An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Measurements
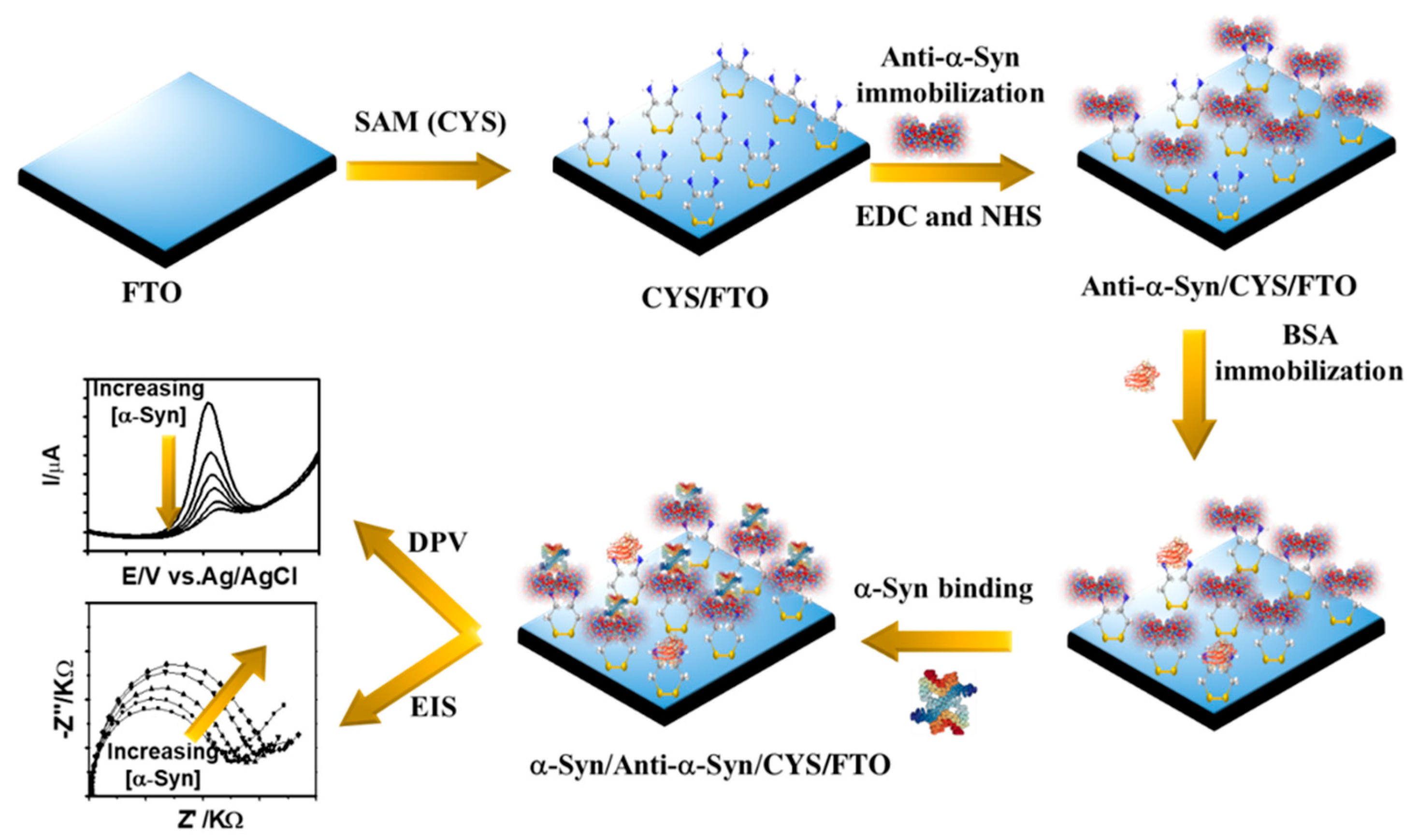
2.3. Preparation of Anti-α-Syn/CYS/FTO Probe
3. Results and Discussion
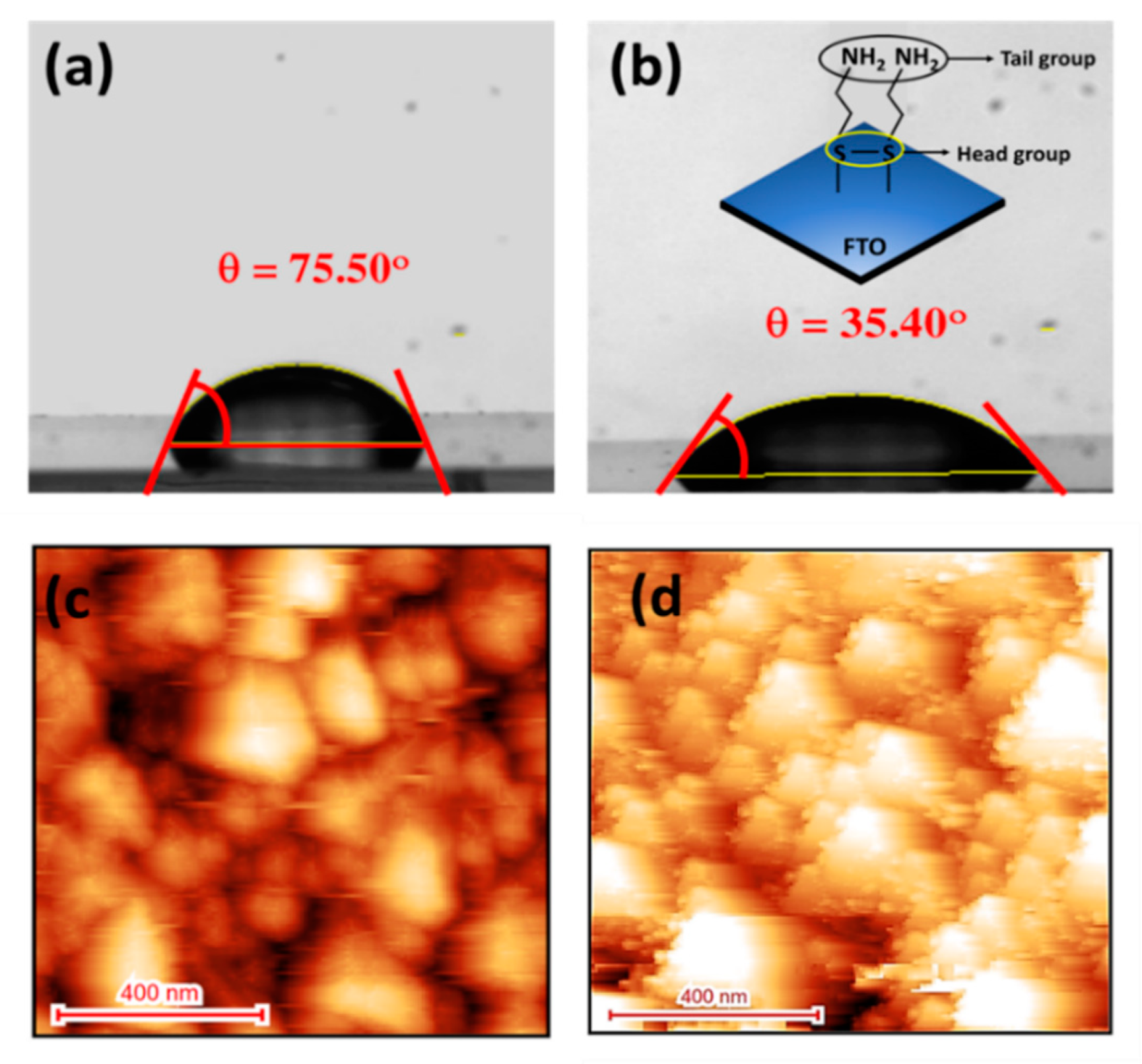
3.1. Contact-Angel and AFM Characterizations of CYS-SAM
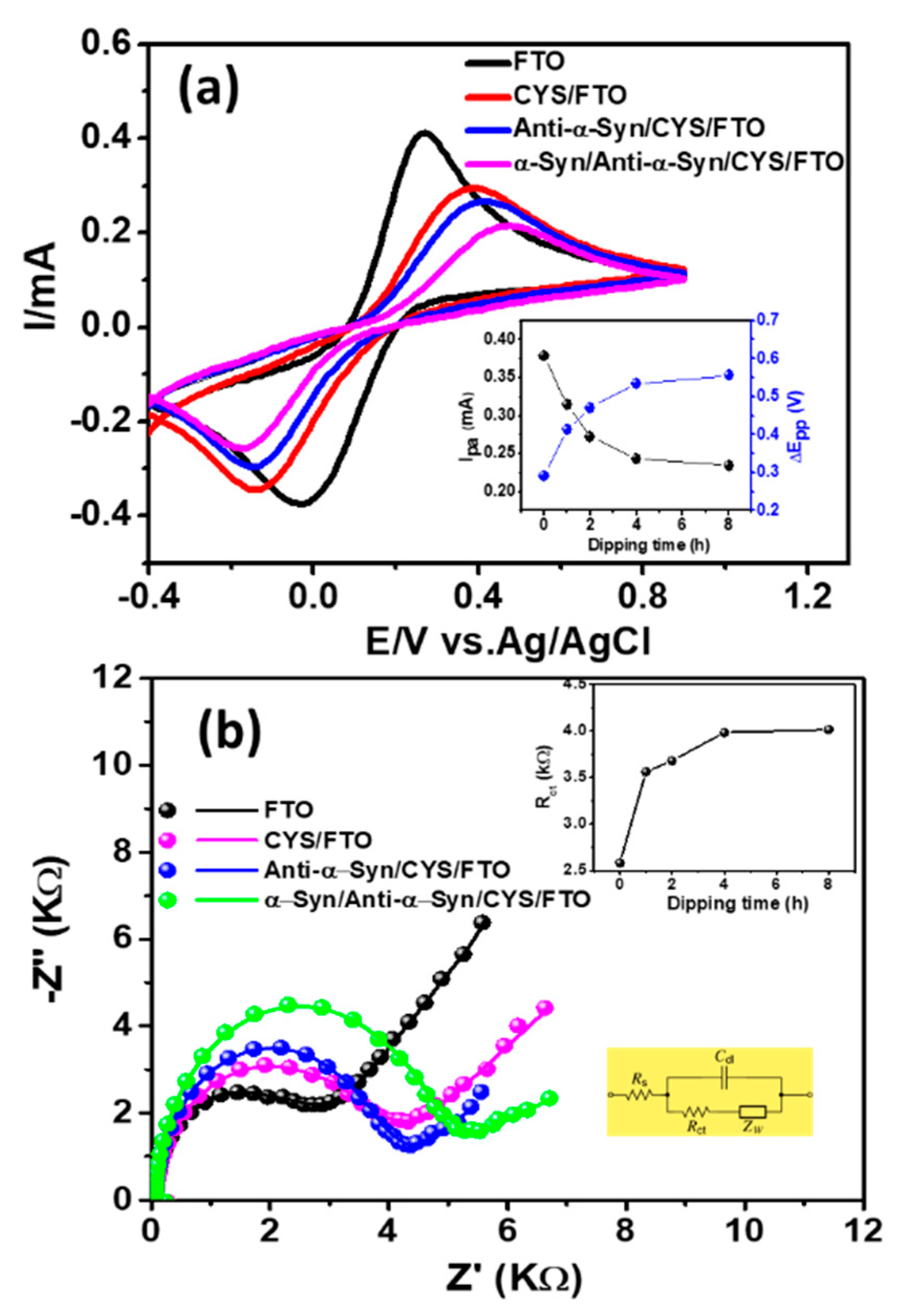
3.2. Electrochemical Characterization
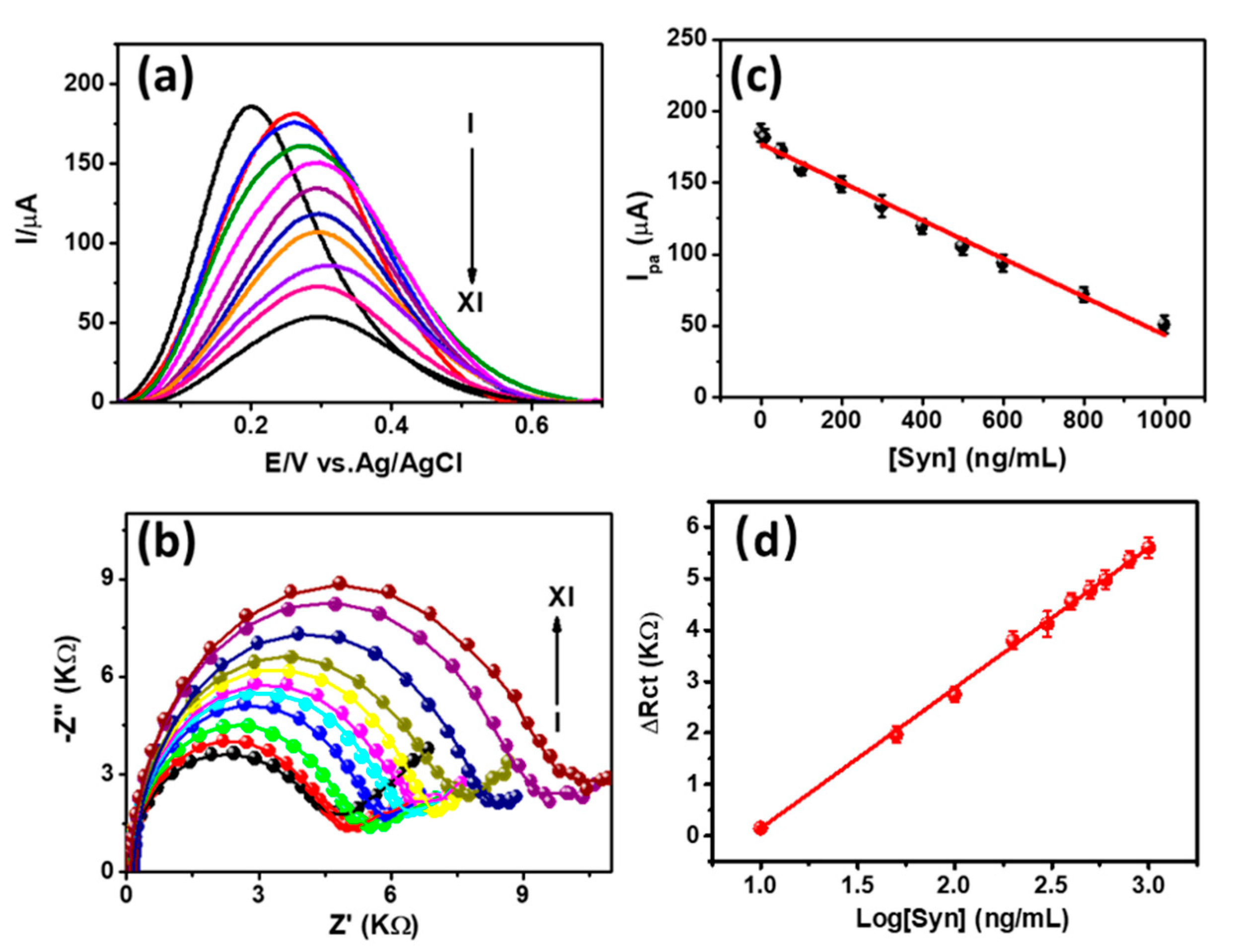
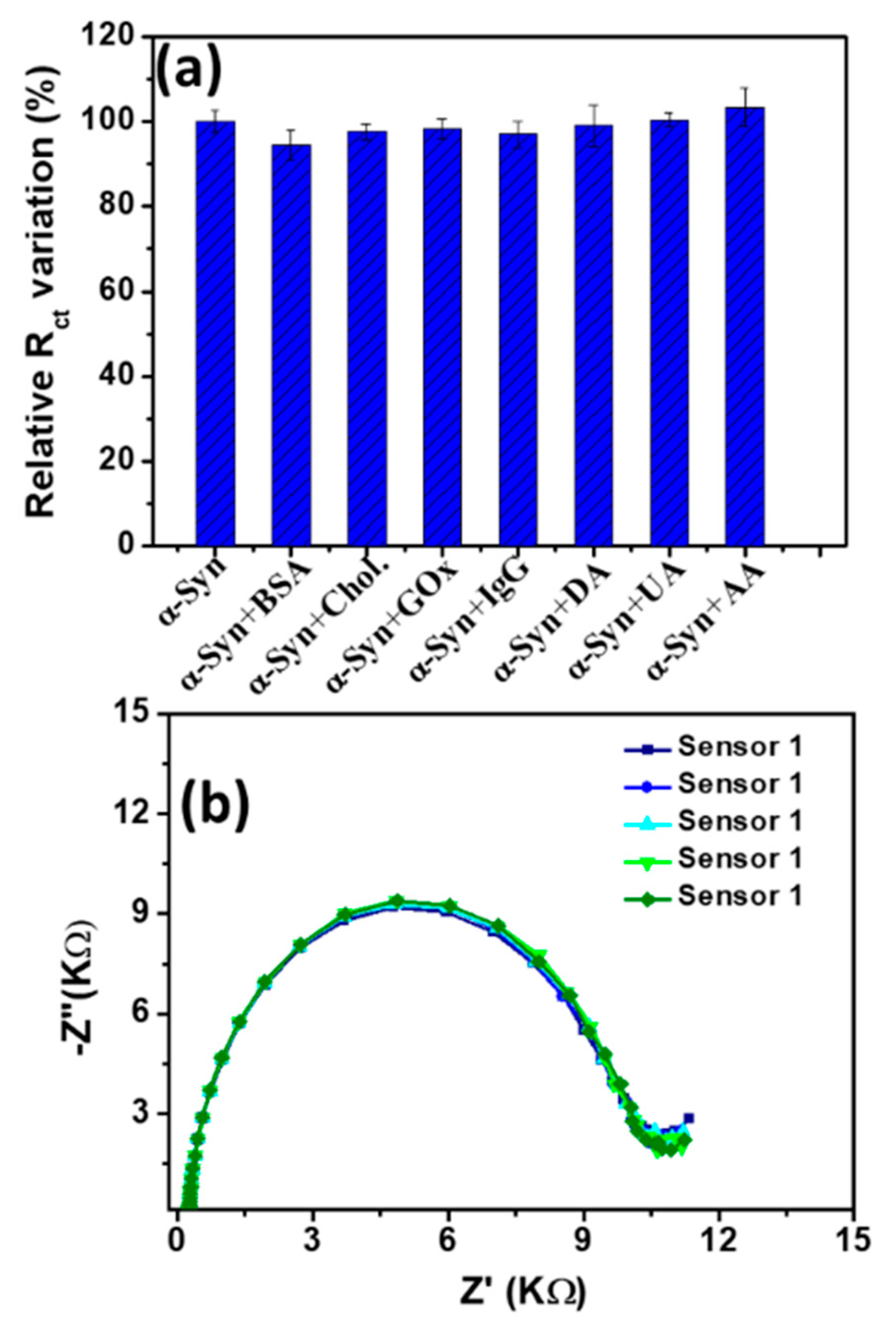
3.3. Detection of α-Syn Antigen and Selectivity Study
3.4. Reproducibility, Stability, and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Zhang, L.; Long, Y.; Zhou, F. Electroanalytical sensors and methods for assays and studies of neurological biomarkers. Electroanalysis 2014, 26, 1236–1248. [Google Scholar] [CrossRef]
- Ganesh, H.V.S.; Chow, A.M.; Kerman, K. Recent advances in biosensors for neurodegenerative disease detection. Trends Anal. Chem. 2016, 79, 363–370. [Google Scholar] [CrossRef]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Rocha, E.M.; Miranda, B.D.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Mochizuki, H.; Choong, C.J.; Masliah, E. A refined concept: α-synuclein dysregulation disease. Neurochem. Int. 2018, 119, 84–96. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Mantri, S.; Morley, J.F.; Siderowf, A.D. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat. Disord. 2019, 64, 20–28. [Google Scholar] [CrossRef]
- Sharma, S.; Moon, C.S.; Khogali, A.; Haidous, A.; Chabenne, A.; Ojo, C.; Jelebinkov, M.; Kurdi, Y.; Ebadi, M. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 2013, 63, 201–229. [Google Scholar] [CrossRef]
- Khodadadian, A.; Hemmati-Dinarvand, M.; Kalantary-Charvadeh, A.; Ghobadi, A.; Mazaheri, M. Candidate biomarkers for Parkinson’s disease. Biomed. Pharmacother. 2018, 104, 699–704. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, A.; Lee, J.J. A conducting poly(N-(1-Naphthyl) ethylenediamine dihydrochloride) nanofibers for the sensitive and interference-free detection of dopamine. J. Electrochem. Soc. 2018, 165, B89–B95. [Google Scholar] [CrossRef]
- Lopes, P.; Dyrnesli, H.; Lorenzen, N.; Otzen, D.; Ferapontova, E.E. Electrochemical analysis of the fibrillation of Parkinson’s disease α-synuclein. Analyst 2014, 139, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-like mechanisms in Parkinson’s disease. Front. Neurosci. 2019, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Slamnoiu, S.; Vlad, C.; Stumbaum, M.; Moise, A.; Lindner, K.; Engel, N.; Vilanova, M.; Diaz, M.; Karreman, C.; Leist, M.; et al. Identification and affinity-quantification of β-amyloid and α-synuclein polypeptides using on-line saw-biosensor-mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 1472–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Lutz, E.A.; Slade, K.M.; Ruf, R.A.S.; Wang, G.-F.; Pielak, G.J. 19F NMR studies of α-synuclein conformation and fibrillation. Biochemistry 2009, 48, 8578–8584. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Silajdžić, E.; Yang, H.; Björkqvist, M.; Kim, S.; Jeong, O.C.; Hansson, O.; Laurell, T. A porous silicon immunoassay platform for fluorometric determination of α-synuclein in human cerebrospinal fluid. Microchim. Acta 2014, 181, 1143–1149. [Google Scholar] [CrossRef]
- Killinger, B.A.; Moszczynska, A. Characterization of α-synuclein multimer stoichiometry in complex biological samples by electrophoresis. Anal. Chem. 2016, 88, 4071–4084. [Google Scholar] [CrossRef]
- An, Y.; Tang, L.; Jiang, X.; Chen, H.; Yang, M.; Jin, L.; Zhang, S.; Wang, C.; Zhang, W. A photoelectrochemical immunosensor based on au-doped TiO2 nanotube arrays for the detection of α-synuclein. Chem. Eur. J. 2010, 16, 14439–14446. [Google Scholar] [CrossRef]
- Sun, K.; Xia, N.; Zhao, L.; Liu, K.; Hou, W.; Liu, L. Aptasensors for the selective detection of alpha-synuclein oligomerby colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2017, 245, 87–94. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Nameghi, M.A.; Ramezani, M.; Alibolandi, M.; Hassanzadeh-Khayat, M.; Emrani, A.S.; Abnous, K. A novel electrochemical aptasensor based on nontarget-induced high accumulation of methylene blue on the surface of electrode for sensing of α-synuclein oligomer. Biosens. Bioelectron. 2019, 123, 14–18. [Google Scholar] [CrossRef]
- Masarik, M.; Stobiecka, A.; Kizek, R.; Jelen, F.; Pechan, Z.; Hoyer, W.; Jovin, T.M.; Subramaniam, V.; Palecek, E. Sensitive electrochemical detection of native and aggregated α-synuclein protein involved in Parkinson’s disease. Electroanalysis 2004, 16, 1172–1181. [Google Scholar] [CrossRef]
- Karaboğa, M.N.S.; Sezgintürk, M.K. Cerebrospinal fluid levels of alpha-synuclein measured using a poly-glutamic acid-modified gold nanoparticle-doped disposable neuro-biosensor system. Analyst 2019, 144, 611–621. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Jiang, X.; Bi, W.; Chen, H.; Jin, L.; Zhang, S.; Wang, C.; Zhang, W. Sensitive electrochemical immunosensor for α-synuclein based on dual signal amplification using PAMAM dendrimer-encapsulated Au and enhanced gold nanoparticle labels. Biosens. Bioelectron. 2012, 32, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Sutradhar, S.C.; Lei, J.; Kim, J.; Kim, D.H.; Lee, Y.H.; Kim, W. Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens. Bioelectron. 2019, 126, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Lei, J.; Choi, I.; Kim, D.H.; Lee, Y.H.; Kim, W. A chemically and electrochemically stable, redox-active and highly sensitive metal azolate framework for non-enzymatic electrochemical detection of glucose. J. Electroanal. Chem. 2019, 840, 263–271. [Google Scholar] [CrossRef]
- Coelho, J.H.; Eisele, A.P.P.; Valezi, C.F.; Mattos, G.J.; Schirmann, J.G.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Sartori, E.R. Exploring the exocellular fungal biopolymer botryosphaeran for laccase-biosensor architecture and application to determine dopamine and spironolactone. Talanta 2019, 204, 475–483. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; de la Guardia, M. Nanomaterial-based electrochemical immunosensors as advanced diagnostic tools. Anal. Methods 2014, 6, 3891–3900. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, H.; Zhou, Y.; Zheng, J. C60 mediated ion pair interaction for label-free electrochemical immunosensing with nanoporous anodic alumina nanochannels. Anal. Chem. 2019, 91, 5125–5132. [Google Scholar] [CrossRef]
- Kim, Y.J.; Rahman, M.M.; Lee, J.J. Ultrasensitive and label-free detection of annexin A3 based on quartz crystal microbalance. Sens. Actuators B Chem. 2013, 177, 172–177. [Google Scholar] [CrossRef]
- Alves, A.C.T.; Gomes, D.J.C.; Silva, J.R.; Silva, G.B. Fluorine-doped tin oxide surfaces modified by self-assembled alkanethiols for thin-film devices. Appl. Surf. Sci. 2013, 279, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Song, X.; Zhang, J.; Shen, H.; Xiong, Q. Switchable wettability in SnO2 nanowires and SnO2@SnO2 heterostructures. J. Phys. Chem. C 2011, 115, 22225–22231. [Google Scholar] [CrossRef]
- Cojocaru, L.; Uchida, S.; Jayaweera, P.V.V.; Kaneko, S.; Wang, H.; Nakazaki, J.; Kubo, T.; Segawa, H. Effect of TiO2 surface treatment on the current–voltage hysteresis of planar-structure perovskite solar cells prepared on rough and flat fluorine-doped tin oxide substrates. Energy Technol. 2017, 5, 1762–1766. [Google Scholar] [CrossRef] [Green Version]
- Lopa, N.S.; Rahman, M.M.; Jang, H.; Sutradhar, S.C.; Ahmed, F.; Ryu, T.; Kim, W. A glassy carbon electrode modified with poly(2,4-dinitrophenylhydrazine) for simultaneous detection of dihydroxybenzene isomers. Microchim. Acta 2018, 185, 23. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Rahman, M.M.; Lee, J.J. Label-free aptasensor for the detection of cardiac biomarker myoglobin based on gold nanoparticles decorated boron nitride nanosheets. Biosens. Bioelectron. 2019, 126, 143–150. [Google Scholar] [CrossRef]
| Electrode Configuration | Sensor Type | Measurement Method | Linear Range (ng/mL) | LOD (ng/mL) | Ref. |
|---|---|---|---|---|---|
| Au–TiO2 NTs | Immunosensor | Photo-electrochemical | 0.05–100 | 0.034 | [18] |
| Thiolated Au | Aptasensor | EIS, SPR | 0.1 nM–0.5 μM | 0.001 | [19] |
| Apt-CS-Au | Aptasensor | CV, DPV | 60 pM–150 nM | 10 pM | [20] |
| Au NP–PGA /ITO | Immunosensor | EIS, CV, SWV | 0.004–2 | 0.135 | [22] |
| PAMAM–Au/C | Immunosensor | EIS, CV | 0.02–200 | 0.0146 | [23] |
| CYS/FTO | Immunosensor | DPV, EIS | 10–1000 | 3.62, 1.13 | This work |
| Sample No. | [α-Syn] Added (ng/mL) | [α-Syn] Found (ng/mL) a | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 50 | 47.9 ± 2.7 | 95.8 ± 5.4 | 1.87 |
| 2 | 200 | 198.4 ± 5.2 | 99.2 ± 2.6 | 0.98 |
| 3 | 500 | 506.5 ± 9.6 | 101.3 ± 1.9 | 3.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, C.-Y.; Rahman, M.M.; Zhang, W.; Lopa, N.S.; Jin, L.; Yoon, S.; Jang, H.; Xu, G.-R.; Kim, W. An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein. Sensors 2020, 20, 617. https://doi.org/10.3390/s20030617
Ge C-Y, Rahman MM, Zhang W, Lopa NS, Jin L, Yoon S, Jang H, Xu G-R, Kim W. An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein. Sensors. 2020; 20(3):617. https://doi.org/10.3390/s20030617
Chicago/Turabian StyleGe, Chuang-Ye, Md. Mahbubur Rahman, Wei Zhang, Nasrin Siraj Lopa, Lei Jin, Sujin Yoon, Hohyoun Jang, Guang-Ri Xu, and Whangi Kim. 2020. "An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein" Sensors 20, no. 3: 617. https://doi.org/10.3390/s20030617
APA StyleGe, C.-Y., Rahman, M. M., Zhang, W., Lopa, N. S., Jin, L., Yoon, S., Jang, H., Xu, G.-R., & Kim, W. (2020). An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein. Sensors, 20(3), 617. https://doi.org/10.3390/s20030617









