Thermal Stability of Type II Modifications by IR Femtosecond Laser in Silica-based Glasses
Abstract
1. Introduction
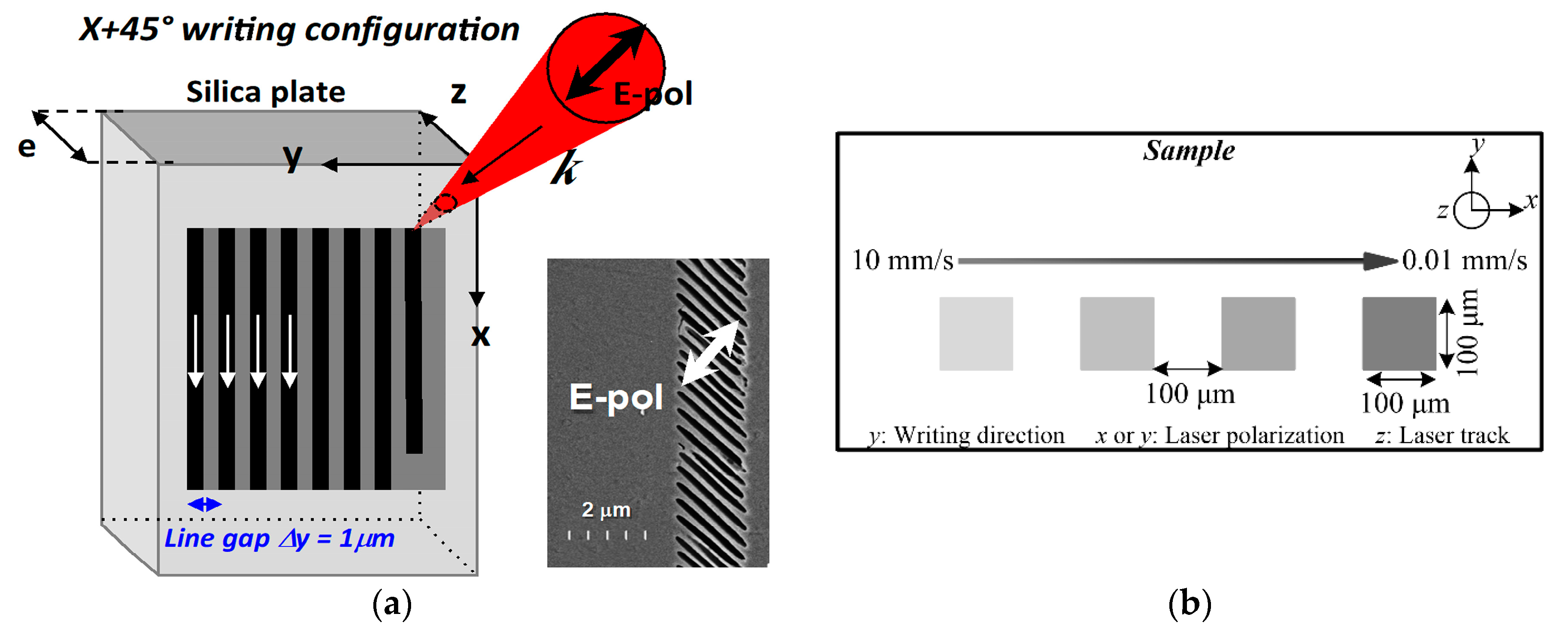
2. Materials and Methods
3. Results
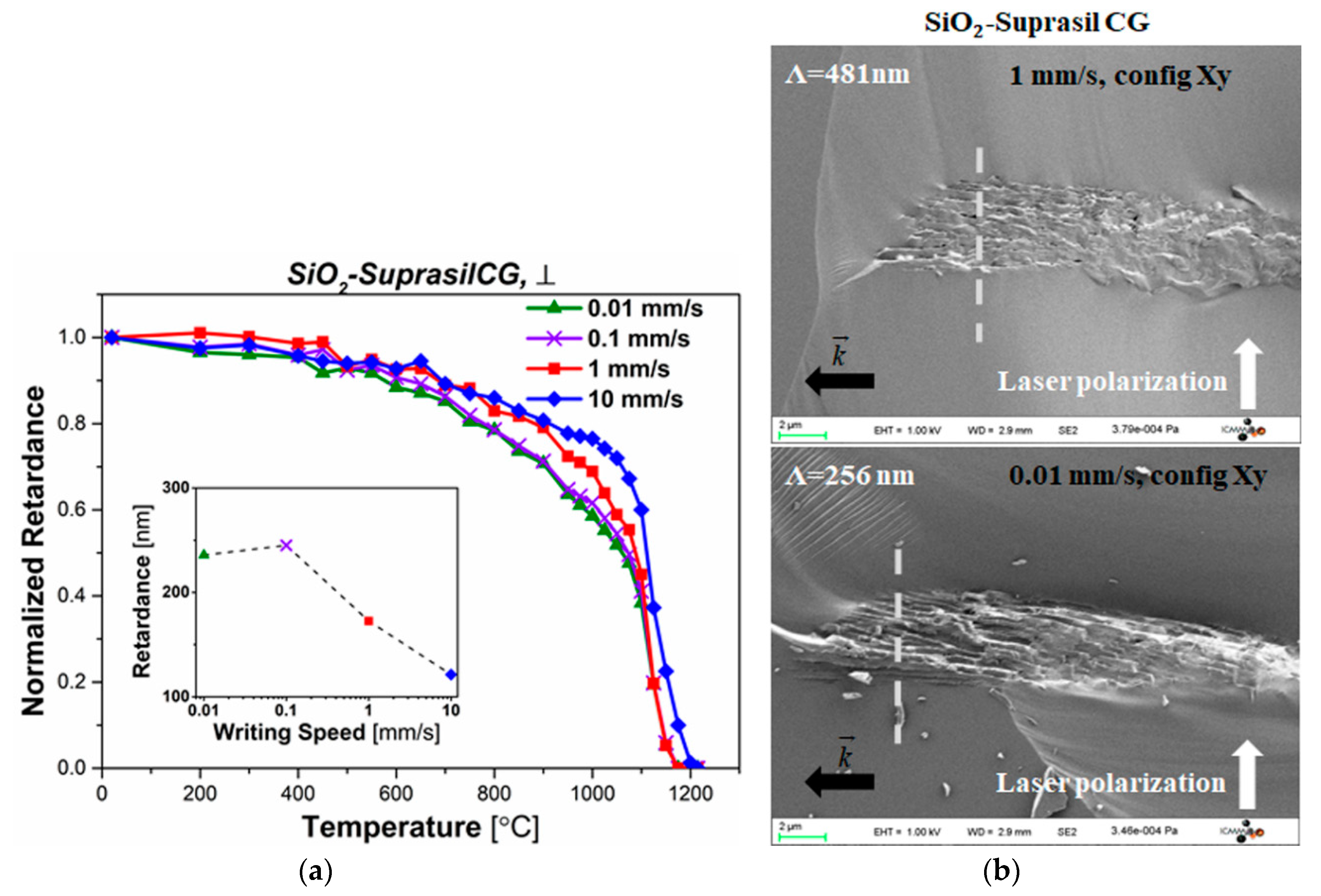
3.1. Impact of the Writing Speed
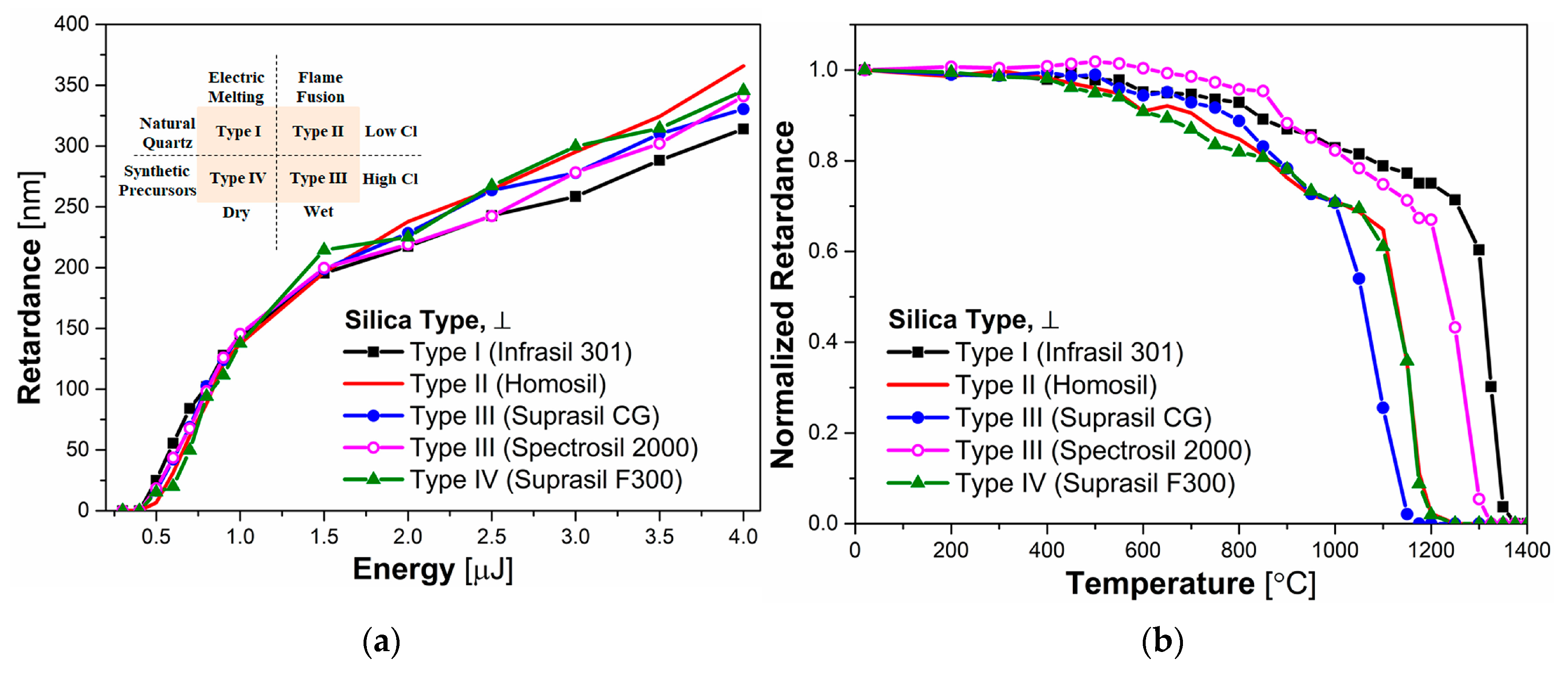
3.2. Impact of Cl and OH Impurities
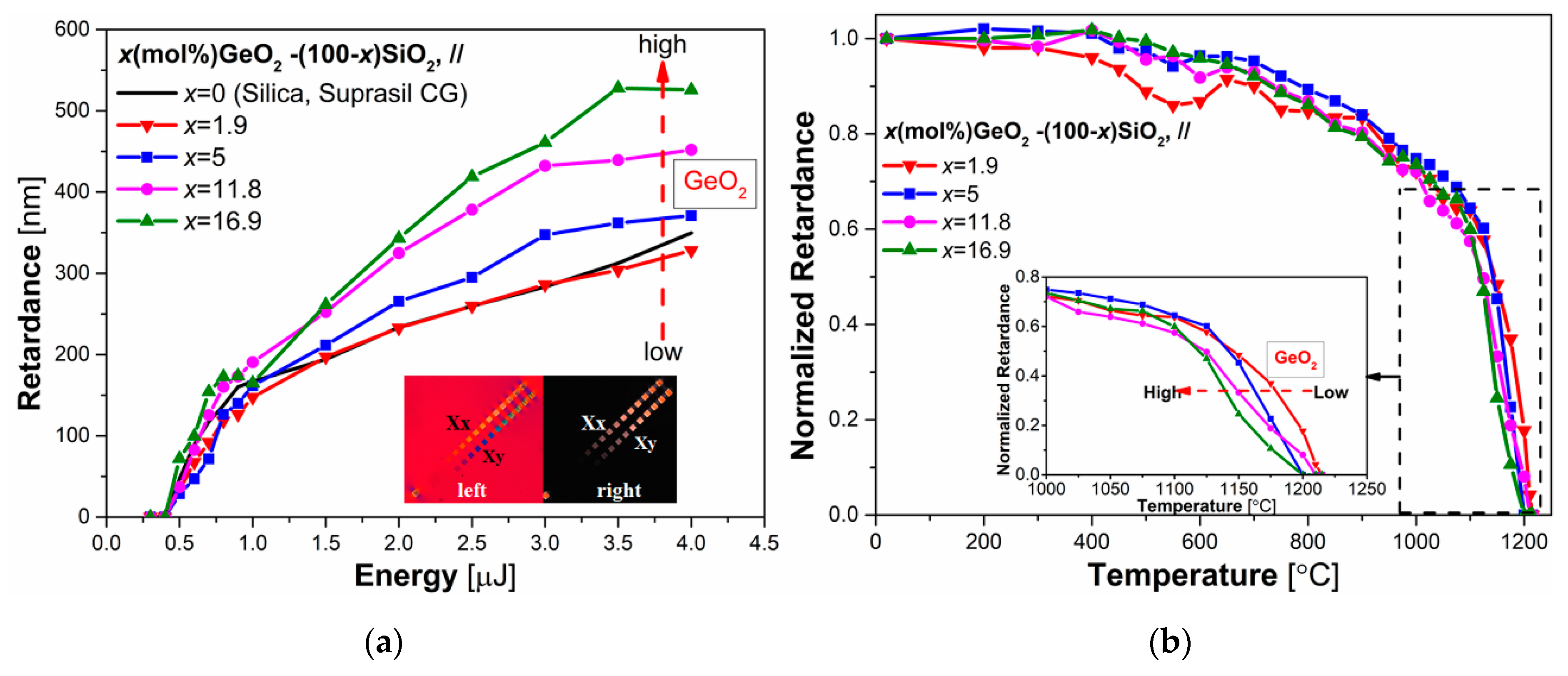
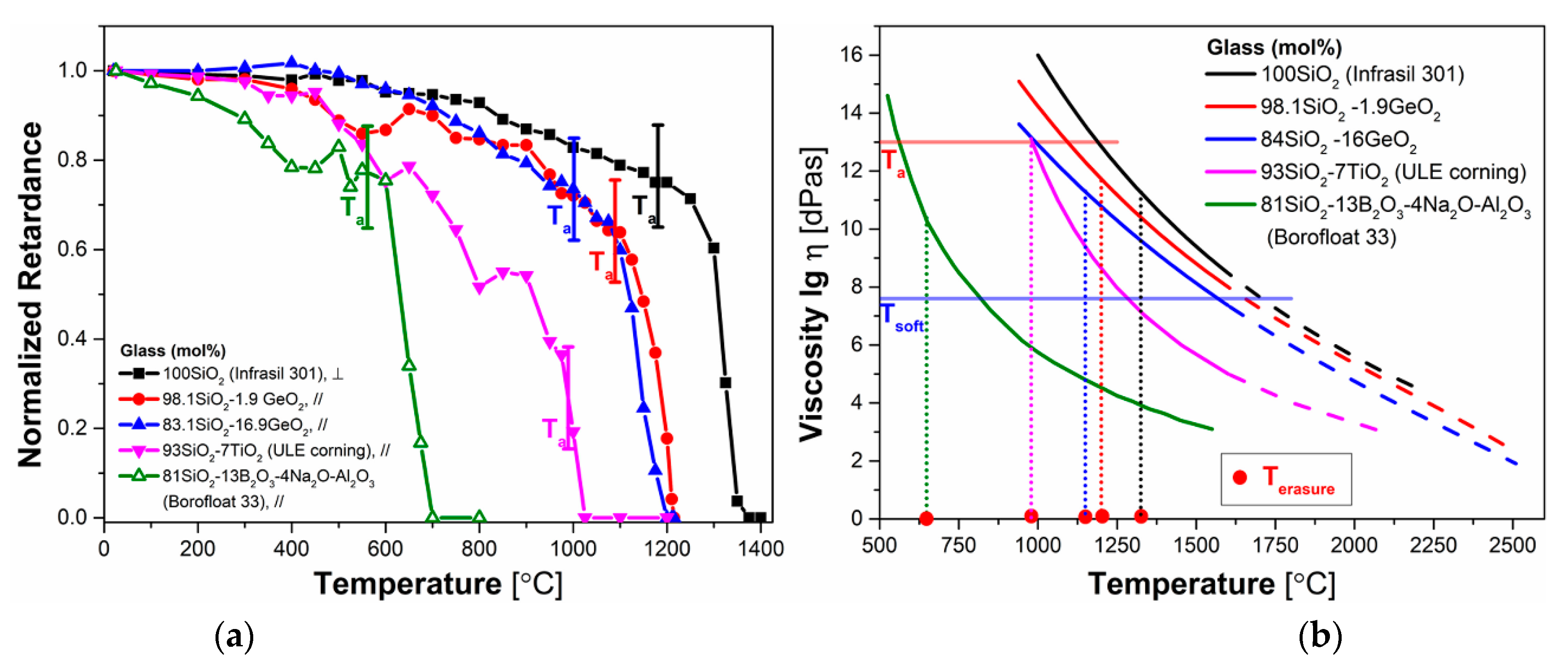
3.3. Influence of GeO2 Content
3.4. Impact of Dopants
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Åslund, M.L.; Canning, J.; Canagasabey, A.; De Oliveira, R.A.; Liu, Y.; Cook, K.; Peng, G.-D. Mapping the thermal distribution within a silica preform tube using regenerated fibre Bragg gratings. Int. J. Heat Mass Transf. 2012, 55, 3288–3294. [Google Scholar] [CrossRef]
- Baldwin, C.S. Brief history of fiber optic sensing in the oil field industry. In Fiber Optic Sensors and Applications XI; SPIE: Baltimore, MD, USA, 2014. [Google Scholar]
- Hill, K.O.; Meltz, G. Fiber bragg grating technology fundamentals and overview. J. Light. Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef]
- Cavillon, M.; Lancry, M.; Poumellec, B.; Wang, Y.T.; Canning, J.; Cook, K.; Hawkins, T.; Dragic, P.; Ballato, J. Overview of high temperature fibre Bragg gratings and potential improvement using highly doped aluminosilicate glass optical fibres. J. Phys. Photonics. 2019, 1, 042001. [Google Scholar] [CrossRef]
- Mihailov, S.J.; Grobnic, D.; Hnatovsky, C.; Walker, R.B.; Lu, P.; Coulas, D.; Ding, H. Extreme environment sensing using femtosecond laser-inscribed fiber Bragg gratings. Sensors 2009, 17, 2909. [Google Scholar] [CrossRef]
- Mihailov, S.J. Fiber bragg grating sensors for harsh environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef]
- Lancry, M.; Poumellec, B.; Canning, J.; Cook, K.; Poulin, J.C.; Brisset, F. Ultrafast nanoporous silica formation driven by femtosecond laser irradiation. Laser Photonics Rev. 2013, 7, 953–962. [Google Scholar] [CrossRef]
- Grobnic, D.; Smelser, C.W.; Mihailov, S.J.; Walker, R.B. Long-term thermal stability tests at 1000 °C of silica fibre bragg gratings made with ultrafast laser radiation. Meas. Sci. Technol. 2006, 17, 1009–1013. [Google Scholar] [CrossRef]
- Bricchi, E.; Kazansky, P.G. Extraordinary stability of anisotropic femtosecond direct-written structures embedded in silica glass. Appl. Phys. Lett. 2006, 88, 111119. [Google Scholar] [CrossRef]
- Wood, D.; Walker, K.; MacChesney, J.; Simpson, J.; Csencsits, R. Germanium chemistry in the MCVD process for optical fiber fabrication. J. Light. Technol. 1987, 5, 277–285. [Google Scholar] [CrossRef]
- Van Uitert, L.G.; Pinnow, D.A.; Williams, J.C.; Rich, T.C.; Jaeger, R.E.; Grodkiewicz, W.H. Borosilicate glasses for fiber optical waveguides. MRS Bull. 1973, 8, 469–476. [Google Scholar] [CrossRef]
- Simpson, J.R.; MacChesney, J.B. Optical fibres with an Al2O3-doped silicate core composition. Electron. Lett. 1983, 19, 261–262. [Google Scholar] [CrossRef]
- Reddy, P.H.; Rahman, M.F.A.; Paul, M.C.; Latiff, A.A.; Rosol, A.H.A.; Das, S.; Dhar, A.; Bhadra, S.K.; Dimyati, K.; Harun, S.W. Titanium dioxide doped fiber as a new saturable absorber for generating mode-locked erbium doped fiber laser. Optik. 2018, 158, 1327–1333. [Google Scholar] [CrossRef]
- Shibata, S.; Nakahara, M. Fluorine and chlorine effects on radiation-induced loss for GeO2-doped silica optical fibers. J. Light. Technol. 1985, 3, 860–863. [Google Scholar] [CrossRef]
- Ainslie, B.J.; France, P.W.; Newns, G.R. Water impurity in low-loss silica fibre. MRS Bull. 1977, 12, 481–487. [Google Scholar] [CrossRef]
- Lancry, M.; Groothoff, N.; Guizard, S.; Yang, W.; Poumellec, B.; Kazansky, P.G.; Canning, J. Femtosecond laser direct processing in wet and dry silica glass. J. Non-Cryst. Solids. 2009, 355, 1057–1061. [Google Scholar] [CrossRef]
- Lancry, M.; Canning, J.; Cook, K.; Heili, M.; Neuville, D.R.; Poumellec, B. Nanoscale femtosecond laser milling and control of nanoporosity in the normal and anomalous regimes of GeO2-SiO2 glasses. Opt. Mater. Express. 2016, 6, 321–330. [Google Scholar] [CrossRef]
- Lancry, M.; Desmarchelier, R.; Guth, N.; Zimmerman, F.; Brisset, F.O.; Nolte, S.; Poumellec, B. Porous Nanogratings and Related Form Birefringence in Silicate and Germanate Glasses. In Proceedings of the Bragg Gratings, Photosensitivity, and Poling in Glass Waveguides, Barcelona, Spain, 27–31 July 2014. [Google Scholar]
- Lancry, M.; Poumellec, B.; Chahid-Erraji, A.; Beresna, M.; Kazansky, P.G. Dependence of the femtosecond laser refractive index change thresholds on the chemical composition of doped-silica glasses. Opt. Mater. Express. 2011, 1, 711–723. [Google Scholar] [CrossRef]
- Lancry, M.; Zimmerman, F.; Desmarchelier, R.; Tian, J.; Brisset, F.; Nolte, S.; Poumellec, B. Nanogratings formation in multicomponent silicate glasses. Appl. Phys. B. 2016, 122, 66. [Google Scholar] [CrossRef]
- Poumellec, B. Links between writing and erasure (or stability) of bragg gratings in disordered media. J. Non-Cryst. Solids. 1998, 239, 108–115. [Google Scholar] [CrossRef]
- Grobnic, D.; Hnatovsky, C.; Mihailov, S.J. Low loss type II regenerative bragg gratings made with ultrafast radiation. Opt. Express. 2016, 24, 28704–28712. [Google Scholar] [CrossRef]
- Desmarchelier, R.; Poumellec, B.; Brisset, F.; Mazerat, S.; Lancry, M. In the heart of femtosecond laser induced nanogratings: From porous nanoplanes to form birefringence. World J. Nano Sci. Eng. 2015, 5, 115–125. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Cicconi, M.R.; Tsuji, Y.; Shimizu, M.; Shimotsuma, Y.; Miura, K.; Peng, G.-D.; Neuville, D.R.; Poumellec, B.; et al. Femtosecond Laser Direct Writing in SiO2-Al2O3 Binary Glasses and Thermal Stability of Type II Permanent Modifications. J. Am. Ceram. Soc. (under review).
- The de Sénarmont Compensator. Available online: https://www.olympus-lifescience.com.cn/fr/microscope-resource/primer/techniques/polarized/desenarmontcompensator/ (accessed on 24 January 2020).
- Fan, C.; Poumellec, B.; Zeng, H.; Lancry, M.; Yang, W.; Bourguignon, B.; Chen, G. Directional writing dependence of birefringence in multicomponent silica-based glasses with ultrashort laser irradiation. J. Laser Micro/Nanoeng. 2011, 6, 158–163. [Google Scholar] [CrossRef]
- Witcher, J.J.; Reichman, W.J.; Fletcher, L.B.; Troy, N.W.; Krol, D.M. Thermal annealing of femtosecond laser written structures in silica glass. Opt. Mater. Express. 2013, 3, 502–510. [Google Scholar] [CrossRef]
- Dogan, Y.; Madsen, C.K. Optimization of ultrafast laser parameters for 3D micromachining of fused silica. Opt Laser Technol. 2019, 123, 105933. [Google Scholar] [CrossRef]
- Brueckner, R. Properties and structure of vitreous silica. I. J. Non-Cryst. Solids. 1970, 5, 123–175. [Google Scholar] [CrossRef]
- Bennett, H.E.; Guenther, A.H.; Newnam, B.E.; Soileau, M.J. Laser Induced Damage in Optical Materials: 1988; NIST: Gaithersburg, MD, USA, 1989; pp. 89–90. [Google Scholar]
- Bricchi, E.; Klappauf, B.G.; Kazansky, P.G. Form birefringence and negative index change created by femtosecond direct writing in transparent materials. Opt. Lett. 2004, 29, 119–121. [Google Scholar] [CrossRef]
- Champagnon, B.; Le Parc, R.; Guenot, P. Relaxation of silica above and below Tg: Light scattering studies. Philos. Mag. B. 2002, 82, 251–255. [Google Scholar] [CrossRef]
- Ho, C.K.; Djie, H.S.; Pita, K.; Ngo, N.Q.; Kam, C.H. Sintering and porosity control of (x) GeO2:(1−x) SiO2 sol-gel derived films for optoelectronic applications. Electrochem. Solid-State Lett. 2004, 7, F96–F98. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sarkar, A.; Schultz, P.C. Relationship between composition, density and refractive index for germania silica glasses. J. Non-Cryst. Solids. 1978, 27, 29–37. [Google Scholar] [CrossRef]
- Poumellec, B.; Lancry, M. Kinetics of thermally activated physical processes in disordered media. Fibers 2015, 3, 206–252. [Google Scholar] [CrossRef]
- Cerkauskaite, A. Ultrafast Laser Nanostructuring for Photonics and Information Technology. Doctoral Dissertation, University of Southampton, England, UK, 2018. [Google Scholar]
- Rudenko, A.; Colombier, J.P.; Itina, T.E. Nanopore-mediated ultrashort laser-induced formation and erasure of volume nanogratings in glass. Phys. Chem. Chem. Phys. 2018, 20, 5887–5899. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags. Steel Res. Int. 2012, 83, 150–153. [Google Scholar] [CrossRef]
- Cai, Z.; Song, B.; Li, L.; Liu, Z.; Cui, X. Effects of B2O3 on viscosity, structure, and crystallization of mold fluxes for casting rare earth alloyed steels. Metals 2018, 8, 2018. [Google Scholar] [CrossRef]
- McMillan, P.W.; Chlebik, A. The effect of hydroxyl ion content on the mechanical and other properties of soda-lime-silica glass. J. Non-Cryst. Solids. 1980, 38, 509–514. [Google Scholar] [CrossRef]
- Haken, U.; Humbach, O.; Ortner, S.; Fabian, H. Refractive index of silica glass: Influence of fictive temperature. J. Non-Cryst. Solids. 2000, 265, 9–18. [Google Scholar] [CrossRef]
- Rajesh, S.; Bellouard, Y. Towards fast femtosecond laser micromachining of fused silica: The effect of deposited energy. Opt. Express 2010, 18, 21490–21497. [Google Scholar] [CrossRef]
- Champion, A.; Bellouard, Y. Direct volume variation measurements in fused silica specimens exposed to femtosecond laser. Opt. Mater. Express. 2012, 2, 789–798. [Google Scholar] [CrossRef]
| Section | Designations | Compositions(mole%) | Impurities1 (ppm) | Ta2 (°C) | |
|---|---|---|---|---|---|
| OH | Cl | ||||
| 3.1 Speed | Suprasil CG® | 100 SiO2 | 830 | < 2500 | 1100 |
| 3.2 OH; Cl | Type I (Infrasil 301®) | 100 SiO2 | 8 | < 0.15 | 1180 |
| Type II (Homosil®) | 380 | < 1 | 1180 | ||
| Type III (Suprasil CG®) | 830 | < 2500 | 1100 | ||
| Type III (Spectrosil 2000®) | 800–1200 | < 0.15 | 1025 | ||
| Type IV (Suprasil F300®) | 0.1 | 2500 | 1110 | ||
| 3.3 GeO2 | Germanosilicate | xGeO2-(1-x) SiO2, x = 1.9, 5, 11.8, 16.9 | < 0.1 | ~2000 | 990–1090 |
| 3.4 Dopants | Infrasil 301® | 100 SiO2 | 8 | < 0.15 | 1180 |
| Germanosilicate | 1.9 GeO2-98.1 SiO2 | < 0.1 | ~2000 | 1090 | |
| Germanosilicate | 16.9 GeO2-83.1 SiO2 | < 0.1 | ~2000 | 990 | |
| ULE corning® | 7 TiO2-93 SiO2 | ----- | ----- | 900 | |
| Borofloat33® | 81 SiO2-13 B2O3-4 Na2O/K2O -2 Al2O3 | ----- | ----- | 663.6 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.-E.; Wang, Y.; Yao, H.; Cavillon, M.; Poumellec, B.; Peng, G.-D.; Lancry, M. Thermal Stability of Type II Modifications by IR Femtosecond Laser in Silica-based Glasses. Sensors 2020, 20, 762. https://doi.org/10.3390/s20030762
Wei S-E, Wang Y, Yao H, Cavillon M, Poumellec B, Peng G-D, Lancry M. Thermal Stability of Type II Modifications by IR Femtosecond Laser in Silica-based Glasses. Sensors. 2020; 20(3):762. https://doi.org/10.3390/s20030762
Chicago/Turabian StyleWei, Shu-En, Yitao Wang, Heng Yao, Maxime Cavillon, Bertrand Poumellec, Gang-Ding Peng, and Matthieu Lancry. 2020. "Thermal Stability of Type II Modifications by IR Femtosecond Laser in Silica-based Glasses" Sensors 20, no. 3: 762. https://doi.org/10.3390/s20030762
APA StyleWei, S.-E., Wang, Y., Yao, H., Cavillon, M., Poumellec, B., Peng, G.-D., & Lancry, M. (2020). Thermal Stability of Type II Modifications by IR Femtosecond Laser in Silica-based Glasses. Sensors, 20(3), 762. https://doi.org/10.3390/s20030762








