Humidity-Resistive Optical NO Gas Sensor Devices Based on Cobalt Tetraphenylporphyrin Dispersed in Hydrophobic Polymer Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the CoTPP(-Polymer) Film
2.3. Film Characterization
2.4. NO Gas Sensing Properties
3. Results and Discussion
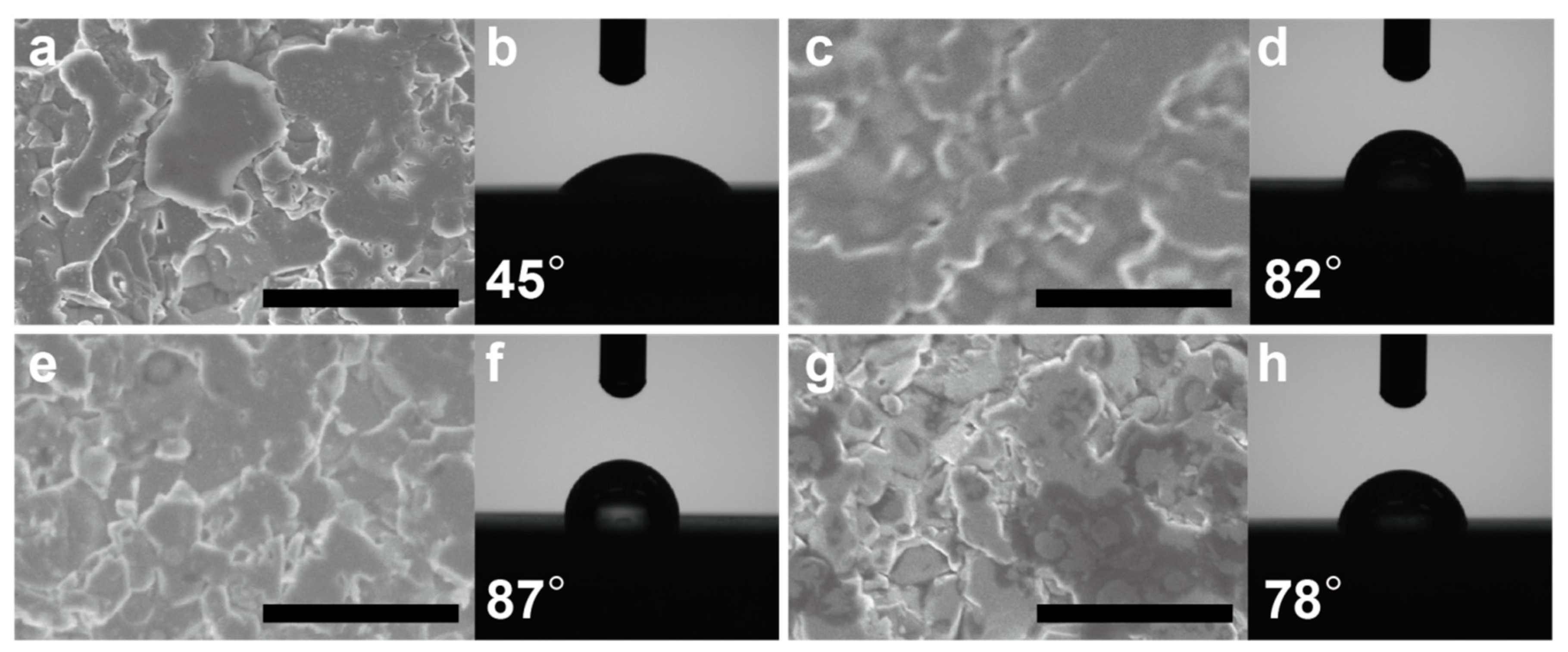
3.1. Structural Investigation and Hydrophobicity of the Coated Film
3.2. Estimation of the Extent of CoTPP Aggregation
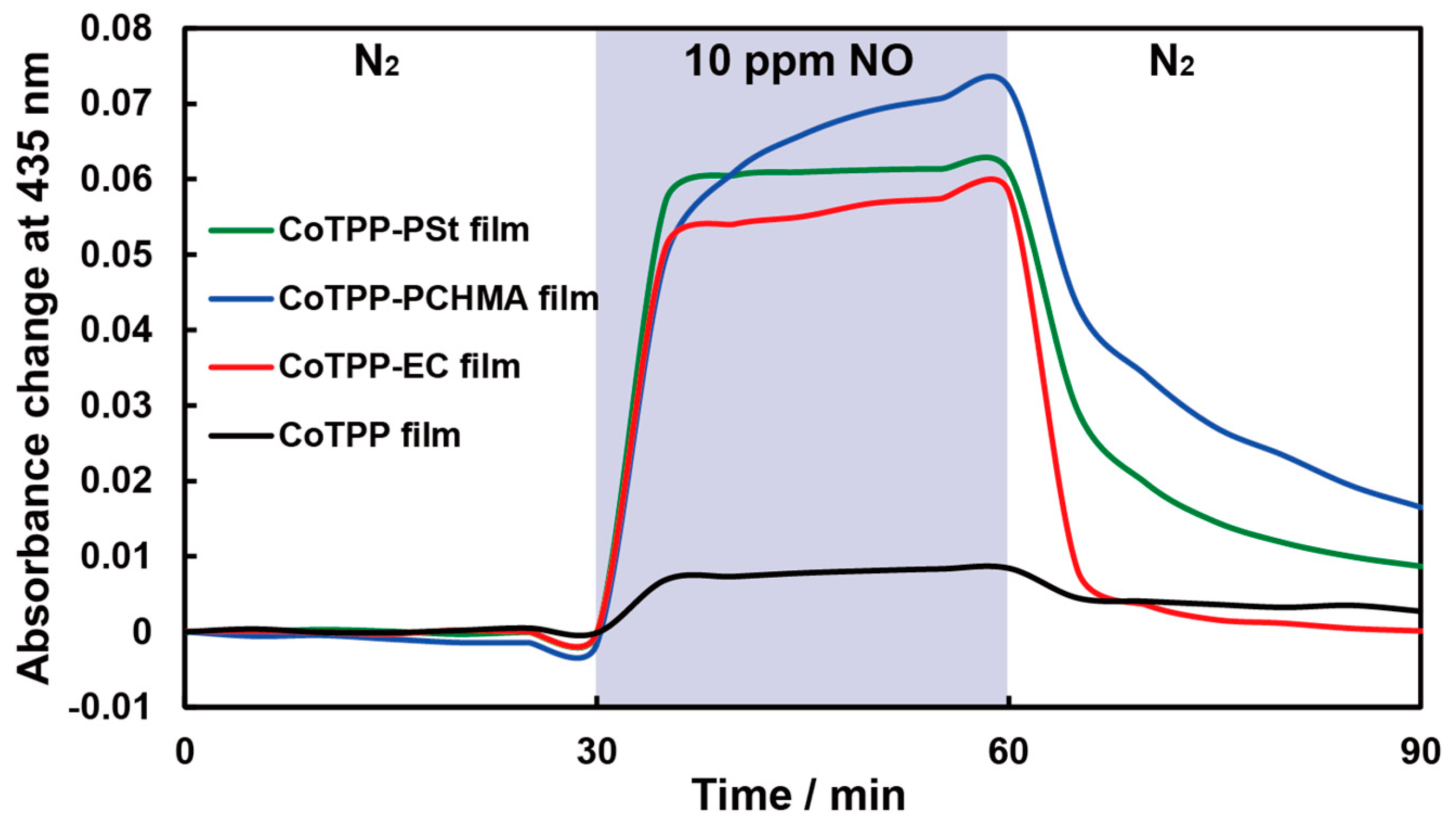
3.3. Sensor Responses to NO Gas
3.4. Humidity Dependence of NO Sensitivity
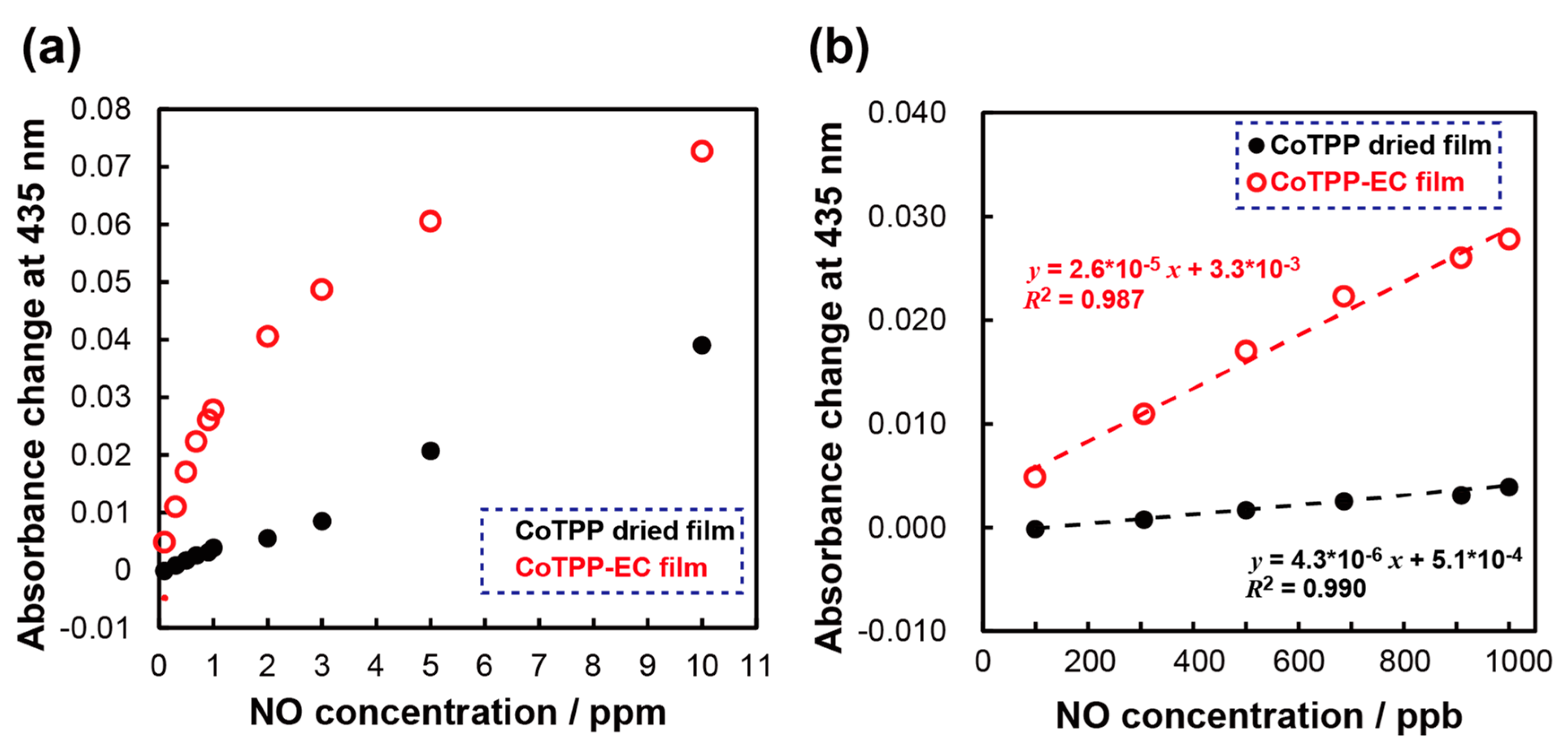
3.5. Calibration Curves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amor, R.E.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28. [Google Scholar] [CrossRef]
- Markar, S.R.; Wiggins, T.; Antonowicz, S.; Chin, S.T.; Romano, A.; Nikolic, K.; Evans, B.; Cunningham, D.; Mughal, M.; Lagergren, J.; et al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol. 2018, 4, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Durán-Acevedo, C.M.; Jaimes-Mogollón, A.L.; Gualdrón-Guerrero, O.E.; Welearegay, T.G.; Martinez-Marín, J.D.; Caceres-Tarazona, J.M.; Sánchez-Acevedo, Z.C.; Beleño-Saenz, K.J.; Cindemir, U.; österlund, L.; et al. Exhaled breath analysis for gastric cancer diagnosis in Colombian patients. Oncotarget 2018, 9, 28805–28817. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Lee, D.S.; Ban, S.W.; Oh, J.; Jung, M.Y.; Kim, S.H.; Park, S.; Persaud, K.; Jheon, S. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens. Actuators B Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Ibrahim, B.; Basanta, M.; Cadden, P.; Singh, D.; Douce, D.; Woodcock, A.; Fowler, S.J. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax 2011, 66, 804–809. [Google Scholar] [CrossRef]
- Schneider, A.; Tilemann, L.; Schermer, T.; Gindner, L.; Laux, G.; Szecsenyi, J.; Meyer, F.J. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement-results of a prospective diagnostic study. Respir. Res. 2009, 10, 15. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef]
- Sachdeva, S.; Agarwal, R.; Agarwal, A. MEMS based tin oxide thin film gas sensor for diabetes mellitus applications. Microsyst. Technol. 2019, 25, 2571–2586. [Google Scholar] [CrossRef]
- Kabir, E.; Raza, N.; Kumar, V.; Singh, J.; Tsang, Y.F.; Lim, D.K.; Szulejko, J.E.; Kim, K.H. Recent Advances in Nanomaterial-Based Human Breath Analytical Technology for Clinical Diagnosis and the Way Forward. Chem. 2019, 5, 3020–3057. [Google Scholar] [CrossRef]
- Zajda, J.; Schmidt, N.J.; Zheng, Z.; Wang, X.; Meyerhoff, M.E. Performance of Amperometric Platinized—Nafion Based Gas Phase Sensor for Determining Nitric Oxide (NO) Levels in Exhaled Human Nasal Breath. Electroanalysis 2018, 30, 1602–1607. [Google Scholar] [CrossRef]
- Mleth, M.; Schubert, J.K.; Gröger, T.; Sabei, B.; Kischkel, S.; Fuchs, P.; Hein, D.; Zimmermann, R.; Miekisch, W. Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal. Chem. 2010, 82, 2541–2551. [Google Scholar] [CrossRef]
- Wang, X.R.; Cassells, J.; Berna, A.Z. Stability control for breath analysis using GC-MS. J. Chromatogr. B 2018, 1097, 27–34. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Schroeder, V.; Kim, B.J.; Swager, T.M. Ionic Liquid-Carbon Nanotube Sensor Arrays for Human Breath Related Volatile Organic Compounds. ACS Sens. 2018, 3, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Moon, H.G.; Lim, C.; Choi, K.; Song, H.S.; Bae, S.; Kim, S.M.; Seo, M.; Lee, T.; Lee, S.; et al. Humidity-Tolerant Single-Stranded DNA-Functionalized Graphene Probe for Medical Applications of Exhaled Breath Analysis. Adv. Funct. Mater. 2017, 27, 1700068. [Google Scholar] [CrossRef]
- Alizadeh, T.; Soltani, L.H. Reduced graphene oxide-based gas sensor array for pattern recognition of DMMP vapor. Sens. Actuators B Chem. 2016, 234, 361–370. [Google Scholar] [CrossRef]
- Manno, D.; Micocci, G.; Serra, A.; Tepore, A.; Valli, L.; Arnold, D.P. Gas sensing properties of meso, meso′-buta-1,3-diyne-bridged Cu(II) octaethylporphyrin dimer Langmuir-Blodgett films. Sens. Actuators B Chem. 1999, 57, 179–182. [Google Scholar] [CrossRef]
- Andringa, A.M.; Spijkman, M.J.; Smits, E.C.P.; Mathijssen, S.G.J.; Hal, P.A.v.; Setayesh, S.; Willard, N.P.; Borshchev, O.V.; Ponomarenko, S.A.; Blom, P.W.M.; et al. Gas sensing with self-assembled monolayer field-effect transistors. Org. Electron. 2010, 11, 895–898. [Google Scholar] [CrossRef]
- Qin, W.; Parzuchowski, P.; Zhang, W.; Meyerhoff, M.E. Optical sensor for amine vapors based on dimer-monomer equilibrium of indium(III) octaethylporphyrin in a polymeric film. Anal. Chem. 2003, 75, 332–340. [Google Scholar] [CrossRef]
- Kang, Y.; Meyerhoff, M.E. Rapid response optical ion/gas sensors using dimer-monomer metalloporphyrin equilibrium in ultrathin polymeric films coated on waveguides. Anal. Chim. Acta 2006, 565, 1–9. [Google Scholar] [CrossRef]
- Tonezzer, M.; Quaranta, A.; Maggioni, G.; Carturan, S.; Mea, G.D. Optical sensing responses of tetraphenyl porphyrins toward alcohol vapours: A comparison between vacuum evaporated and spin-coated thin films. Sens. Actuators B Chem. 2007, 122, 620–626. [Google Scholar] [CrossRef]
- Biring, S.; Sadhu, A.S.; Deb, M. An effective optical dual gas sensor for simultaneous detection of oxygen and ammonia. Sensors 2019, 19, 5124. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Rossi, R.; Alvisi, M.; Signore, M.A.; Serra, E.; Paolesse, R.; D’Amico, A.; Di Natale, C. Metalloporphyrins-modified carbon nanotubes networked films-based chemical sensors for enhanced gas sensitivity. Sens. Actuators B Chem. 2010, 144, 387–394. [Google Scholar] [CrossRef]
- Liu, S.F.; Moh, L.C.H.; Swager, T.M. Single-walled carbon nanotube-metalloporphyrin chemiresistive gas sensor arrays for volatile organic compounds. Chem. Mater. 2015, 27, 3560–3563. [Google Scholar] [CrossRef]
- Dunbar, A.D.F.; Brittle, S.; Richardson, T.H.; Hutchinson, J.; Hunter, C.A. Detection of volatile organic compounds using porphyrin derivatives. J. Phys. Chem. B 2010, 114, 11697–11702. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Pang, W.; Zhang, H.; Zhang, D.; Duan, X. Detection of Volatile Organic Compounds Using Microfabricated Resonator Array Functionalized with Supramolecular Monolayers. ACS Appl. Mater. Interfaces 2015, 7, 17893–17903. [Google Scholar] [CrossRef]
- Okajima, T.; Yamamoto, Y.; Ouchi, Y.; Seki, K. NEXAFS spectra of metallotetraphenylporphyrins with adsorbed nitrogen monoxide. J. Electron. Spectrosc. Relat. Phenom. 2001, 114, 849–854. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hanazato, Y. Nitrogen Monoxide Adsorption and Contact Decomposition Properties of Co(II) Complexes. Nippon Kagaku Kaishi 1998, 1998, 338–345. [Google Scholar] [CrossRef][Green Version]
- Miki, H.; Matsubara, F.; Nakashima, S.; Ochi, S.; Nakagawa, K.; Matsuguchi, M.; Sadaoka, Y. A fractional exhaled nitric oxide sensor based on optical absorption of cobalt tetraphenylporphyrin derivatives. Sens. Actuators B Chem. 2016, 231, 458–468. [Google Scholar] [CrossRef]
- Itagaki, Y.; Yamanaka, S.; Sadaoka, Y. HCl detection using polymer-porphyrin composite coated optical fiber sensor. Sens. Lett. 2011, 9, 114–117. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiba, S.; Yamada, K.; Matsuguchi, M. Humidity-Resistive Optical NO Gas Sensor Devices Based on Cobalt Tetraphenylporphyrin Dispersed in Hydrophobic Polymer Matrix. Sensors 2020, 20, 1295. https://doi.org/10.3390/s20051295
Shiba S, Yamada K, Matsuguchi M. Humidity-Resistive Optical NO Gas Sensor Devices Based on Cobalt Tetraphenylporphyrin Dispersed in Hydrophobic Polymer Matrix. Sensors. 2020; 20(5):1295. https://doi.org/10.3390/s20051295
Chicago/Turabian StyleShiba, Shunsuke, Kohei Yamada, and Masanobu Matsuguchi. 2020. "Humidity-Resistive Optical NO Gas Sensor Devices Based on Cobalt Tetraphenylporphyrin Dispersed in Hydrophobic Polymer Matrix" Sensors 20, no. 5: 1295. https://doi.org/10.3390/s20051295
APA StyleShiba, S., Yamada, K., & Matsuguchi, M. (2020). Humidity-Resistive Optical NO Gas Sensor Devices Based on Cobalt Tetraphenylporphyrin Dispersed in Hydrophobic Polymer Matrix. Sensors, 20(5), 1295. https://doi.org/10.3390/s20051295




