A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
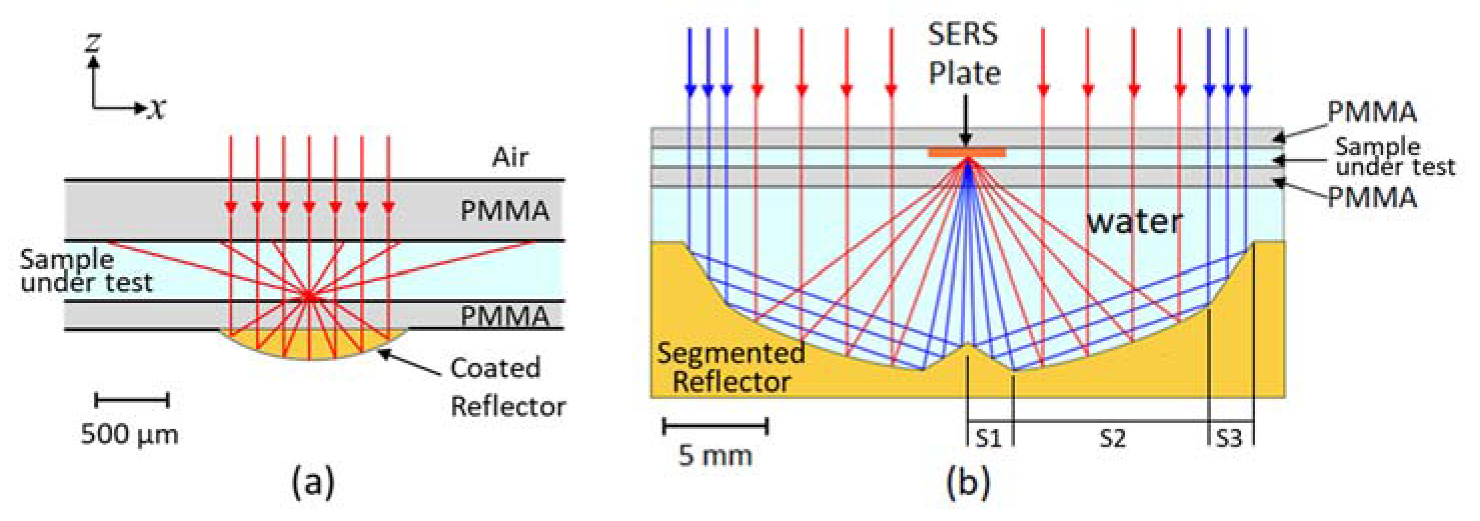
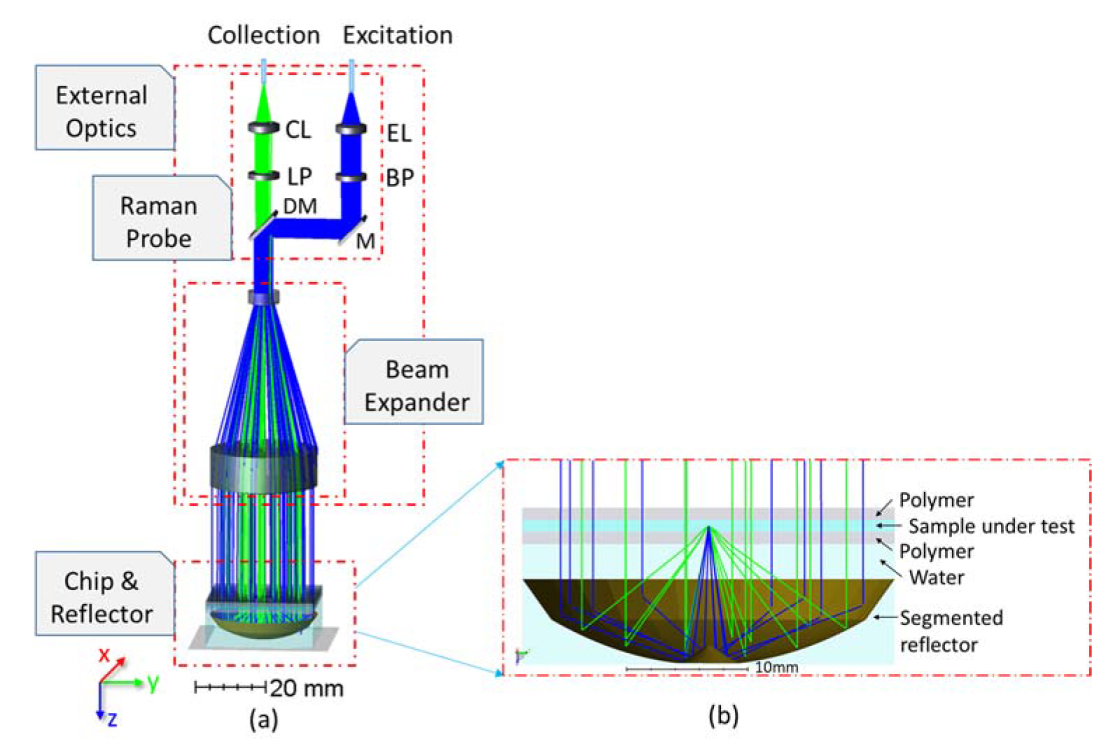
2.1. Design of the Microfluidic Chip and Freeform Segmented Reflector
2.2. Non-Sequential Simulation
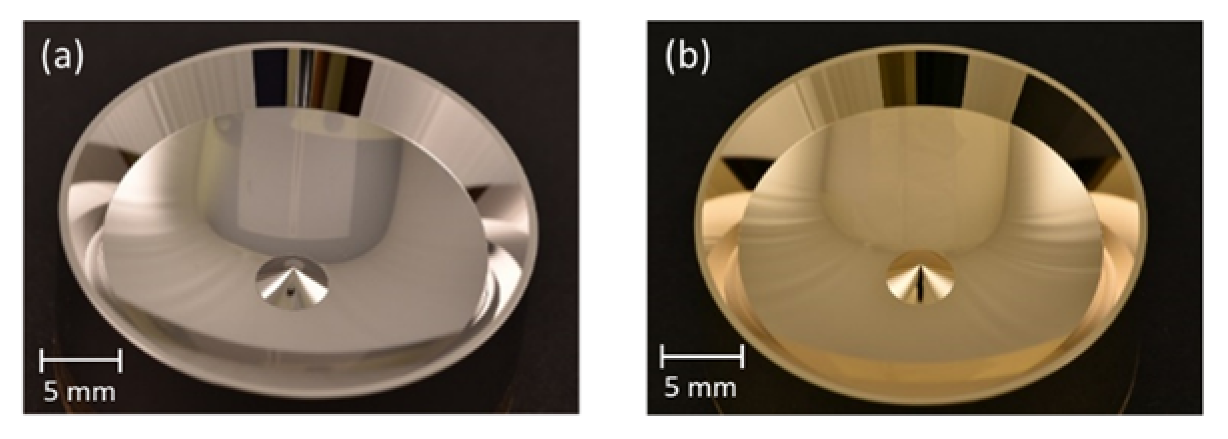
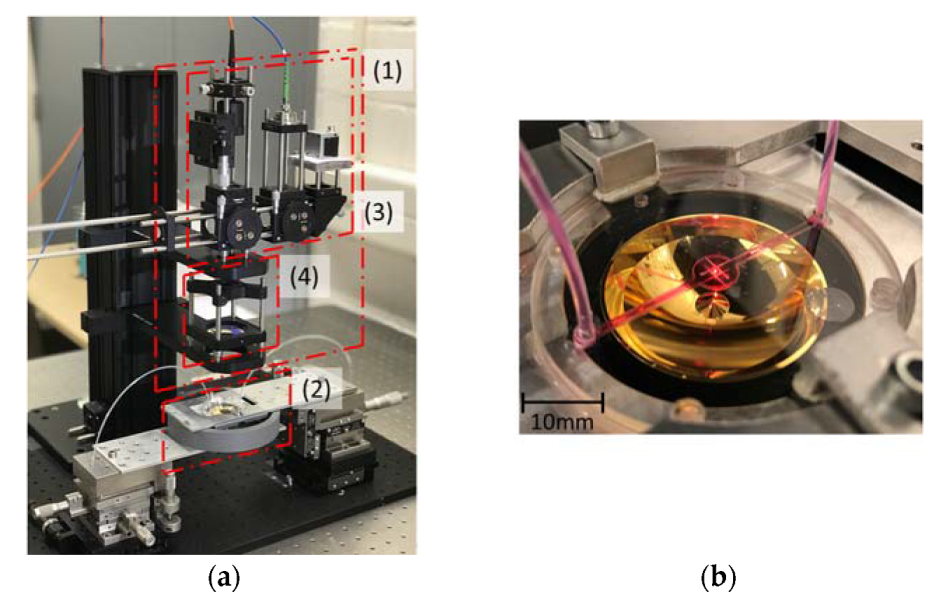
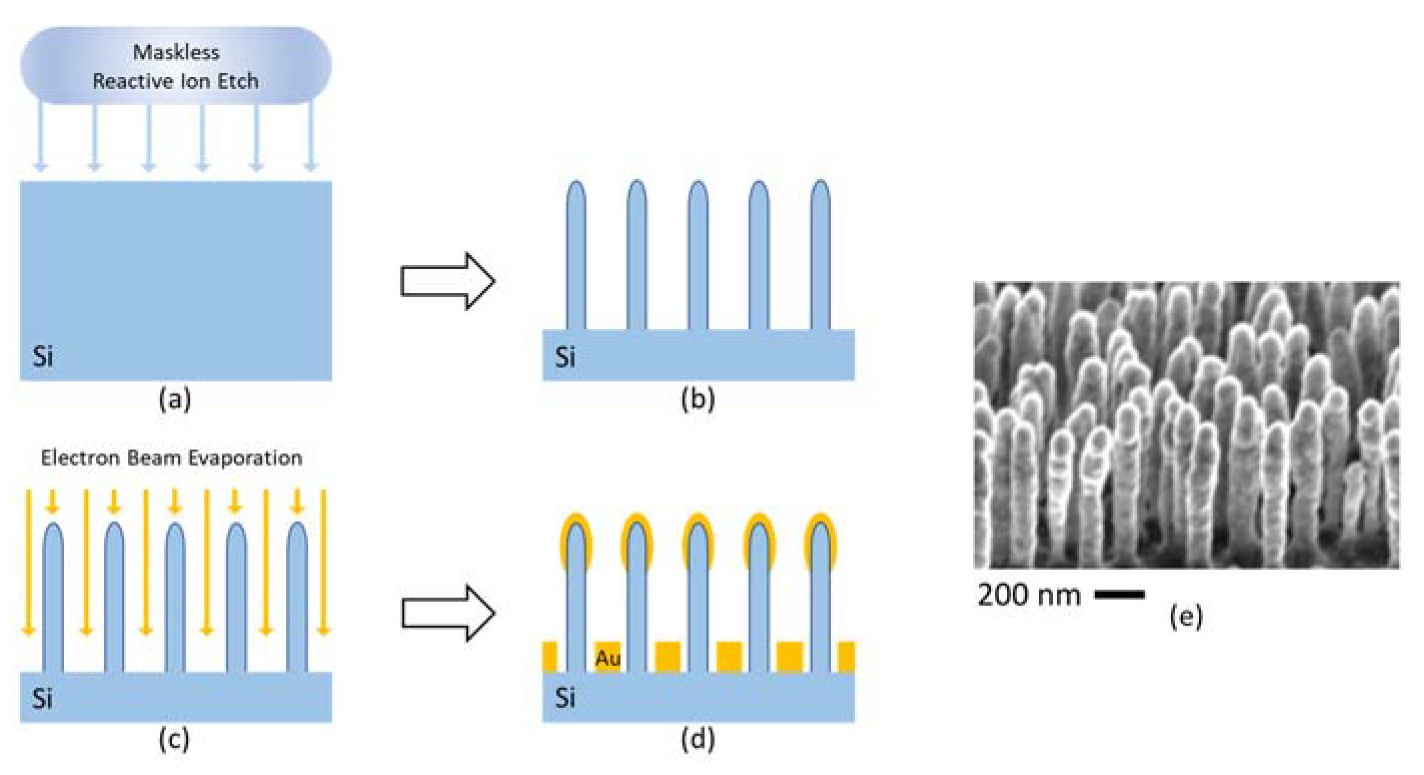
2.3. Fabrication of the Segmented Reflector and the Microfluidic Chip
2.4. Preparation of the SERS Substrates and SERS Chip
3. Results and Discussion
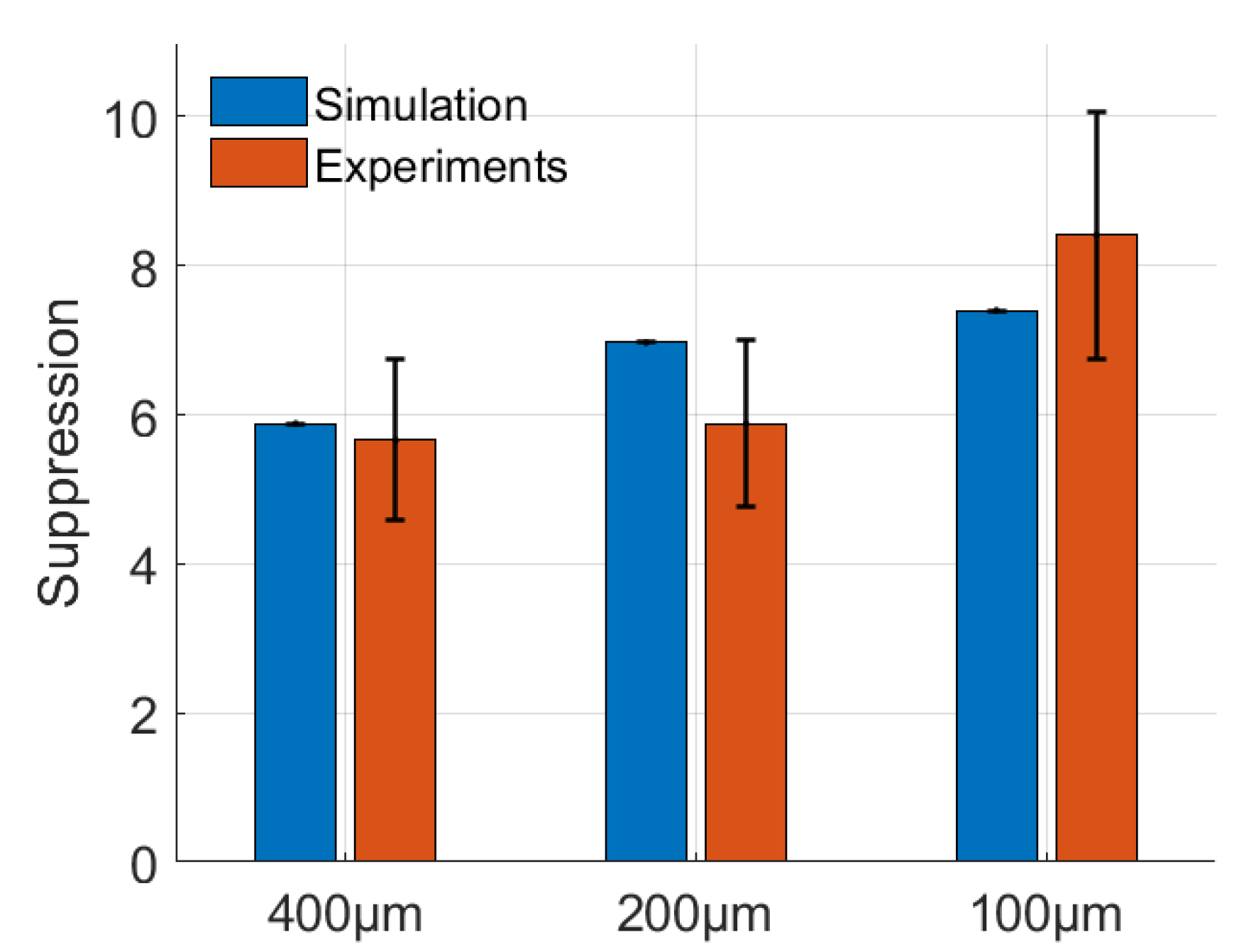
3.1. Suppression Factor
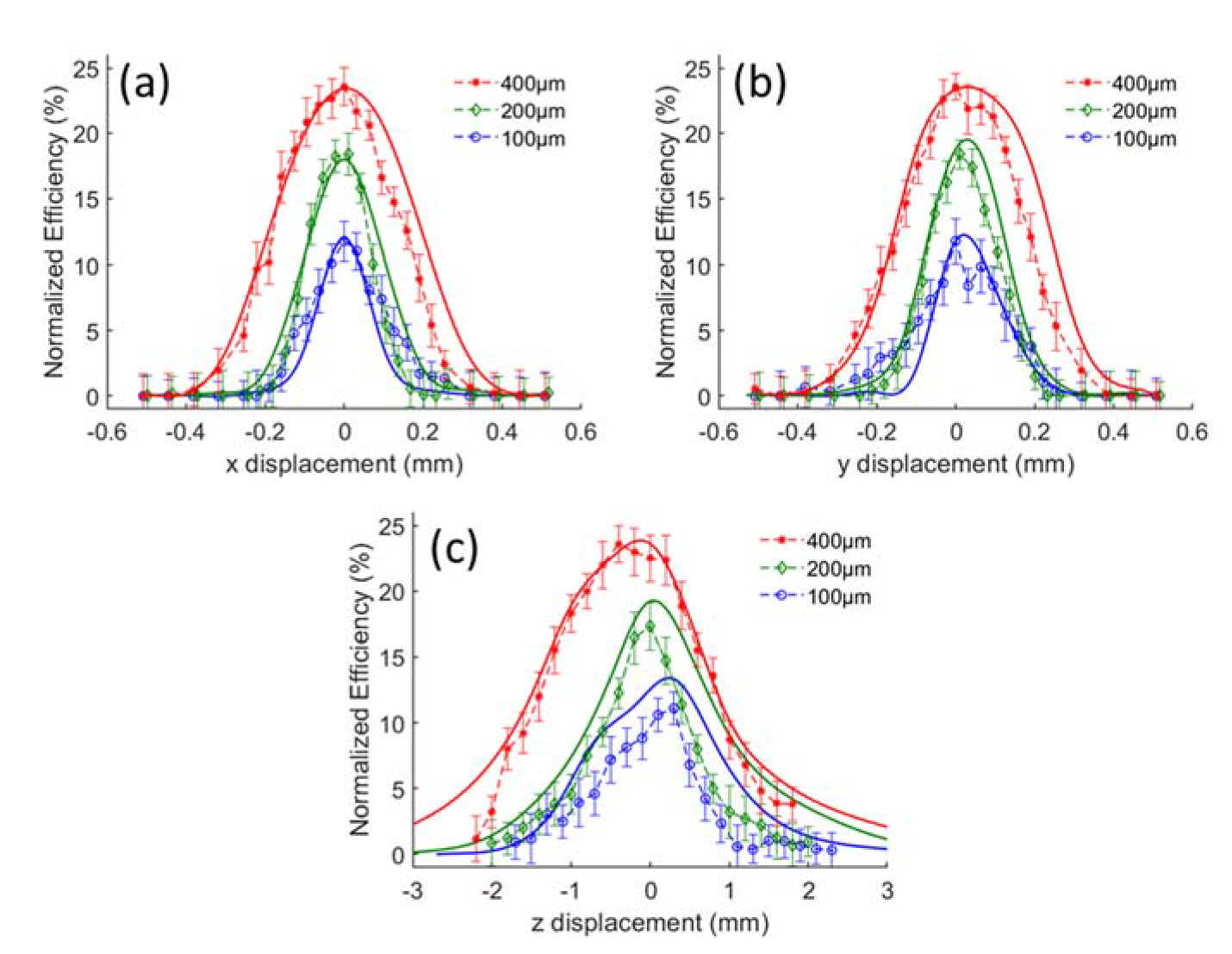
3.2. Alignment Tolerance Analysis
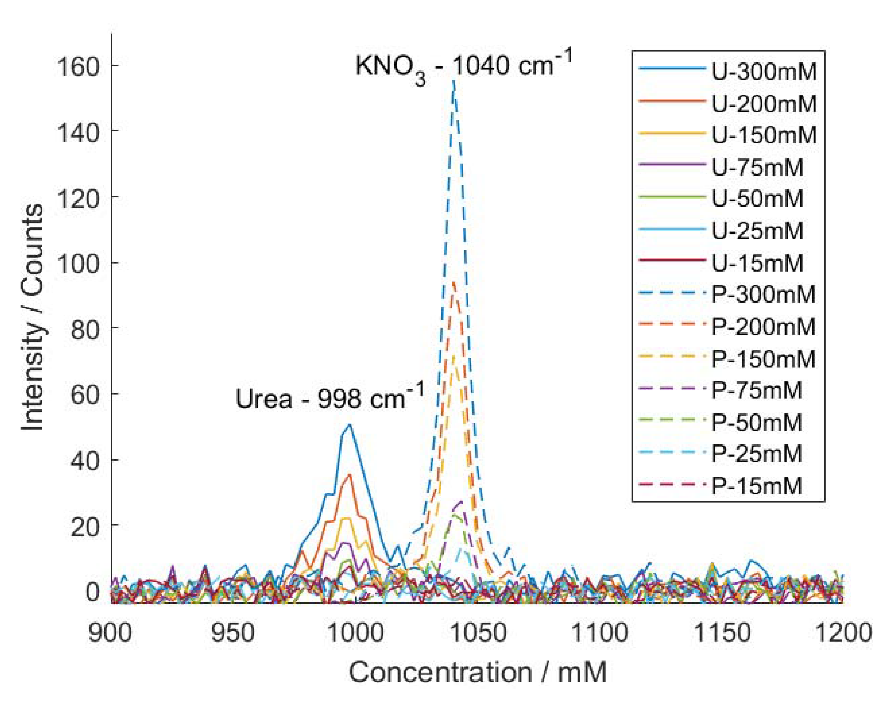
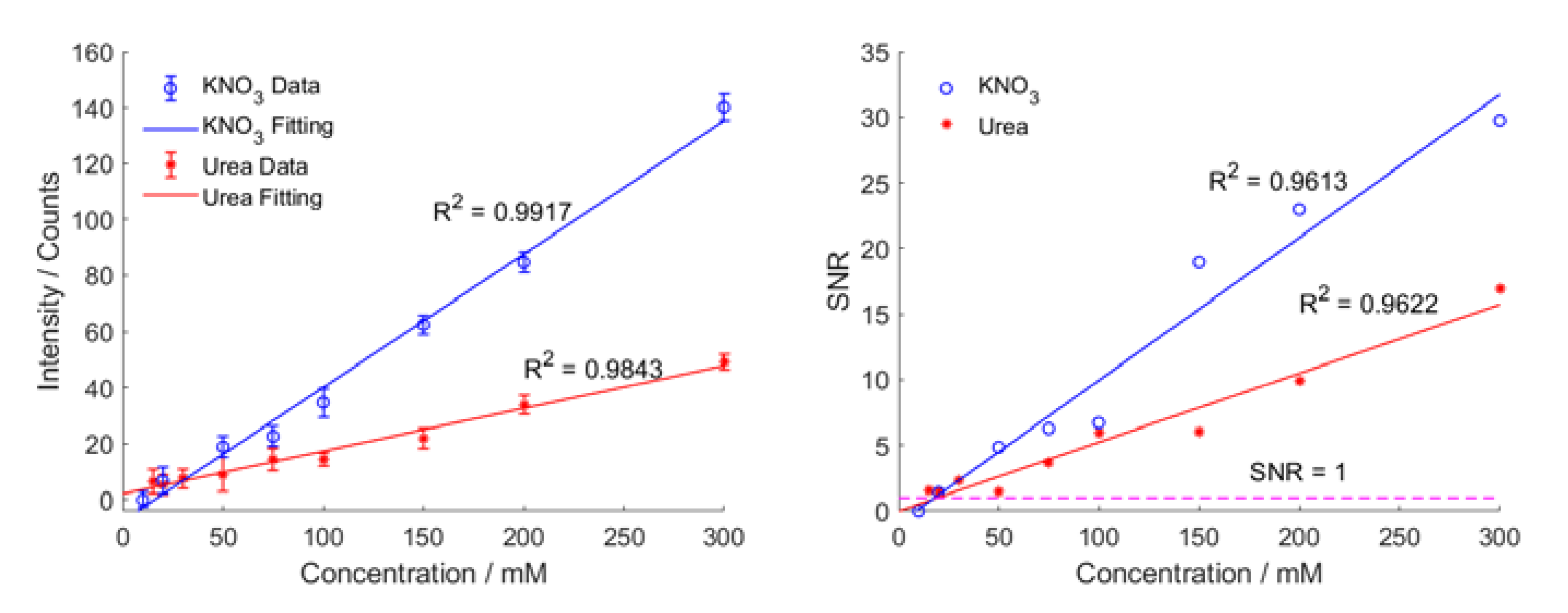
3.3. Limit of Detection for Conventional Raman Analysis
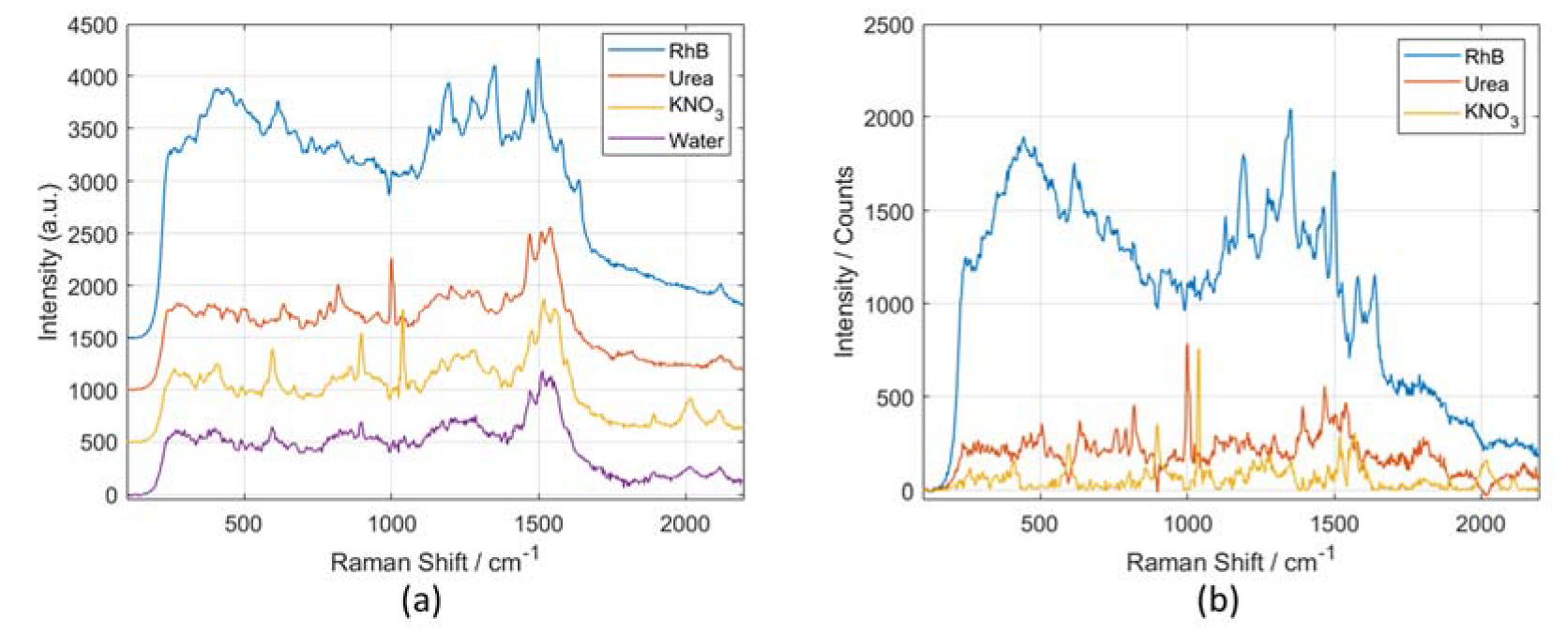
3.4. Using the Microfluidic Chip and Segmented Reflector in Combination with a SERS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raman, C.V. A new radiation. India J. Phys. 1928, 2, 387–398. [Google Scholar] [CrossRef]
- Tu, Q.; Chang, C. Diagnostic applications of Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 545–558. [Google Scholar] [CrossRef]
- Lim, I.-I.S.; Njoki, P.N.; Park, H.-Y.; Wang, X.; Wang, L.; Mott, D.; Zhong, C.-J. Gold and magnetic oxide/gold core/shell nanoparticles as bio-functional nanoprobes. Nanotechnology 2008, 19, 305102. [Google Scholar] [CrossRef] [PubMed]
- Aoki, P.H.B.; Furini, L.N.; Alessio, P.; Aliaga, A.E.; Constantino, C.J.L. Surface-enhanced Raman scattering (SERS) applied to cancer diagnosis and detection of pesticides, explosives, and drugs. Rev. Anal. Chem. 2013, 32, 55–76. [Google Scholar] [CrossRef]
- Matteini, P.; Cottat, M.; Tavanti, F.; Panfilova, E.; Scuderi, M.; Nicotra, G.; Menziani, M.C.; Khlebtsov, N.; de Angelis, M.; Pini, R. Site-Selective Surface-Enhanced Raman Detection of Proteins. ACS Nano 2017, 11, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Popp, J. The many facets of Raman spectroscopy for biomedical analysis. Anal. Bioanal. Chem. 2015, 407, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Habermehl, A.; Strobel, N.; Eckstein, R.; Bolse, N.; Mertens, A.; Hernandez-Sosa, G.; Eschenbaum, C.; Lemmer, U. Lab-on-chip, surface-enhanced Raman analysis by aerosol jet printing and roll-to-roll hot embossing. Sensors 2017, 17, 2401. [Google Scholar] [CrossRef]
- Liu, L.; Devoe, D.L.; White, I.M. Microfluidic SERS Using a 3-Dimensional Porous Monolith as a SERS-Active Solid Phase in a Microchannel. In Proceedings of the Conference on Lasers and Electro-Optics 2010, San Jose, CA, USA, 16–21 May 2010; pp. 6–7. [Google Scholar]
- Uusitalo, S.; Hiltunen, J.; Karioja, P.; Siitonen, S.; Kontturi, V.; Myllylä, R.; Kinnunen, M.; Meglinski, I. Performance and flow dynamics studies of polymeric optofluidic sers sensors. J. Eur. Opt. Soc. 2015, 10, 15043. [Google Scholar] [CrossRef]
- Khijwania, S.K.; Tiwari, V.S.; Yueh, F.Y.; Singh, J.P. A fiber optic Raman sensor for hydrocarbon detection. Sens. Actuators, B Chem. 2007, 125, 563–568. [Google Scholar] [CrossRef]
- Ashok, P.C.; Singh, G.P.; Tan, K.M.; Dholakia, K. Fiber probe based microfluidic Raman spectroscopy. Opt. Express 2010, 18, 7642–7649. [Google Scholar] [CrossRef]
- Ashok, P.C.; Singh, G.P.; Rendall, H.A.; Krauss, T.F.; Dholakia, K. Waveguide confined Raman spectroscopy for microfluidic interrogation. Lab Chip 2011, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Dochow, S.; Beleites, C.; Henkel, T.; Mayer, G.; Albert, J.; Clement, J.; Krafft, C.; Popp, J. Quartz microfluidic chip for tumour cell identification by Raman spectroscopy in combination with optical traps. Anal. Bioanal. Chem. 2013, 405, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- De Coster, D.; Loterie, D.; Ottevaere, H.; Vervaeke, M.; Van Erps, J.; Missinne, J.; Thienpont, H. Free-form optics enhanced confocal Raman spectroscopy for optofluidic lab-on-chips. IEEE J. Sel. Top. Quantum Electron. 2015, 21. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Short, M.; Zeng, H. Real-time in vivo cancer diagnosis using Raman spectroscopy. J. Biophotonics 2015, 8, 527–545. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, G.; Zheng, P.; Cushing, S.; Wu, N.; Huang, Q. A Surface-Enhanced Raman Scattering Sensor Integrated with Battery-Controlled Fluidic Device for Capture and Detection of Trace Small Molecules. Sci. Rep. 2015, 5, 12865. [Google Scholar] [CrossRef]
- Perozziello, G.; Candeloro, P.; De Grazia, A.; Esposito, F.; Allione, M.; Coluccio, M.L.; Tallerico, R.; Valpapuram, I.; Tirinato, L.; Das, G.; et al. Microfluidic device for continuous single cells analysis via Raman spectroscopy enhanced by integrated plasmonic nanodimers. Opt. Express 2016, 24, 180–190. [Google Scholar] [CrossRef]
- Kline, N.D.; Tripathi, A.; Mirsafavi, R.; Pardoe, I.; Moskovits, M.; Meinhart, C.; Guicheteau, J.A.; Christesen, S.D.; Fountain, A.W., III. Optimization of Surface-Enhanced Raman Spectroscopy Conditions for Implementation into a Micro fluidic Device for Drug Detection. Anal. Chem. 2016, 88, 10513–10522. [Google Scholar] [CrossRef]
- Pallaoro, A.; Hoonejani, M.R.; Braun, G.B.; Meinhart, C.D.; Moskovits, M. Rapid Identification by Surface-Enhanced Raman Spectroscopy of Cancer Cells at Low Concentrations Flowing in a Microfluidic Channel. ACS Nano 2015, 9, 4328–4336. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.J.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Blackie, E. Quantification of the Enhancement Factor in Surface-Enhanced Raman Scattering. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2010. [Google Scholar]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Basic Properties of the Electromagnetic Field. In Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, 4th ed.; Pergamon Press: Oxford, UK, 1970; pp. 36–47. [Google Scholar]
- Henyey, L.G.; Greenstein, J.L. Diffuse radiation in the galaxy. Astrophys. J. 1941, 93, 70–83. [Google Scholar] [CrossRef]
- Michels, R.; Foschum, F.; Kienle, A. Optical properties of fat emulsions. Opt. Express 2008, 16, 5907–5926. [Google Scholar] [CrossRef] [PubMed]
- Binzoni, T.; Leung, T.S.; Gandjbakhche, A.H.; Rüfenacht, D.; Delpy, D.T. The use of the Henyey-Greenstein phase function in Monte Carlo simulations in biomedical optics. Phys. Med. Biol. 2006, 51, 313–322. [Google Scholar] [CrossRef]
- Berk, A.; Bernstein, L.S.; Anderson, G.P.; Acharya, P.K.; Robertson, D.C.; Chetwynd, J.H.; Adler-Golden, S.M. MODTRAN cloud and multiple scattering upgrades with application to AVIRIS. Remote Sens. Environ. 1998, 65, 367–375. [Google Scholar] [CrossRef]
- Nafie, L.; Stein, P.; Fanconi, B.; Peticolas, W.L. Angular Dependence of Raman Scattering Intensity. J. Chem. Phys. 1970, 52, 1584–1588. [Google Scholar] [CrossRef]
- Gangadhara, S. Zemax OpticStudio Knowledgebase - Bulk Scattering with the Rayleigh Model. Available online: https://customers.zemax.com/os/resources/learn/knowledgebase/bulk-scattering-with-the-rayleigh-model (accessed on 5 February 2008).
- Barycka, I.; Hołodnik, B.; Misiuk, A. NiP as a New Material for Thick Film Technology. Electrocompon. Sci. Technol. 1981, 7, 221–226. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Hübner, J.; Boisen, A. Large area fabrication of leaning silicon nanopillars for Surface Enhanced Raman Spectroscopy. Adv. Mater. 2012, 24, OP11–OP18. [Google Scholar] [CrossRef]
- Li, M.; Zhao, F.; Zeng, J.; Qi, J.; Lu, J.; Shih, W. Microfluidic surface-enhanced Raman scattering sensor with monolithically integrated nanoporous gold disk arrays for rapid and label-free biomolecular detection. J. Biomed. Opt. 2014, 19, 111611. [Google Scholar] [CrossRef]
- Tran, C.T.K.; Tran, H.T.T.; Bui, H.T.T.; Dang, T.Q.; Nguyen, L.Q. Determination of low level nitrate/nitrite contamination using SERS-active Ag/ITO substrates coupled to a self-designed Raman spectroscopy system. J. Sci. Adv. Mater. Devices 2017, 2, 172–177. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Rintoul, L.; Kloprogge, J.T. Raman spectroscopy of urea and urea-intercalated kaolinites at 77 K. Spectrochim. Acta Part A 2000, 56, 1681–1691. [Google Scholar] [CrossRef]
- Frost, R.L.; Erickson, K.L. Vibrational spectroscopic study of the nitrate containing hydrotalcite Mbobomkulite. Spectrochim. Acta Part A 2005, 61, 2919–2925. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Sun, X.; Li, Y. Surface Enhanced Raman Scattering Effects of Silver Colloids with Different Shapes. J. Phys. Chem. B 2005, 109, 12544–12548. [Google Scholar] [CrossRef]
- Luo, Z.; Fang, Y.; Yao, J. A New Approach for Non-destructive Detection of Dye Molecules by Combination of Terahertz Time-domain Spectra and Raman. Spectra. Trends Appl. Sci. Res. 2007, 2, 295–303. [Google Scholar]



| MMF (µm) | Max Efficiency | Confocality - FWHM (µm) | ||
|---|---|---|---|---|
| x | y | z | ||
| 100 | 13.4% | 41.8 | 35.2 | 120.0 |
| 200 | 19.3% | 57.8 | 57.4 | 124.6 |
| 400 | 23.8% | 100.0 | 100.4 | 155.6 |
| Analyte | Literature (cm−1) | SERS (cm−1) | Assignment |
|---|---|---|---|
| Urea [34] | 1012 S | 1000 S | ν (N-C-N) |
| 1474 W | 1467 M | ν (N-C-N) | |
| 1542 W | 1540 M | δ (NH2) | |
| Potassium nitrate [35] | 1050 S | 1040 S | ν (NO3) |
| 1343 W | 1357 W | ν (NO3) | |
| Rhodamine B [36,37] | 619 S | 615.9 S | ν (Aromatic C-C) |
| 1130 W | 1130 W | δ (Aromatic C-H) | |
| 1199 M | 1190 S | δ (Aromatic C-H) | |
| 1284 S | 1274 M | δ (C-C) | |
| 1360 S | 1351 S | ν (Aromatic C-C) | |
| 1508 S | 1499 S | ν (Aromatic C-C) | |
| 1591 W | 1581 S | ν (C=C) | |
| 1644 S | 1638 S | ν (Aromatic C-C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Stenbæk Schmidt, M.; Thienpont, H.; Ottevaere, H. A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy. Sensors 2020, 20, 1250. https://doi.org/10.3390/s20051250
Liu Q, Stenbæk Schmidt M, Thienpont H, Ottevaere H. A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy. Sensors. 2020; 20(5):1250. https://doi.org/10.3390/s20051250
Chicago/Turabian StyleLiu, Qing, Michael Stenbæk Schmidt, Hugo Thienpont, and Heidi Ottevaere. 2020. "A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy" Sensors 20, no. 5: 1250. https://doi.org/10.3390/s20051250
APA StyleLiu, Q., Stenbæk Schmidt, M., Thienpont, H., & Ottevaere, H. (2020). A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy. Sensors, 20(5), 1250. https://doi.org/10.3390/s20051250






