Neurosci. 2014, 8, 1–9. [Google Scholar] [CrossRef][Green Version]
- Moritz, W.; Yoshinobu, T.; Finger, F.; Krause, S.; Martin-Fernandez, M.; Schöning, M.J. High resolution LAPS using amorphous silicon as the semiconductor material. Sens. Actuators B 2004, 103, 436–441. [Google Scholar] [CrossRef]
- Bucher, V.; Brunner, B.; Leibrock, C.; Schubert, M.; Nisch, W. Electrical properties of a light-addressable microelectrode chip with high electrode density for extracellular stimulation and recording of excitable cells. Biosens. Bioelectron. 2001, 16, 205–210. [Google Scholar] [CrossRef]
- Bucher, V.; Schubert, M.; Kern, D.; Nisch, W. Light-addressed sub-μm electrodes for extracellular recording and stimulation of excitable cells. Microelectron. Eng. 2001, 57, 705–712. [Google Scholar] [CrossRef]
- Bucher, V.; Brugger, J.; Kern, D.; Kim, G.M.; Schubert, M.; Nisch, W. Electrical properties of light-addressed sub-μm electrodes fabricated by use of nanostencil-technology. Microelectron. Eng. 2002, 61, 971–980. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Takahashi, H.; Takayama, Y.; Warisawa, S.; Mitsuishi, M.; Nakao, M.; Jimbo, Y. Light-addressable planar electrode with hydrogenated amorphous silicon and low-conductive passivation layer for stimulation of cultured neurons. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 648–651. [Google Scholar]
- Suzurikawa, J.; Takahashi, H.; Kanzaki, R.; Nakao, M.; Takayama, Y.; Jimbo, Y. Light-addressable electrode with hydrogenated amorphous silicon and low-conductive passivation layer for stimulation of cultured neurons. Appl. Phys. Lett. 2007, 90, 1–3. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Jimbo, Y.; Kanzaki, R.; Takahashi, H. Light-addressed stimulation under Ca2+ imaging of cultured neurons. IEEE Trans. Biomed. Eng. 2009, 56, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Suzurikawa, J.; Kanzaki, R.; Nakao, M.; Jimbo, Y.; Takahashi, H. Optimization of thin-film configuration for light-addressable stimulation electrode. Electron. Commun. Jpn. 2011, 94, 61–68. [Google Scholar] [CrossRef]
- Suzurikawa, J.; Nakao, M.; Jimbo, Y.; Kanzaki, R.; Takahashi, H. A light addressable electrode with a TiO2 nanocrystalline film for localized electrical stimulation of cultured neurons. Sens. Actuators B 2014, 192, 393–398. [Google Scholar] [CrossRef]
- Huang, Y.; Mason, A.J. Lab-on-CMOS integration of microfluidics and electrochemical sensors. Lab Chip 2013, 13, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Breuer, L.; Mang, T.; Schöning, M.J.; Thoelen, R.; Wagner, T. Investigation of the spatial resolution of a laser-based stimulation process for light-addressable hydrogels with incorporated graphene oxide by means of IR thermography. Sens. Actuators A 2017, 268, 126–132. [Google Scholar] [CrossRef]
- Miyamoto, K.i.; Ichimura, H.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Chemical imaging of the concentration profile of ion diffusion in a microfluidic channel. Sens. Actuators B 2013, 189, 240–245. [Google Scholar] [CrossRef]
- Miyamoto, K.i.; Itabashi, A.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. High-speed chemical imaging inside a microfluidic channel. Sens. Actuators B 2014, 194, 521–527. [Google Scholar] [CrossRef]
- An, R.; Massa, K.; Wipf, D.O.; Minerick, A.R. Solution pH change in non-uniform alternating current electric fields at frequencies above the electrode charging frequency. Biomicrofluidics 2014, 8, 1–13. [Google Scholar] [CrossRef]
- Cheng, L.J.; Chang, H.C. Microscale pH regulation by splitting water. Biomicrofluidics 2011, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Chang, H.C. Switchable pH actuators and 3D integrated salt bridges as new strategies for reconfigurable microfluidic free-flow electrophoretic separation. Lab Chip 2014, 14, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Macounová, K.; Cabrera, C.R.; Holl, M.R.; Yager, P. Generation of natural pH gradients in microfluidic channels for use in isoelectric focusing. Anal. Chem. 2000, 72, 3745–3751. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M. Detecting Both Physical and (Bio-)Chemical Parameters by Means of ISFET Devices. Electroanalysis 2004, 16, 1863–1872. [Google Scholar] [CrossRef]
- Hafeman, D.G.; Harkins, J.B.; Witkowski, C.E.; Lewis, N.S.; Warmack, R.J.; Brown, G.M.; Thundat, T. Optically directed molecular transport and 3D isoelectric positioning of amphoteric biomolecules. Proc. Natl. Acad. Sci. USA 2006, 103, 6436–6441. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsueh, H.J.; Jiang, Y.L. Light-addressable electrodeposition of cell-encapsulated alginate hydrogels for a cellular microarray using a digital micromirror device. Biomicrofluidics 2011, 5, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Lai, H.S.S.; Liu, Y.T.; Li, W.J.; Shen, Y.J. Three-dimensional calcium alginate hydrogel assembly via tiopc-based light-induced controllable electrodeposition. Micromachines 2017, 8, 192. [Google Scholar] [CrossRef]
- Huang, S.H.; Wei, L.S.; Chu, H.T.; Jiang, Y.L. Light-addressed electrodeposition of enzyme-entrapped chitosan membranes for multiplexed enzyme-based bioassays using a digital micromirror device. Sensors 2013, 13, 10711–10724. [Google Scholar] [CrossRef]
- Huang, S.H.; Wei, L.S. Light-addressed electrodeposition of polysaccharide chitosan membranes on a photoconductive substrate. Int. J. Autom. Smart Technol. 2014, 4, 196–201. [Google Scholar]
- Andreeva, D.V.; Melnyk, I.; Baidukova, O.; Skorb, E.V. Local pH gradient initiated by light on TiO2 for light-triggered modulation of polyhistidine-tagged proteins. ChemElectroChem 2016, 3, 1306–1310. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Brezesinski, G.; Möhwald, H.; Fratzl, P.; Schacher, F.H.; Poznyak, S.K.; Andreeva, D.V.; Skorb, E.V. Light-induced water splitting causes high-amplitude oscillation of pH-sensitive layer-by-layer assemblies on TiO2. Angew. Chem. Int. Ed. 2016, 55, 13001–13004. [Google Scholar] [CrossRef] [PubMed]
- Maltanava, H.M.; Poznyak, S.K.; Andreeva, D.V.; Quevedo, M.C.; Bastos, A.C.; Tedim, J.; Ferreira, M.G.; Skorb, E.V. Light-induced proton pumping with a semiconductor: Vision for photoproton lateral separation and robust manipulation. ACS Appl. Mater. Interfaces 2017, 9, 24282–24289. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.H.; Ciampi, S.; Yang, Y.; Tavallaie, R.; Zhu, Y.; Zarei, L.; Gonçales, V.R.; Gooding, J.J. Connecting electrodes with light: One wire, many electrodes. Chem. Sci. 2015, 6, 6769–6776. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B. Ferrocene-terminated monolayers covalently bound to hydrogen-terminated silicon surfaces. Toward the development of charge storage and communication devices. Acc. Chem. Res. 2010, 43, 1509–1518. [Google Scholar] [CrossRef]
- Fabre, B. Functionalization of oxide-free silicon surfaces with redox-active assemblies. Chem. Rev. 2016, 116, 4808–4849. [Google Scholar] [CrossRef]
- Yang, Y.; Ciampi, S.; Choudhury, M.H.; Gooding, J.J. Light activated electrochemistry: Light intensity and pH dependence on electrochemical performance of anthraquinone derivatized silicon. J. Phys. Chem. C 2016, 120, 2874–2882. [Google Scholar] [CrossRef]
- Yang, Y.; Ciampi, S.; Zhu, Y.; Gooding, J.J. Light-activated electrochemistry for the two-dimensional interrogation of electroactive regions on a monolithic surface with dramatically improved spatial resolution. J. Phys. Chem. C 2016, 120, 13032–13038. [Google Scholar] [CrossRef]
- Lian, J.; Yang, Y.; Wang, W.; Parker, S.G.; Gonçales, V.R.; Tilley, R.D.; Gooding, J.J. Amorphous silicon on indium tin oxide: A transparent electrode for simultaneous light activated electrochemistry and optical microscopy. Chem. Commun. 2019, 55, 123–126. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 1–11. [Google Scholar] [CrossRef]
- Skompska, M.; Zarębska, K. Electrodeposition of ZnO nanorod arrays on transparent conducting substrates—A review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Micheels, R.H.; Darrow, A.D.; Rauh, R.D. Photoelectrochemical deposition of microscopic metal film patterns on Si and GaAs. Appl. Phys. Lett. 1981, 39, 418–420. [Google Scholar] [CrossRef]
- Dasog, M.; Carim, A.I.; Yalamanchili, S.; Atwater, H.A.; Lewis, N.S. Profiling photoinduced carrier generation in semiconductor microwire arrays via photoelectrochemical metal deposition. Nano Lett. 2016, 16, 5015–5021. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Honda, K. Photoelectrochemical imaging processes using semiconductor electrodes. Chem. Lett. 1978, 7, 1197–1200. [Google Scholar] [CrossRef]
- Baba, R.; Konda, R.; Fujishima, A.; Honda, K. Photoelectrochemical deposition of metals on TiO2 powders in the presence of alcohols. Chem. Lett. 1986, 15, 1307–1310. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, Y.R.; Ha, K.; Lee, J.K.; Lee, J.G.; Jang, W.; Lee, J.Y.; Bae, J.H.; Chung, T.D. Light-guided electrodeposition of non-noble catalyst patterns for photoelectrochemical hydrogen evolution. Energy Environ. Sci. 2015, 8, 3654–3662. [Google Scholar] [CrossRef]
- Seo, D.; Lim, S.Y.; Lee, J.; Yun, J.; Chung, T.D. Robust and high spatial resolution light addressable electrochemistry using hematite (α-Fe2O3) Photoanodes. ACS Appl. Mater. Interfaces 2018, 10, 33662–33668. [Google Scholar] [CrossRef]
- Zhong, D.K.; Cornuz, M.; Sivula, K.; Grätzel, M.; Gamelin, D.R. Photo-assisted electrodeposition of cobalt–phosphate (Co–Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ. Sci. 2011, 4, 1759–1764. [Google Scholar] [CrossRef]
- McDonald, K.J.; Choi, K.S. Photodeposition of Co-based oxygen evolution catalysts on α-Fe2O3 photoanodes. Chem. Mater. 2011, 23, 1686–1693. [Google Scholar] [CrossRef]
- Vogel, Y.B.; Gonçales, V.R.; Al-Obaidi, L.; Gooding, J.J.; Darwish, N.; Ciampi, S. Nanocrystal inks: Photoelectrochemical printing of Cu2O nanocrystals on silicon with 2D control on polyhedral shapes. Adv. Funct. Mater. 2018, 1804791, 1–9. [Google Scholar] [CrossRef]
- Funt, B.L.; Tan, S.R. The photoelectrochemical initiation of polymerization of styrene. J. Polym. Sci.: Polym. Chem. Ed. 1984, 22, 605–608. [Google Scholar] [CrossRef]
- Janáky, C.; de Tacconi, N.R.; Chanmanee, W.; Rajeshwar, K. Bringing conjugated polymers and oxide nanoarchitectures into intimate contact: Light-induced electrodeposition of polypyrrole and polyaniline on nanoporous WO3 or TiO2 nanotube array. J. Phys. Chem. C 2012, 116, 19145–19155. [Google Scholar] [CrossRef]
- Okano, M.; Itoh, K.; Fujishima, A. Incorporation of dye molecules in polypyrrole films in the process of photoelectrochemical deposition. Chem. Lett. 1987, 16, 2129–2130. [Google Scholar] [CrossRef]
- Okano, M.; Kikuchi, E.; Itoh, K.; Fujishima, A. Photoelectrochemical polymerization of pyrrole on ZnO and its application to conducting pattern generation. J. Electrochem. Soc. 1988, 135, 1641–1645. [Google Scholar] [CrossRef]
- Okano, M.; Baba, R.; Itoh, K.; Fujishima, A. New aspects in area-selective electrode reactions on illuminated semiconductors. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; Volume 47, pp. 375–387. [Google Scholar]
- Takagi, S.; Makuta, S.; Veamatahau, A.; Otsuka, Y.; Tachibana, Y. Organic/inorganic hybrid electrochromic devices based on photoelectrochemically formed polypyrrole/TiO2 nanohybrid films. J. Mater. Chem. 2012, 22, 22181–22189. [Google Scholar] [CrossRef]
- Welden, R.; Scheja, S.; Schöning, M.J.; Wagner, P.; Wagner, T. Electrochemical Evaluation of Light-Addressable Electrodes Based on TiO2 for the Integration in Lab-on-Chip Systems. Phys. Status Solidi (a) 2018, 215, 1800150. [Google Scholar] [CrossRef]
- Janáky, C.; Chanmanee, W.; Rajeshwar, K. Mechanistic aspects of photoelectrochemical polymerization of polypyrrole on a TiO2 nanotube array. Electrochim. Acta 2014, 122, 303–309. [Google Scholar] [CrossRef]
- Kierstan, M.; Bucke, C. The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels. Biotechnol. Bioeng. 1977, 19, 387–397. [Google Scholar] [CrossRef]
- Gurtner, C.; Edman, C.F.; Formosa, R.E.; Heller, M.J. Photoelectrophoretic transport and hybridization of DNA oligonucleotides on unpatterned silicon substrates. J. Am. Chem. Soc. 2000, 122, 8589–8594. [Google Scholar] [CrossRef]
- Chow, B.Y.; Emig, C.J.; Jacobson, J.M. Photoelectrochemical synthesis of DNA microarrays. Proc. Natl. Acad. Sci. USA 2009, 106, 15219–15224. [Google Scholar] [CrossRef]
- Jacobs, J.W.; Rikken, J.M. Photoelectrochemically-induced gold deposition on p-GaAs electrodes part I. Nucleation and growth. J. Electrochem. Soc. 1989, 136, 3633–3640. [Google Scholar] [CrossRef]
- Ngaboyamahina, E.; Cachet, H.; Pailleret, A.; Sutter, E. Photo-assisted electrodeposition of an electrochemically active polypyrrole layer on anatase type titanium dioxide nanotube arrays. Electrochim. Acta 2014, 129, 211–221. [Google Scholar] [CrossRef]



| Type of Material | Deposited Material | Type of Semiconductor | References |
|---|---|---|---|
| Metal (oxide) | Au | p-Si | [101,102] |
| Au | p-GaAs | [101,121] | |
| Ag | p-GaP | [103] | |
| Cu, Ni | p-Si | [101] | |
| Cu, Ni | p-GaAs | [101] | |
| Au, Cu, Pt, Ag | TiO2 | [104] | |
| Pt, Au, Ni–Mo | a:Si | [105] | |
| Co–Pi | -Fe2O3 | [106,107,108] | |
| Cu2O | a:Si | [105] | |
| Polymer | Polystyrene | TiO2 | [110] |
| Polyaniline | WO3 | [111] | |
| Polyaniline | TiO2 | [111] | |
| Polypyrrol | TiO2 | [56,111,112,114,115,122] | |
| Polypyrrol | n-Si | [55,92] | |
| Polypyrrol | ZnO | [113] | |
| Others | Calcium alginate gels | a:Si | [110] |
| Calcium alginate gels | TiOPc | [85,86] | |
| Chitosan | a:Si | [87,88] | |
| Polyhistidine-tagged proteins | TiO2 | [89] | |
| DNA oligonucleotids | n-Si | [119] | |
| DNA oligonucleotids | a:Si | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welden, R.; Schöning, M.J.; Wagner, P.H.; Wagner, T. Light-addressable Electrodes for Dynamic and Flexible Addressing of Biological Systems and Electrochemical Reactions. Sensors 2020, 20, 1680. https://doi.org/10.3390/s20061680
Welden R, Schöning MJ, Wagner PH, Wagner T. Light-addressable Electrodes for Dynamic and Flexible Addressing of Biological Systems and Electrochemical Reactions. Sensors. 2020; 20(6):1680. https://doi.org/10.3390/s20061680
Chicago/Turabian StyleWelden, Rene, Michael J. Schöning, Patrick H. Wagner, and Torsten Wagner. 2020. "Light-addressable Electrodes for Dynamic and Flexible Addressing of Biological Systems and Electrochemical Reactions" Sensors 20, no. 6: 1680. https://doi.org/10.3390/s20061680
APA StyleWelden, R., Schöning, M. J., Wagner, P. H., & Wagner, T. (2020). Light-addressable Electrodes for Dynamic and Flexible Addressing of Biological Systems and Electrochemical Reactions. Sensors, 20(6), 1680. https://doi.org/10.3390/s20061680







