Calibration of Electrochemical Sensors for Nitrogen Dioxide Gas Detection Using Unmanned Aerial Vehicles
Abstract
1. Introduction
2. Methodology for Calibrating Electrochemical Sensors for NO2 Gas Detection on Board UAV’s
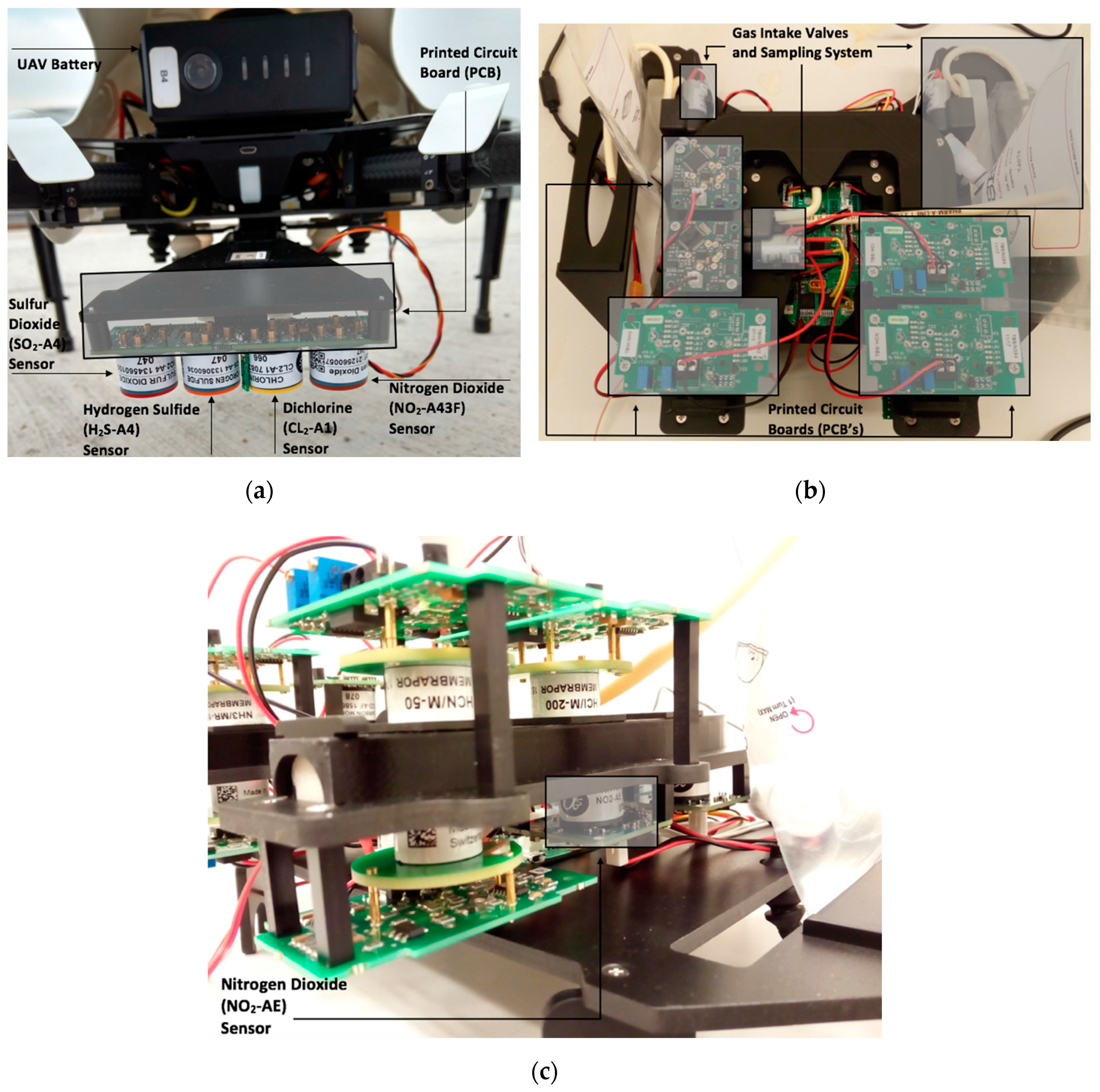
2.1. Materials and Instrumentation
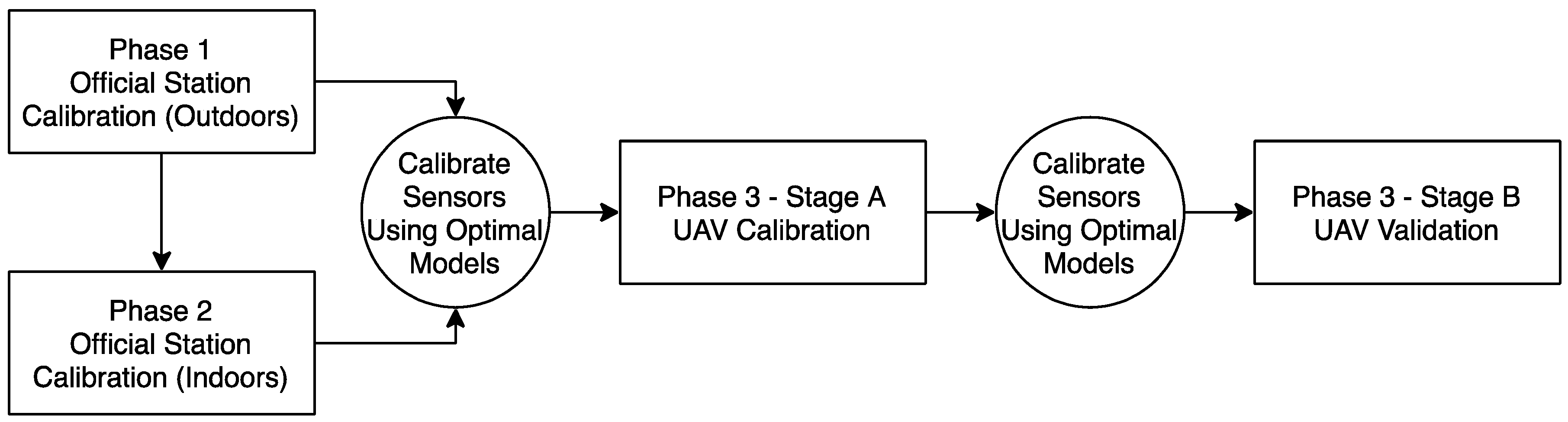
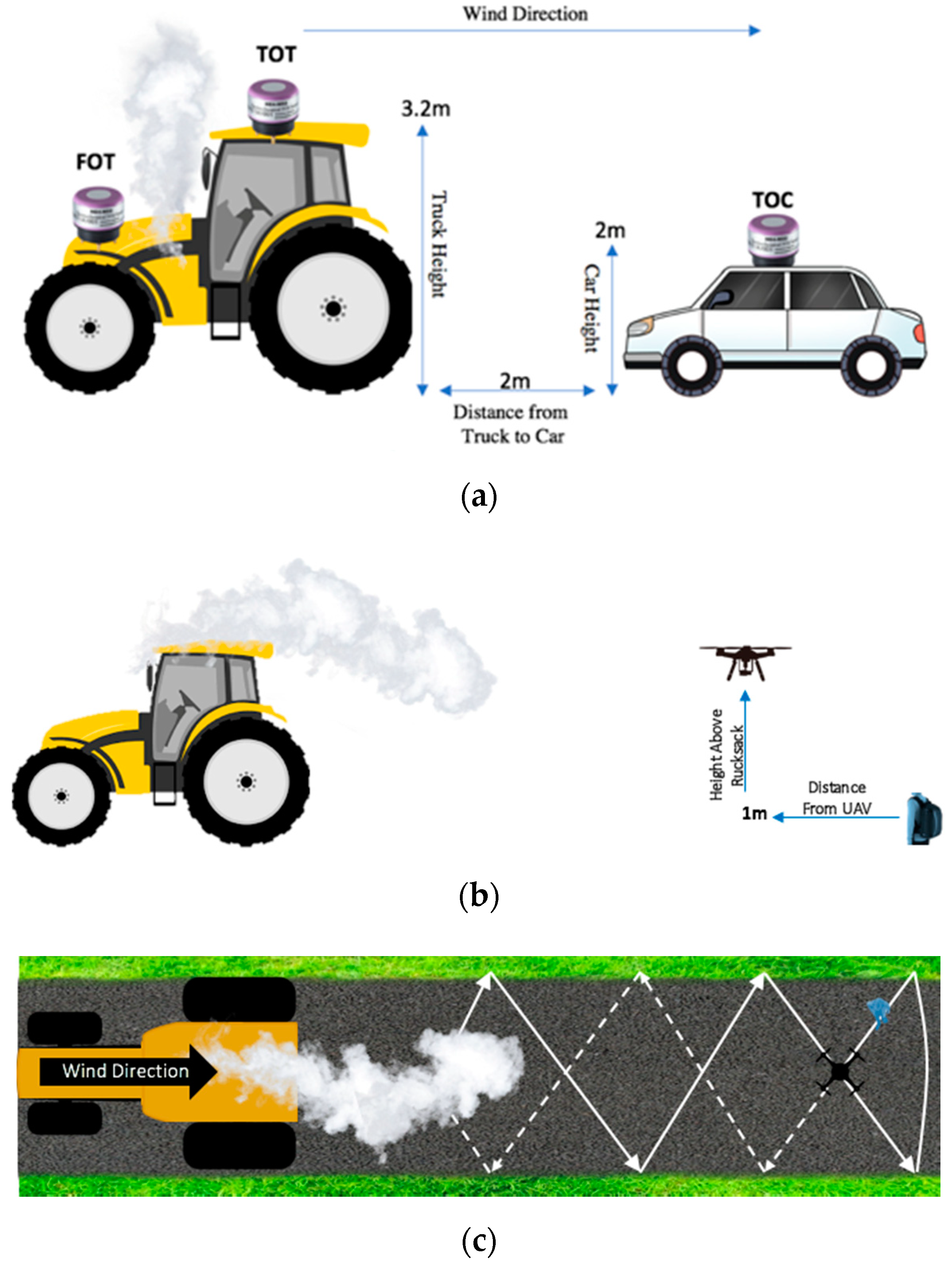
2.2. Experiment Setup
- (1)
- Phase 1: On 26 November 2018 both sensors were placed on the rooftop of the Amsterdam Vondelpark air quality monitoring station and were left there for a total calibration duration of eight days. They were positioned next to the stations’ sensors that measure NO2 and underneath a designed housing so that they would not be exposed to rain or other debris during the calibration process. For unknown reasons, the NO2-A43F sensor stopped recording after 19 h. As a result of having too few observations, this sensor is not calibrated during this Phase. A data split for the NO2-AE sensor occurs at approximately 48-h intervals between the start and end time.
- (2)
- Phase 2: Approximately two hours after Phase 1 ended, the sensors were taken to GGD Amsterdam’s indoor facility to begin Phase 2. Both sensors were exposed to different gases at different concentrations starting on 4 December 2018 at 13:00 and ending at 10:13 on 12 December 2018, which was controlled and monitored by the Environment S.A AC32 E-Series particulate analyzer – a micro-sensor designed specifically for controlling NOx gases. The sensors were left on throughout this entire duration. The period at which the sensors were exposed only to NO2 was from 10:35–12:00 (85 min) on 11 December 2018. Startup time is therefore not an issue during this phase. The data is split at approximately 21-min intervals between the start and end time. For this calibration, ground truth readings are 0.045 ppm from 10:35–11:24 and 0.06 from 11:24–12:00. T and RH are not controlled by the micro-sensor and are instead managed by the air-tight conditions of the testing room, which results in consistent readings of T and RH throughout the entire calibration period.
- (3)
- Phase 3: On 16 July 2019, the sensors were taken to the Twente Safety Campus in Enschede, The Netherlands. It was necessary to travel to this location in order to use an emission source capable of releasing enough gas for the sensors to detect. This phase is broken down into two stages: Stage A, which was inspired by previous research activities to calibrate the sensors, and Stage B, which is meant for validating the calibration [28]. The NO2-A43F and NO2-AE sensors both go through the same Stage A-Stage B processes. A complete overview of Phases 1, 2 and 3 are represented in Figure 3 below.
2.3. Data Filtering
2.4. Modelling
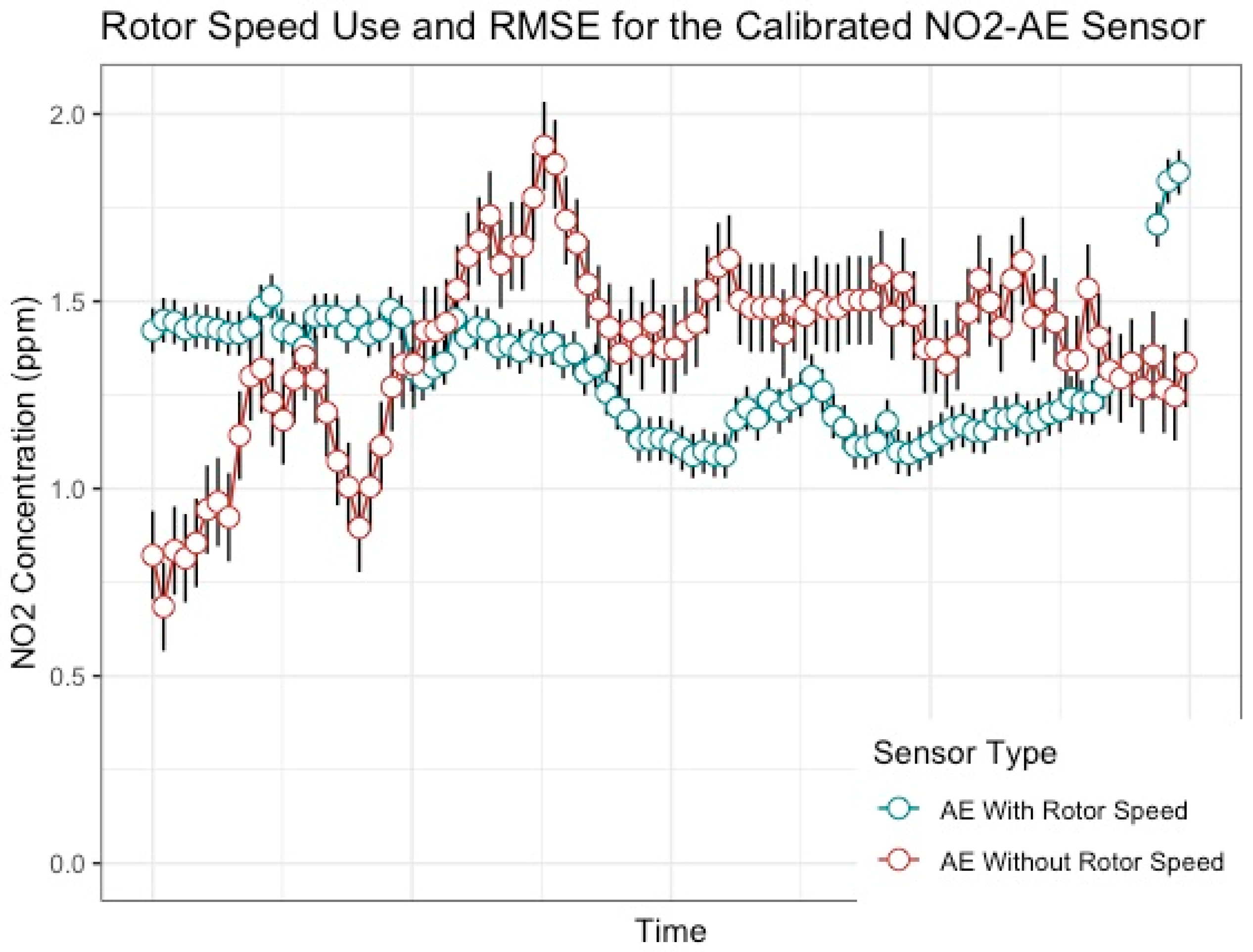
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rotatori, M.; Salvatori, R.; Salzano, R. Planning Air Pollution Monitoring Networks in Industrial Areas by Means of Remote Sensed Images and GIS Techniques. Air Qual. Monit. Assess. Manag. 2011. [Google Scholar] [CrossRef]
- Kizel, F.; Etzion, Y.; Shafran-Nathan, R.; Levy, I.; Fishbain, B.; Bartonova, A.; Broday, D.M. Node-to-node field calibration of wireless distributed air pollution sensor network. Environ. Pollut. 2018, 233, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Li, X. A Cost-effective Wireless Sensor Network System for Indoor Air Quality Monitoring Applications. Procedia Comput. Sci. 2014, 34, 165–171. [Google Scholar] [CrossRef]
- Mead, M.I.; Popoola, O.; Stewart, G.; Landshoff, P.V.; Calleja, M.; Hayes, M.J.; Baldovi, J.; McLeod, M.; Hodgson, T.; Dicks, J.; et al. The use of electrochemical sensors for monitoring urban air quality in low-cost, high-density networks. Atmos. Environ. 2013, 70, 186–203. [Google Scholar] [CrossRef]
- Mijling, B.; Jiang, Q.; De Jonge, D.; Bocconi, S. Field calibration of electrochemical NO2 sensors in a citizen science context. Atmos. Meas. Tech. 2018, 11, 1297–1312. [Google Scholar] [CrossRef]
- Rüdiger, J.; Tirpitz, J.-L.; De Moor, J.M.; Bobrowski, N.; Gutmann, A.; Liuzzo, M.; Ibarra, M.; Hoffmann, T. Implementation of electrochemical, optical and denuder-based sensors and sampling techniques on UAV for volcanic gas measurements: Examples from Masaya, Turrialba and Stromboli volcanoes. Atmos. Meas. Tech. 2018, 11, 2441–2457. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Villani, M.G.; Aleixandre, M.; Bonavitacola, F. Field calibration of a cluster of low-cost available sensors for air quality monitoring. Part A: Ozone and nitrogen dioxide. Sens. Actuators B Chem. 2015, 215, 249–257. [Google Scholar] [CrossRef]
- Thongplang, J. The Challenges with Electrochemical NO2 Sensors in Outdoor Air Monitoring. 23 July 2018. Available online: www.aeroqual.com/challenges-electrochemical-no2-sensors-outdoor-air-monitoring (accessed on 22 December 2018).
- Gerboles, M.; Spinelle, L.; Signorini, M.; Kotsev, A. AirSensEUR: And Open Data/Software/Hardware Multi-Sensor Platform for Air Quality Monitoring: Part B: Host, Influx Datapush and Assembling of AirSensEUR; EU SCIENCE HUB: Luxembourg, Luxembourg, 2016. [Google Scholar] [CrossRef]
- Villa, T.F.; Salimi, F.; Morton, K.; Morawska, L.; Gonzalez, F. Development and Validation of a UAV Based System for Air Pollution Measurements. Sensors 2016, 16, 2202. [Google Scholar] [CrossRef]
- Valente, J.; Munniks, S.; De Man, I.; Kooistra, L. Validation of a small flying e-nose system for air pollutants control: A plume detection case study from an agricultural machine. In Proceedings of the 2018 IEEE International Conference on Robotics and Biomimetics (ROBIO), Kuala Lumpur, Malaysia, 12–15 December 2018; pp. 1993–1998. [Google Scholar] [CrossRef]
- Zhou, F.; Gu, J.; Chen, W.; Ni, X. Measurement of SO2 and NO2 in Ship Plumes Using Rotary Unmanned Aerial System. Atmosphere 2019, 10, 657. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, Z.; Wu, Z.; Marinello, F. Spatial Variation of NO2 and Its Impact Factors in China: An Application of Sentinel-5P Products. Remote Sens. 2019, 11, 1939. [Google Scholar] [CrossRef]
- Jamali, S.; Klingmyr, D.; Tagesson, T. Global-Scale Patterns and Trends in Tropospheric NO2 Concentrations, 2005–2018. Remote Sens. 2020, 12, 3526. [Google Scholar] [CrossRef]
- Cross, E.S.; Williams, L.R.; Lewis, D.K.; Magoon, G.R.; Onasch, T.B.; Kaminsky, M.L.; Worsnop, D.R.; Jayne, J.T. Use of electrochemical sensors for measurement of air pollution: Correcting interference response and validating measurements. Atmos. Meas. Tech. 2017, 10, 3575–3588. [Google Scholar] [CrossRef]
- Bigi, A.; Mueller, M.; Grange, S.K.; Ghermandi, G.; Hueglin, C. Performance of NO, NO2 low cost sensors and three calibration approaches within a real world application. Atmos. Meas. Tech. 2018, 11, 3717–3735. [Google Scholar] [CrossRef]
- Deepak, P.; Shrivastava, K.; Prathik, K.; Ganesh, G.; Puneet, S.; Mishra, V. Artificial Neural Network for Automated Gas Sensor Calibration. Int. J. Adv. Comput. Eng. Netw. 2016. Available online: www.iraj.in/journal/journal_file/journal_pdf/3-299-147806450969-71.pdf (accessed on 22 December 2018).
- Esposito, E.; De Vito, S.; Salvato, M.; Bright, V.; Jones, R.; Popoola, O. Dynamic neural network architectures for on field stochastic calibration of indicative low cost air quality sensing systems. Sens. Actuators B Chem. 2016, 231, 701–713. [Google Scholar] [CrossRef]
- Yamamoto, K.; Togami, T.; Yamaguchi, N.; Ninomiya, S. Machine Learning-Based Calibration of Low-Cost Air Temperature Sensors Using Environmental Data. Sensors 2017, 17, 1290. [Google Scholar] [CrossRef]
- Jensen, C.K. Assessing the Applicability of Low-Cost Electrochemical Gas Sensors for Urban Air Quality Monitoring. IT University of Copenhagen; Pervasive Interaction Technology Laboratory: Copenhagen, Denmark, 2016; Available online: fritzing.org/media/fritzing-repo/projects/n/noxo3-sensor/other_files/Christian_kandidatprojekt_Rettet3.pdf (accessed on 22 December 2018).
- Robor Electronics. 2020. Available online: www.robor.nl (accessed on 2 December 2020).
- Alphasense: Technical Specification, NO2-A43F Nitrogen Dioxide Sensor 4-Electrode. July 2019. Available online: www.alphasense.com/WEB1213/wp-content/uploads/2017/03/NO2-AE.pdf (accessed on 2 December 2020).
- Alphasense: Technical Specification, NO2-AE Nitrogen Dioxide Sensor High Concentration. March 2017. Available online: www.alphasense.com/WEB1213/wp-content/uploads/2019/09/NO2-A43F.pdf (accessed on 2 December 2020).
- DRON Expert the Netherlands: Thermal Solutions. 2019. Available online: Dronexpert.nl/thermal (accessed on 2 December 2020).
- Realterm: Serial Terminal. Available online: www.realterm.sourceforge.io (accessed on 2 December 2020).
- Stroke Reader ActiveX, FAQ. 2020. Available online: https://strokescribe.com/en/serial-port-faq.html (accessed on 2 December 2020).
- Twente Safety Campus: Homepage. 2020. Available online: www.twentesafetycampus.nl/nl/homepage (accessed on 2 December 2020).
- Valente, J.; Almeida, R.; Kooistra, L. A Comprehensive Study of the Potential Application of Flying Ethylene-Sensitive Sensors for Ripeness Detection in Apple Orchards. Sensors 2019, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.O.; Valente, J.; Kooistra, L.; Munniks, S.; Peters, R.J.B. Experimental Flight Patterns Evaluation for a UAV-Based Air Pollutant Sensor. Micromachines 2020, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Gray, M.J.; Kaminski, R.M.; Weerakkody, G. Predicting Seed Yield of Moist-Soil Plants. J. Wildl. Manag. 1999, 63, 1261. [Google Scholar] [CrossRef]
- Veerasamy, R.; Rajak, H.; Jain, A.; Sivadasan, S.; Varghese, C.P.; Agrawal, R.K. Validation of QSAR Models—Strategies and Importance. Int. J. Drug Design Discov. 2011. Available online: www.researchgate.net/publication/284566093_Validation_of_QSAR_Models_Strategies_and_importance (accessed on 22 December 2018).
- Malaver, A.J.R.; Gonzalez, L.; Motta, N.; Villa, T. Design and flight testing of an integrated solar powered UAV and WSN for remote gas sensing. In Proceedings of the 2015 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2015; pp. 1–6. [Google Scholar] [CrossRef]

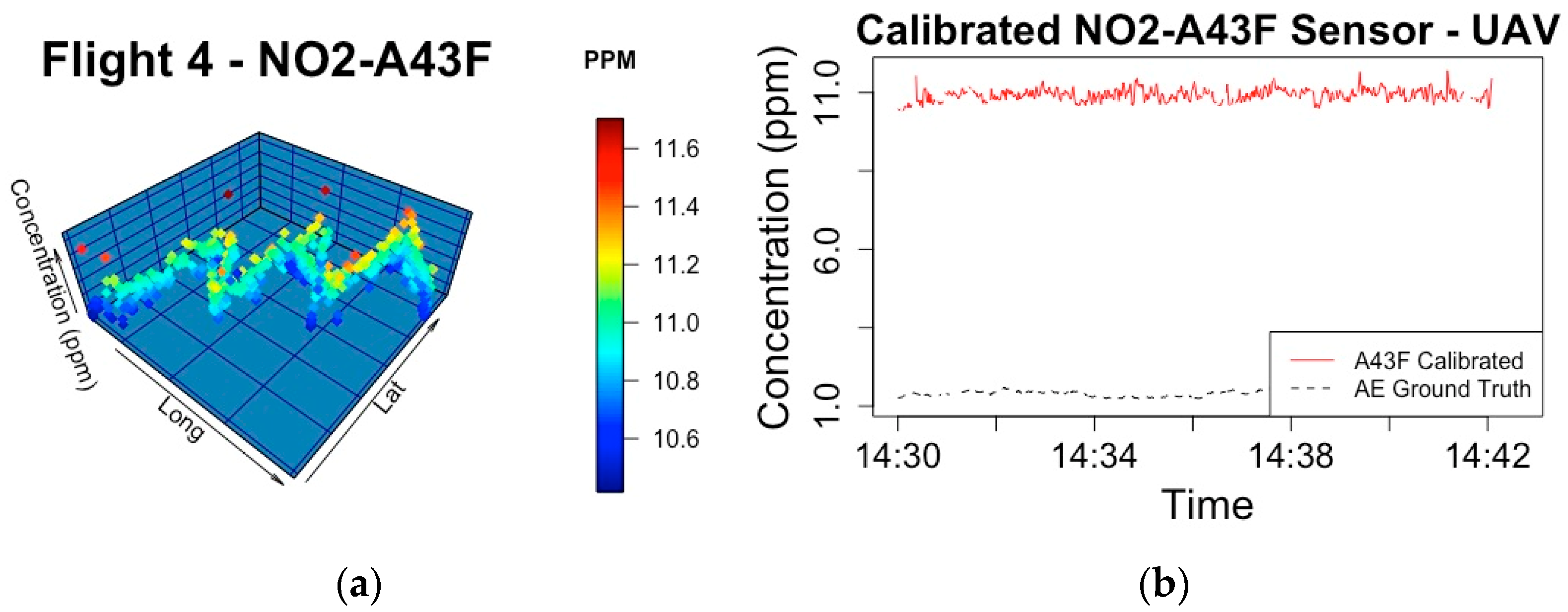
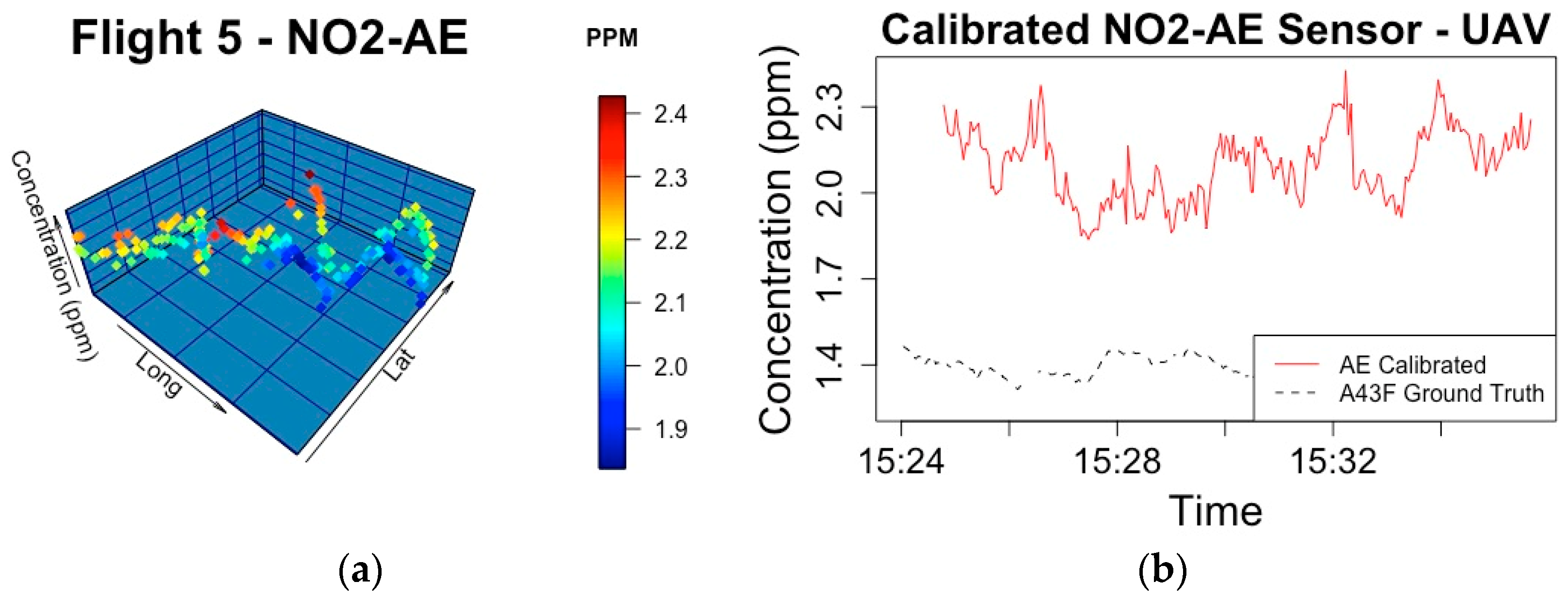
| Phase | Sensor | Ground Truth | Split/Sensor Placement | Date/Time | Model Components | Predictive R2 | RMSE |
|---|---|---|---|---|---|---|---|
| 1 | NO2-AE | Amsterdam Vondelpark | 3rd Quarter | 30/11/18 07:00:00–02/12/18 08:00:00 | Signal and RH | 0.77 | 0.0021 |
| 2 | NO2-AE | GGD Amsterdam | 2nd Quarter | 11/12/18 10:55:51–11/12/18 11:16:31 | Signal3, T2, RH2 | 0.97 | 0.0099 |
| 2 | NO2-A43F | GGD Amsterdam | All Data (no split) | 11/12/18 10:35:00–11/12/18 12:00:00 | Signal, T, RH | 0.81 | 0.0030 |
| 3 | NO2-AE | Calibrated NO2-AE | FOT | 16/07/19 11:26:59–16/07/19 11:31:20 | Signal | 0.21 | 0.1962 |
| 3 | NO2-AE | Calibrated NO2-AE | TOT | 16/07/19 11:31:21–16/07/19 11:35:22 | Signal, RH | 0.75 | 0.1194 |
| 3 | NO2-AE | Calibrated NO2-AE | TOC | 16/07/19 11:35:23–16/07/19 11:43:20 | Signal, T, Speed | 0.80 | 0.0624 |
| 3 | NO2-A43F | Calibrated NO2-A43F | FOT | 16/07/19 16:09:03–16/07/19 16:12:16 | Signal, T, RH, Speed | 0.50 | 0.0507 |
| 3 | NO2-A43F | Calibrated NO2-A43F | TOT | 16/07/19 16:12:18–16/07/19 16:13:58 | Signal | −0.04 | 0.1092 |
| 3 | NO2-A43F | Calibrated NO2-A43F | TOC | 16/07/19 16:14:01–16/07/19 16:17:45 | Signal, T, RH | 0.43 | 0.2217 |
| Flight Number | UAV Sensor | Ground Truth Sensor | RMSE |
|---|---|---|---|
| 4 | NO2-A43F | NO2-AE | 9.5584 |
| 5 | NO2-AE | NO2-A43F | 0.7520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawrence, R.; Munniks, S.; Valente, J. Calibration of Electrochemical Sensors for Nitrogen Dioxide Gas Detection Using Unmanned Aerial Vehicles. Sensors 2020, 20, 7332. https://doi.org/10.3390/s20247332
Mawrence R, Munniks S, Valente J. Calibration of Electrochemical Sensors for Nitrogen Dioxide Gas Detection Using Unmanned Aerial Vehicles. Sensors. 2020; 20(24):7332. https://doi.org/10.3390/s20247332
Chicago/Turabian StyleMawrence, Raphael, Sandra Munniks, and João Valente. 2020. "Calibration of Electrochemical Sensors for Nitrogen Dioxide Gas Detection Using Unmanned Aerial Vehicles" Sensors 20, no. 24: 7332. https://doi.org/10.3390/s20247332
APA StyleMawrence, R., Munniks, S., & Valente, J. (2020). Calibration of Electrochemical Sensors for Nitrogen Dioxide Gas Detection Using Unmanned Aerial Vehicles. Sensors, 20(24), 7332. https://doi.org/10.3390/s20247332







