An Unmanned Aerial Vehicle-Based Gas Sampling System for Analyzing CO2 and Atmospheric Particulate Matter in Laboratory
Abstract
1. Introduction
2. Materials and Methods
2.1. The Unmanned Aerial Vehicle-Based Gas Sampling System
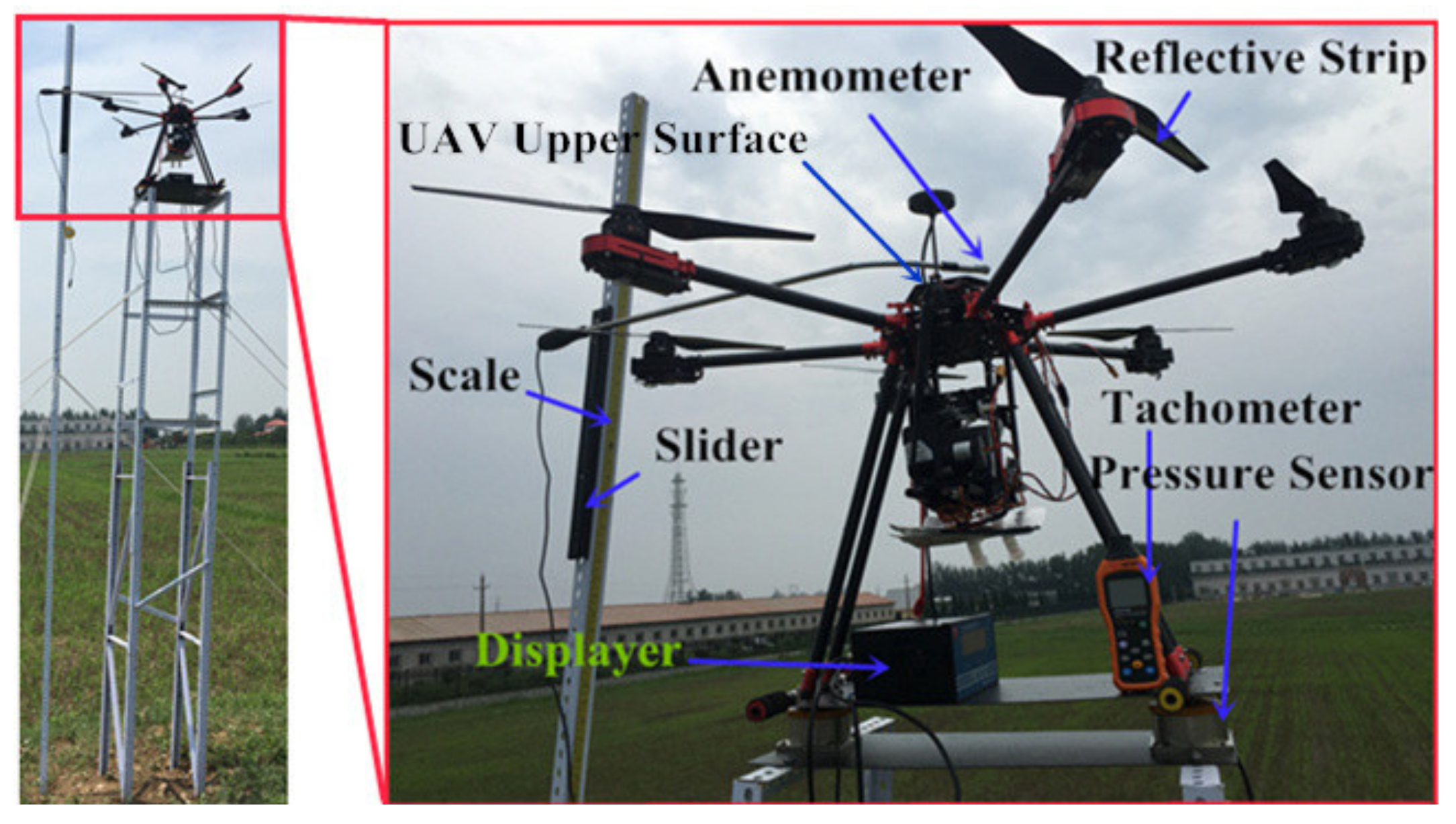
2.1.1. The Unmanned Aerial Vehicle
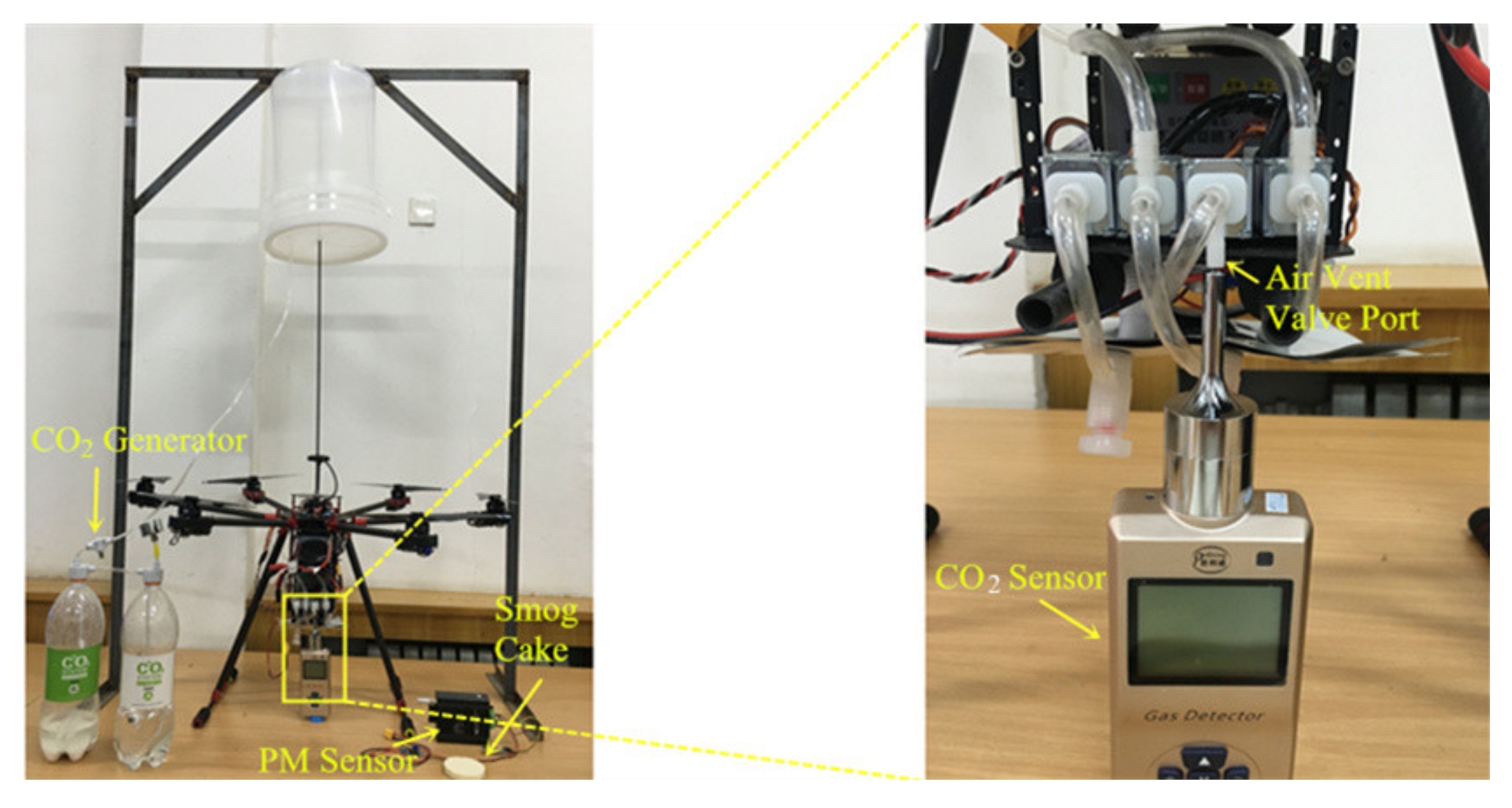
2.1.2. Gas Sampling Equipment
2.2. Rotation Speed and Propeller Gas Flow Measurement
2.3. Original Gas Discharge Checking System
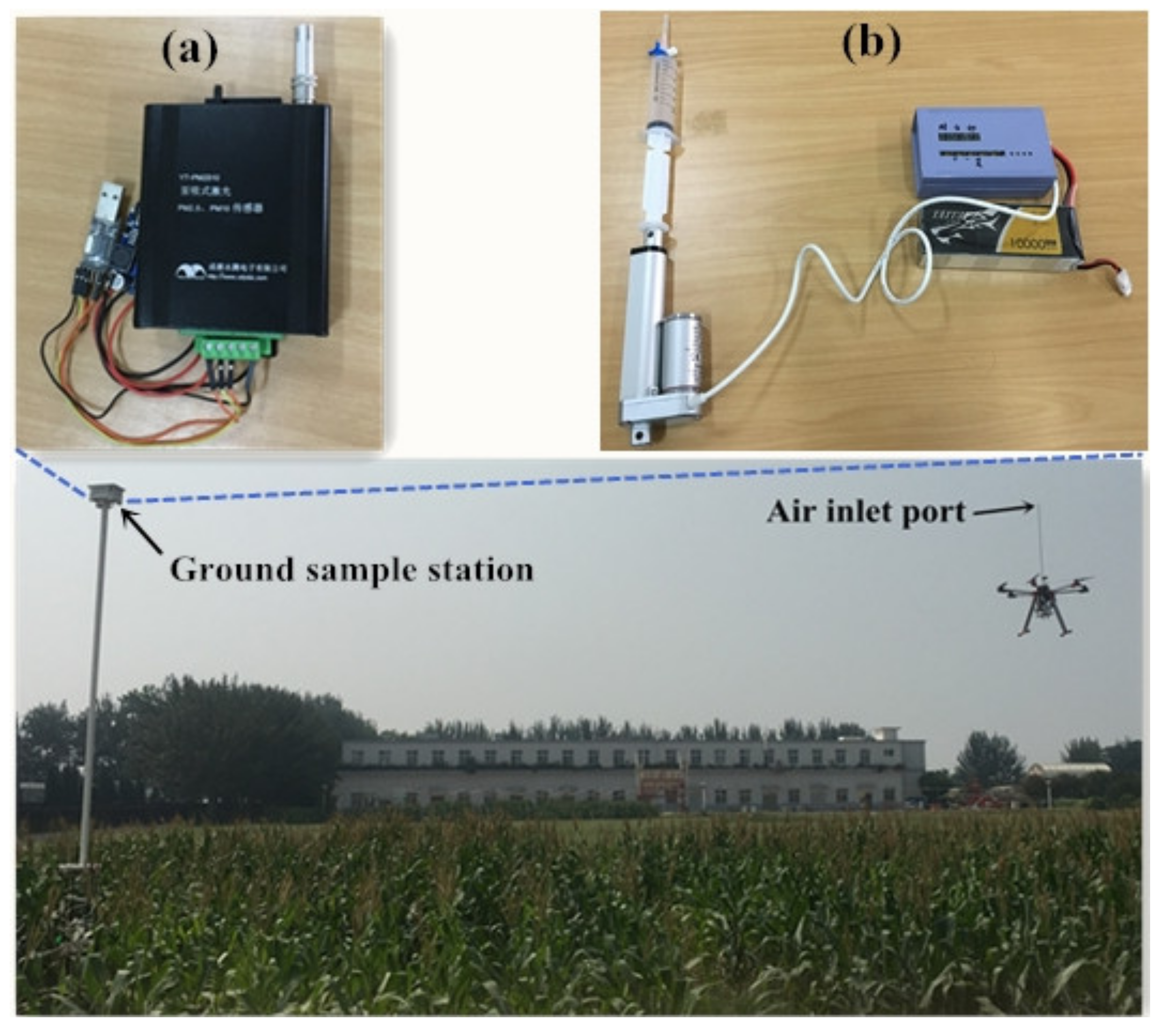
2.4. Ground Gas Sampling Station and Flight Design
3. Results and Discussion
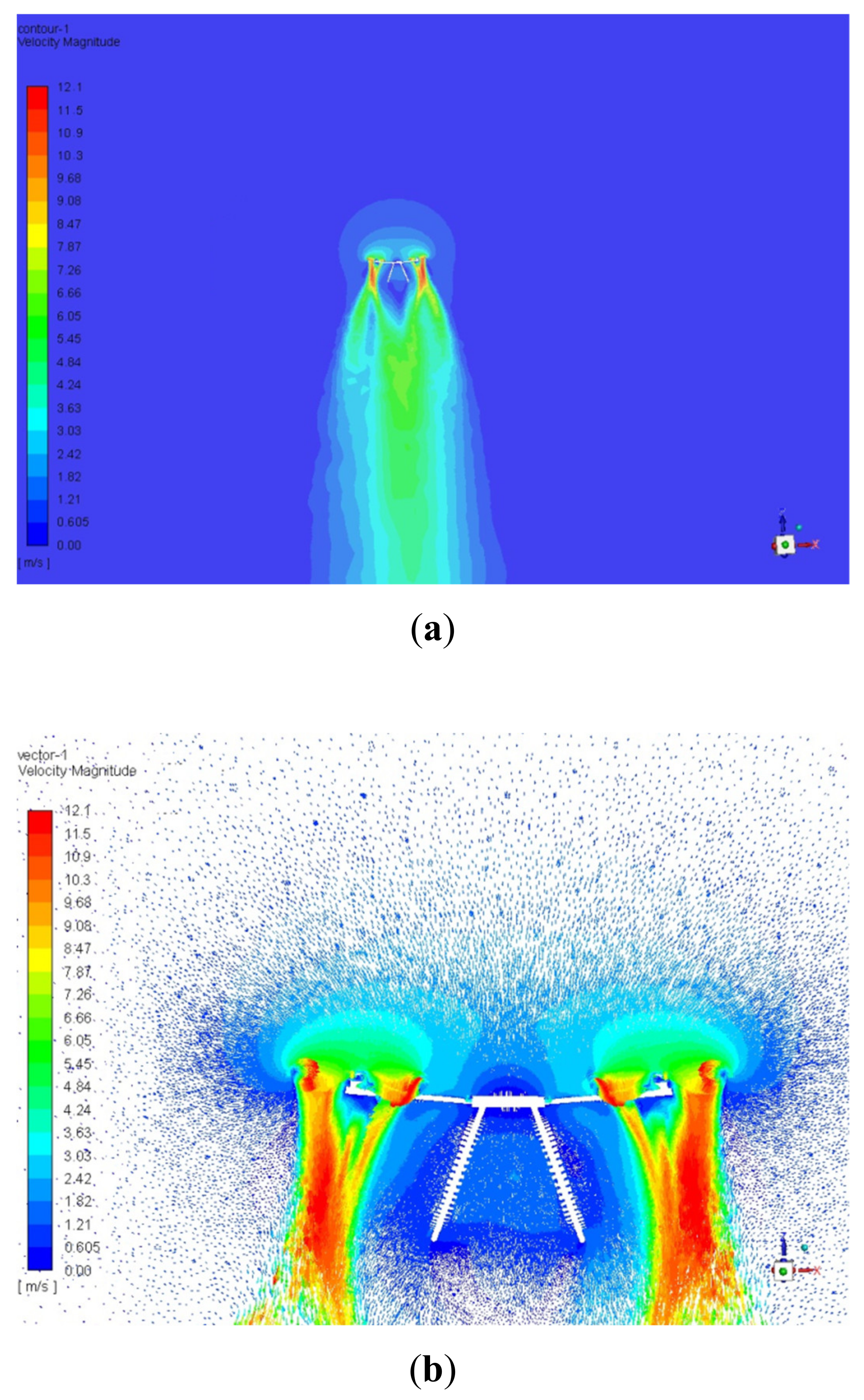
3.1. Unmanned Aerial Vehicle Air Turbulence in Hover Mode and Position of the Unmanned Aerial Vehicle-Based Gas Sampling System Intake Port
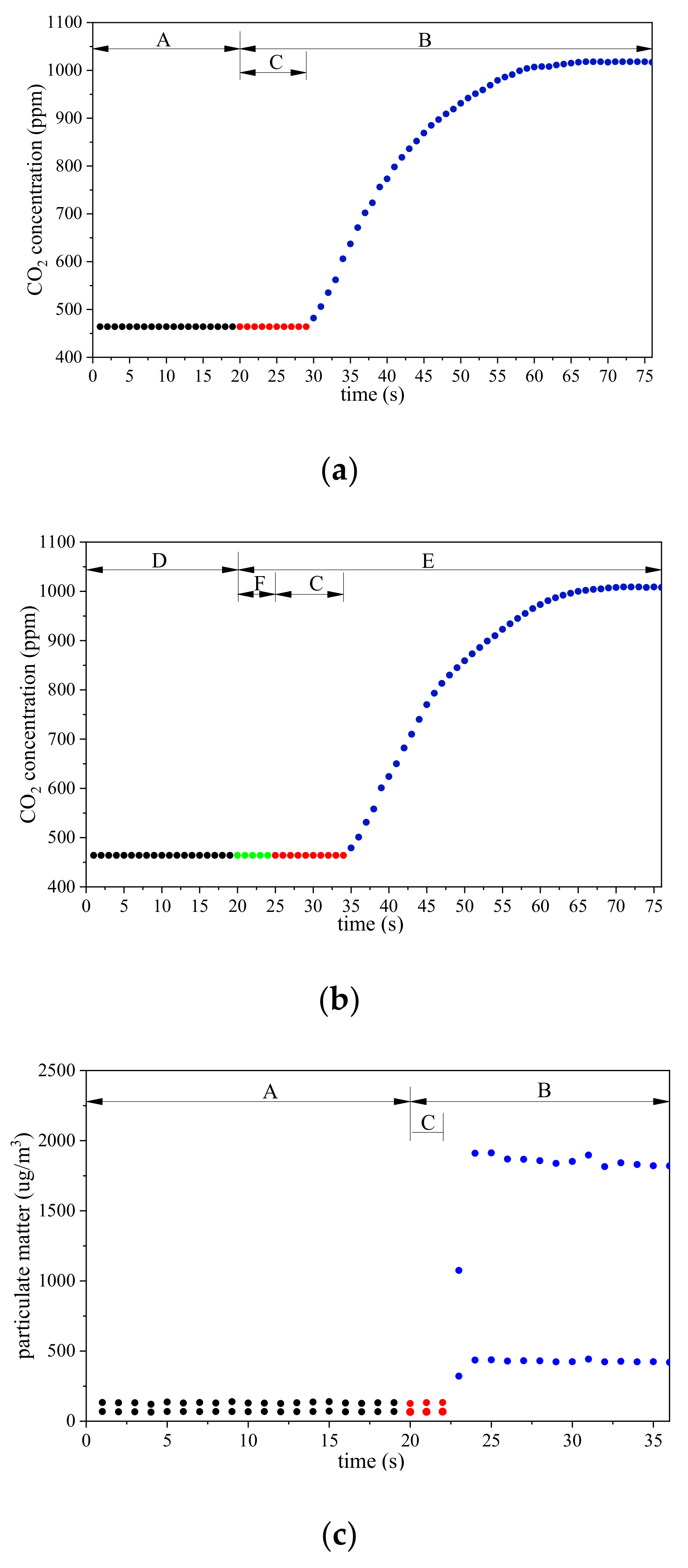
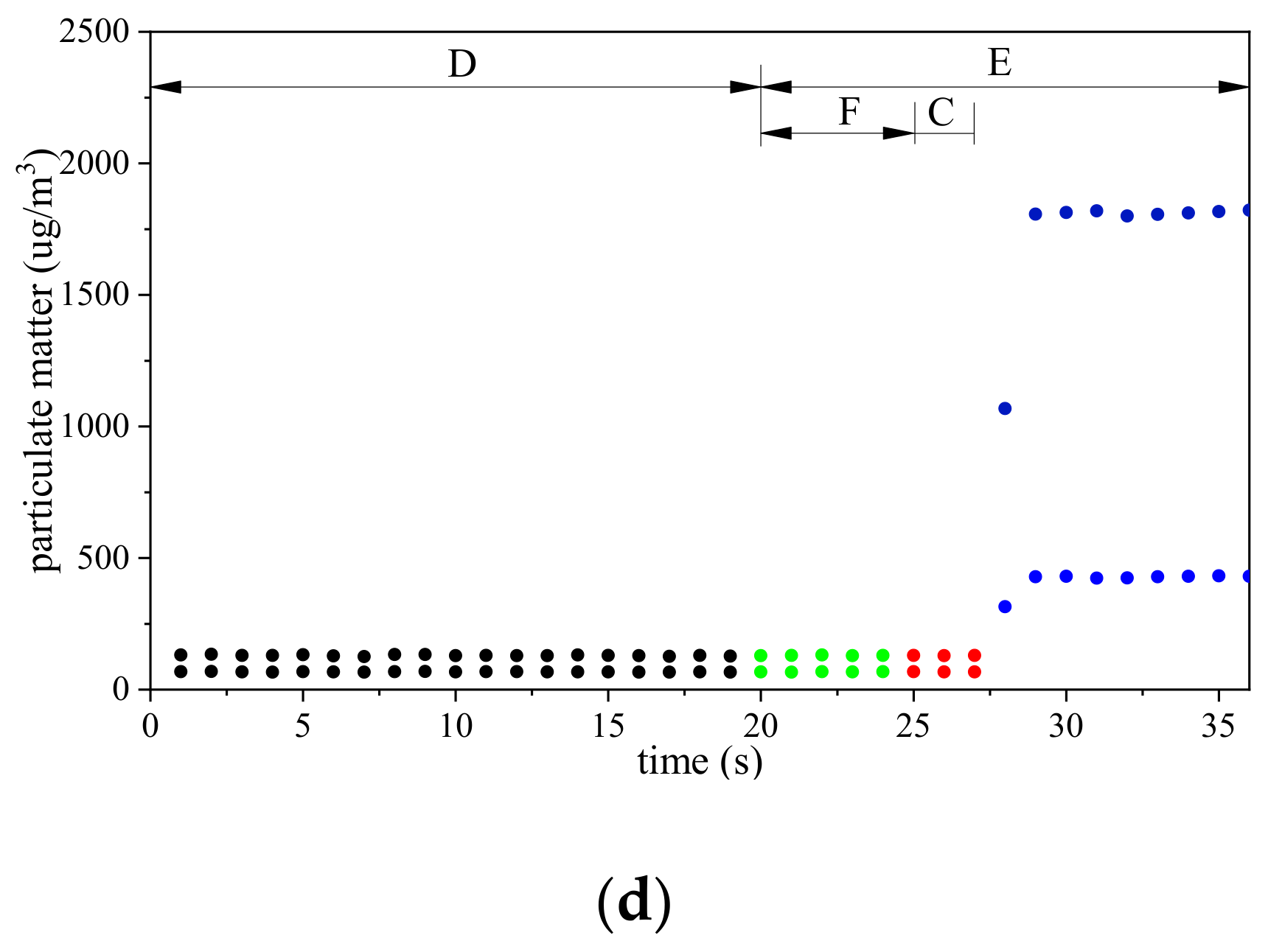
3.2. Time Required to Discharge Residual Gas in Unmanned Aerial Vehicle-Based Gas Sampling System
3.3. Accuracy Validation of the Unmanned Aerial Vehicle-Based Gas Sampling System
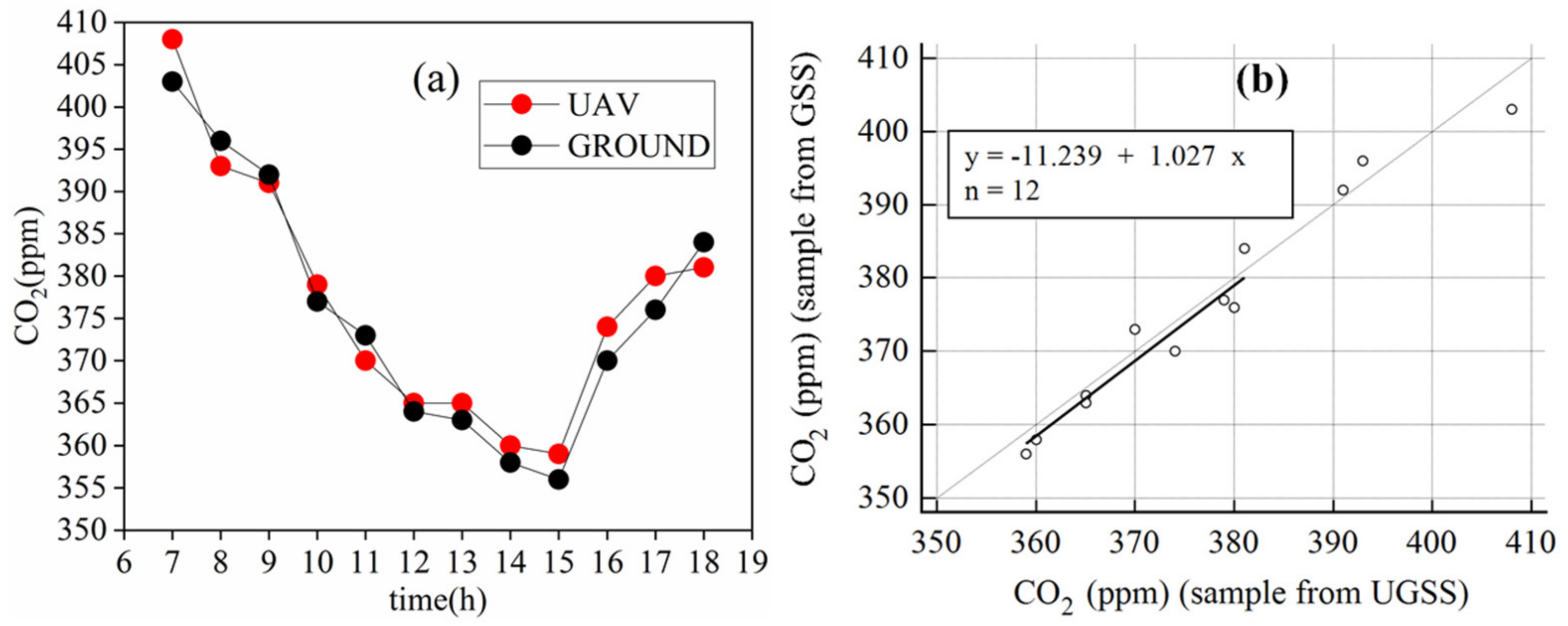
3.3.1. Accuracy Verification of CO2 Concentration Collected Using Unmanned Aerial Vehicle-Based Gas Sampling System
3.3.2. Accuracy Verification of Particulate Matter Concentration Collected Using the Unmanned Aerial Vehicle-Based Gas Sampling System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mukherjee, M.; Shu, L.; Hu, L.K.; Hancke, G.P.; Zhu, C.S. Sleep scheduling in industrial wireless sensor networks for toxic gas monitoring. IEEE Wirel. Commun. 2017, 24, 106–112. [Google Scholar] [CrossRef]
- Thomas, G.W.; Sousan, S.; Tatum, M.; Liu, X.X.; Zuidema, C.; Fitzpatrick, M.; Koehler, K.A.; Peters, T.M. Low-cost, distributed environmental monitors for factory worker health. Sensors 2018, 18, 1411. [Google Scholar] [CrossRef]
- Bennetzen, E.H.; Smith, P.; Porter, J.R. Agricultural production and greenhouse gas emissions from world regions–The major trends over 40 years. Glob. Environ. Chang. Hum. Policy Dimens. 2016, 37, 43–55. [Google Scholar] [CrossRef]
- De Rosa, D.; Rowlings, D.W.; Biala, J.; Scheer, C.; Basso, B.; Grace, P.R. N2O and CO2 emissions following repeated application of organic and mineral N fertiliser from a vegetable crop rotation. Sci. Total Environ. 2018, 637, 813–824. [Google Scholar] [CrossRef]
- Liu, C.Y.; Yao, Z.S.; Wang, K.; Zheng, X.H.; Li, B.G. Net ecosystem carbon and greenhouse gas budgets in fiber and cereal cropping systems. Sci. Total Environ. 2018, 647, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, S.; Bertolini, T.; Valente, A.; Chiti, T.; Valentini, R. Nitrous oxide emissions from soil of an African rain forest in Ghana. Biogeosciences 2013, 10, 4179–4187. [Google Scholar] [CrossRef]
- Werner, C.; Butterbach-Bahl, K.; Haas, E.; Hickler, T.; Kiese, R. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Glob. Biogeochem. Cycle 2007, 21. [Google Scholar] [CrossRef]
- Bjerg, B.; Zhang, G.Q.; Madsen, J.; Rom, H.B. Methane emission from naturally ventilated livestock buildings can be determined from gas concentration measurements. Environ. Monit. Assess. 2012, 184, 5989–6000. [Google Scholar] [CrossRef]
- Lush, L.; Wilson, R.P.; Holton, M.D.; Hopkins, P.; Marsden, K.A.; Chadwick, D.R.; King, A.J. Classification of sheep urination events using accelerometers to aid improved measurements of livestock contributions to nitrous oxide emissions. Comput. Electron. Agric. 2018, 150, 170–177. [Google Scholar] [CrossRef]
- McGinn, S.M.; Flesch, T.K. Ammonia and greenhouse gas emissions at beef cattle feedlots in Alberta Canada. Agric. For. Meteorol. 2018, 258, 43–49. [Google Scholar] [CrossRef]
- Schuck, T.J.; Brenninkmeijer, C.A.M.; Slemr, F.; Xueref-Remy, I.; Zahn, A. Greenhouse gas analysis of air samples collected onboard the CARIBIC passenger aircraft. Atmos. Meas. Tech. 2009, 2, 449–464. [Google Scholar] [CrossRef]
- Van Vuuren, E.; Naude, Y. Air Sampling: A Mini-denuder Sampling Device for Direct Desorption in an Inlet of a Gas Chromatograph for the Analyses of Airborne Gaseous Phase Pollutants and Airborne Particulate Phase Pollutants. S. Afr. J. Chem. Suid Afr. Tydskr. Chem. 2019, 72, 55–58. [Google Scholar] [CrossRef]
- Stavert, A.R.; O’Doherty, S.; Stanley, K.; Young, D.; Manning, A.J.; Lunt, M.F. UK greenhouse gas measurements at two new tall towers for aiding emissions verification. Atmos. Meas. Tech. 2019, 12, 4495–4518. [Google Scholar] [CrossRef]
- Danilov, A.S.; Smirnov, U.D.; Pashkevich, M.A. The system of the ecological monitoring of environment which is based on the usage of UAV. Russ. J. Ecol. 2015, 46, 14–19. [Google Scholar] [CrossRef]
- Haas, P.; Balistreri, C.; Pontelandolfo, P.; Triscone, G. Development of an unmanned aerial vehicle UAV for air quality measurement in urban areas. In Proceedings of the 32nd AIAA Applied Aerodynamics Conference, Atlanta, GA, USA, 16–20 June 2014. [Google Scholar]
- Toscano, P.; Gioli, B.; Dugheri, S.; Salvini, A.; Matese, A.; Bonacchi, A.; Zaldei, A.; Cupelli, V.; Miglietta, F. Locating industrial VOC sources with aircraft observations. Environ. Pollut. 2011, 159, 1174–1182. [Google Scholar] [CrossRef]
- Ras, M.R.; Marce, R.M.; Borrull, F. Volatile organic compounds in air at urban and industrial areas in the Tarragona region by thermal desorption and gas chromatography-mass spectrometry. Environ. Monit. Assess. 2010, 161, 389–402. [Google Scholar] [CrossRef]
- Malaver Rojas, J.A.; Motta, N.; Gonzalez, L.F.; Corke, P. Towards the development of a gas sensor system for monitoring pollutant gases in the low troposphere using small unmanned aerial vehicles. In Proceedings of the Workshop on Robotics for Environmental Monitoring, Sydney, NSW, Australia, 11 July 2012. [Google Scholar]
- Peng, Z.R.; Wang, D.S.; Wang, Z.Y.; Gao, Y.; Lu, S.J. A study of vertical distribution patterns of PM2.5 concentrations based on ambient monitoring with unmanned aerial vehicles: A case in Hangzhou, China. Atmos. Environ. 2015, 123, 357–369. [Google Scholar] [CrossRef]
- Rudiger, J.; Tirpitz, J.L.; de Moor, J.M.; Bobrowski, N.; Gutmann, A.; Liuzzo, M.; Ibarra, M.; Hoffmann, T. Implementation of electrochemical, optical and denuder-based sensors and sampling techniques on UAV for volcanic gas measurements: Examples from Masaya, Turrialba and Stromboli volcanoes. Atmos. Meas. Tech. 2018, 11, 2441–2457. [Google Scholar] [CrossRef]
- Andersen, T.; Scheeren, B.; Peters, W.; Chen, H.L. A UAV-based active AirCore system for measurements of greenhouse gases. Atmos. Meas. Tech. 2018, 11, 2683–2699. [Google Scholar] [CrossRef]
- Neumann, P.P.; Asadi, S.; Lilienthal, A.J.; Bartholmai, M.; Schiller, J.H. Autonomous gas-sensitive microdrone wind vector estimation and gas distribution mapping. IEEE Robot. Autom. Mag. 2012, 19, 50–61. [Google Scholar] [CrossRef]
- Rodriguez-Mata, A.E.; Gonzalez-Hernandez, I.; Rangel-Peraza, J.G.; Salazar, S.; Leal, R.L. Wind-gust compensation algorithm based on high-gain residual observer to control a quadrotor aircraft: Real-time verification task at fixed point. Int. J. Control Autom. Syst. 2018, 16, 856–866. [Google Scholar] [CrossRef]
- Yang, F.B.; Xue, X.Y.; Zhang, L.; Sun, Z. Numerical simulation and experimental verification on downwash air flow of six-rotor agricultural unmanned aerial vehicle in hover. Int. J. Agric. Biol. Eng. 2017, 10, 41–53. [Google Scholar] [CrossRef]
- Roldan, J.J.; Joossen, G.; Sanz, D.; del Cerro, J.; Barrientos, A. Mini-UAV based sensory system for measuring environmental variables in greenhouses. Sensors 2015, 15, 3334–3350. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.F.; Salimi, F.; Morton, K.; Morawska, L.; Gonzalez, F. Development and validation of a UAV based system for air pollution measurements. Sensors 2016, 16, 2202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, Y.Q.; Zhao, J.; Zhao, J.B. Optimal location of a particulate matter sampling head outside an unmanned aerial vehicle. Particuology 2017, 32, 153–159. [Google Scholar] [CrossRef]
- Alvarado, M.; Gonzalez, F.; Erskine, P.; Cliff, D.; Heuff, D. A methodology to monitor airborne PM10 dust particles using a small unmanned aerial vehicle. Sensors 2017, 17, 343. [Google Scholar] [CrossRef]
- Berman, E.S.F.; Fladeland, M.; Liem, J.; Kolyer, R.; Gupta, M. Greenhouse gas analyzer for measurements of carbon dioxide, methane, and water vapor aboard an unmanned aerial vehicle. Sens. Actuator B Chem. 2012, 169, 128–135. [Google Scholar] [CrossRef]
- Jawhar, I.; Mohamed, N.; Al-Jaroodi, J.; Zhang, S. A framework for using unmanned aerial vehicles for data collection in linear wireless sensor networks. J. Intell. Robot. Syst. 2014, 74, 437–453. [Google Scholar] [CrossRef]
- Frank, M. Fluid Mechanics, 7th ed.; WCB McGraw-Hill: New York, NY, USA, 2011; pp. 25–39. [Google Scholar]
- Song, C.; Zhang, J.; Wang, Y.; Wang, Y.; Zhao, Z. Emission of CO2, CH4 and N2O from freshwater marsh in northeast of China. J. Environ. Manag. 2008, 88, 428–436. [Google Scholar] [CrossRef]
- Xi, M.; Zhang, X.; Kong, F. CO2 exchange under different vegetation covers in a coastal wetland of Jiaozhou Bay, China. Ecol. Eng. 2019, 137, 26–33. [Google Scholar] [CrossRef]



| Part | Model | Dimensions | Performance | Manufacturer |
|---|---|---|---|---|
| Micro pump | FNY6003 | 928348 (mm) | maximum flow: 3L/min | Xin Wei Cheng Co., Ltd. China |
| Speed governor | KS-1 | 804525 (mm) | Flow regulation range: 0-3 L/min | Xin Wei Cheng Co., Ltd. China |
| Electromagnetic valves | PYF3 | 302012 (mm) | 2-position 2-way | Pin Cheng Co., Ltd. China |
| Air bag | E-Switch | 150150 (mm) | 2 valves, 0.5 (L) | Sunrise Instrument Co.,Ltd. China |
| Blade Number | Rotation Direction | Rotation Speed (rpm) |
|---|---|---|
| Blade 1 | Clockwise | 5649 |
| Blade 2 | Counter-clockwise | 5647 |
| Blade 3 | Clockwise | 5651 |
| Blade 4 | Counter-clockwise | 5654 |
| Blade 5 | Clockwise | 5653 |
| Blade 6 | Counter-clockwise | 5647 |
| Location Range on the Central Axis above UAV Upper Surface (cm) | Air Speed Range (m/s) |
|---|---|
| 0–3 | 0–0.605 |
| 3–60 | 1.82–2.42 |
| 60–70 | 1.21–1.82 |
| 70–120 | 0.605–1.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Han, W.; Peng, M.; Zhang, M.; Yao, X.; Liu, W.; Wang, T. An Unmanned Aerial Vehicle-Based Gas Sampling System for Analyzing CO2 and Atmospheric Particulate Matter in Laboratory. Sensors 2020, 20, 1051. https://doi.org/10.3390/s20041051
Li C, Han W, Peng M, Zhang M, Yao X, Liu W, Wang T. An Unmanned Aerial Vehicle-Based Gas Sampling System for Analyzing CO2 and Atmospheric Particulate Matter in Laboratory. Sensors. 2020; 20(4):1051. https://doi.org/10.3390/s20041051
Chicago/Turabian StyleLi, Chaoqun, Wenting Han, Manman Peng, Mengfei Zhang, Xiaomin Yao, Wenshuai Liu, and Tonghua Wang. 2020. "An Unmanned Aerial Vehicle-Based Gas Sampling System for Analyzing CO2 and Atmospheric Particulate Matter in Laboratory" Sensors 20, no. 4: 1051. https://doi.org/10.3390/s20041051
APA StyleLi, C., Han, W., Peng, M., Zhang, M., Yao, X., Liu, W., & Wang, T. (2020). An Unmanned Aerial Vehicle-Based Gas Sampling System for Analyzing CO2 and Atmospheric Particulate Matter in Laboratory. Sensors, 20(4), 1051. https://doi.org/10.3390/s20041051





