Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does
Abstract
1. Background
1.1. Similarities and Differences between FW and BW
1.2. Assessing Cortical Activation by Using EEG Measurements
1.3. Mu Rhythm
2. Methods
2.1. Participants
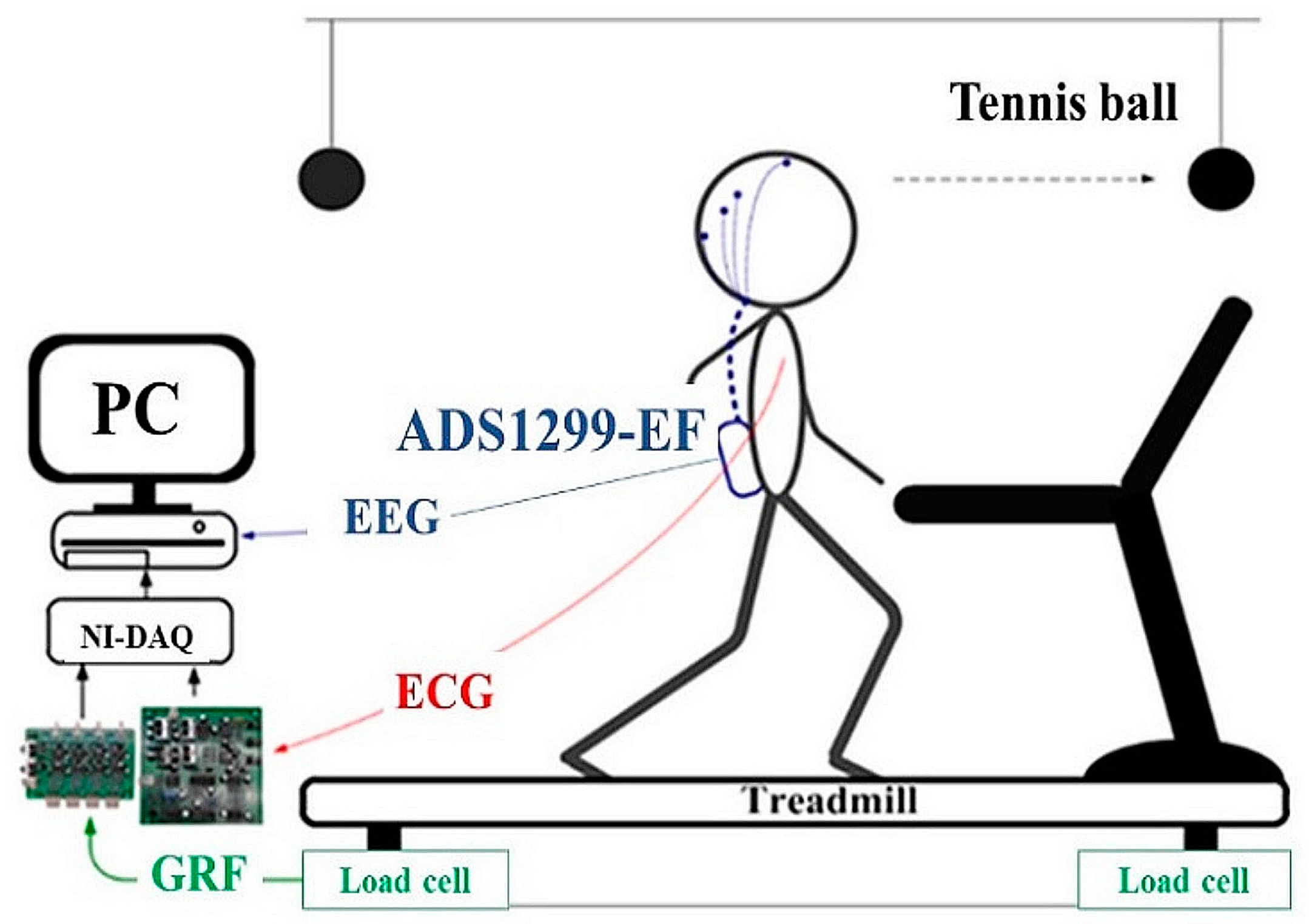
2.2. Experimental Setup and Protocol
2.3. EEG Signal Processing
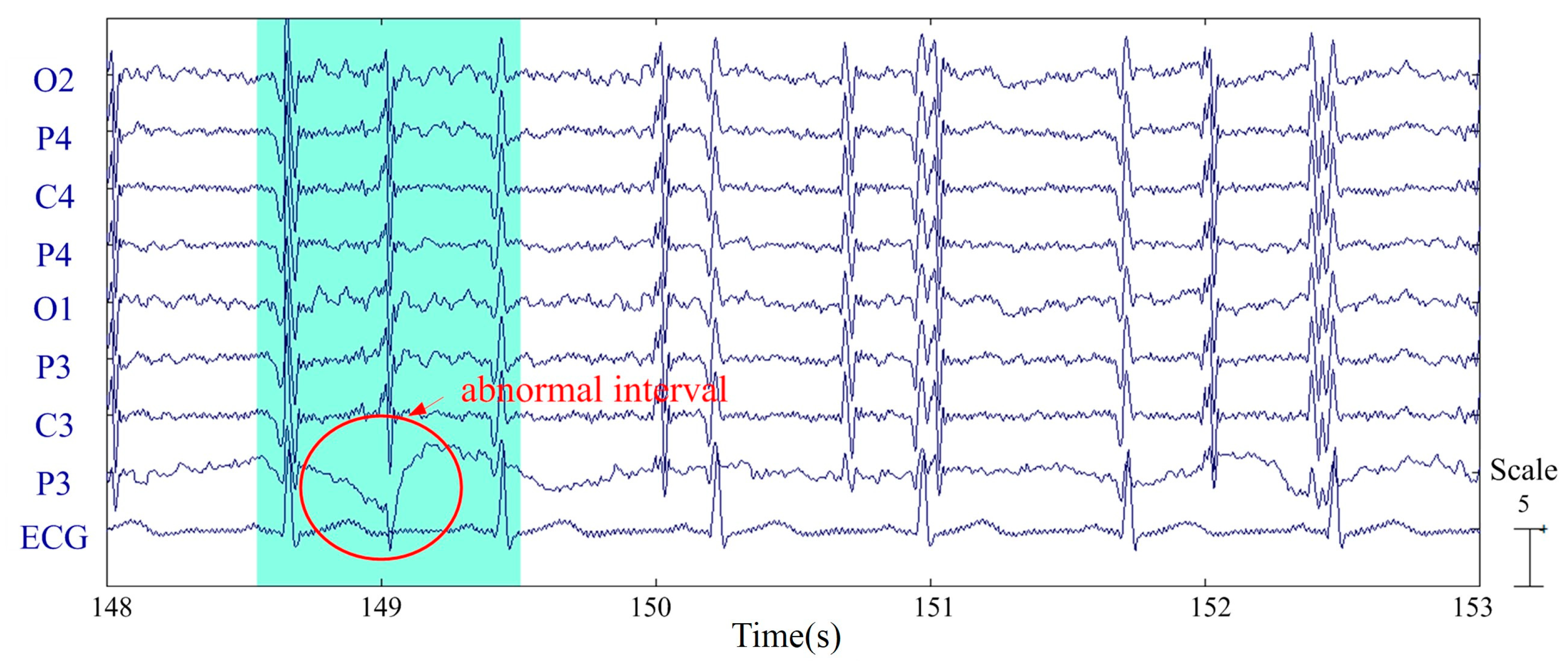
2.3.1. Preprocessing
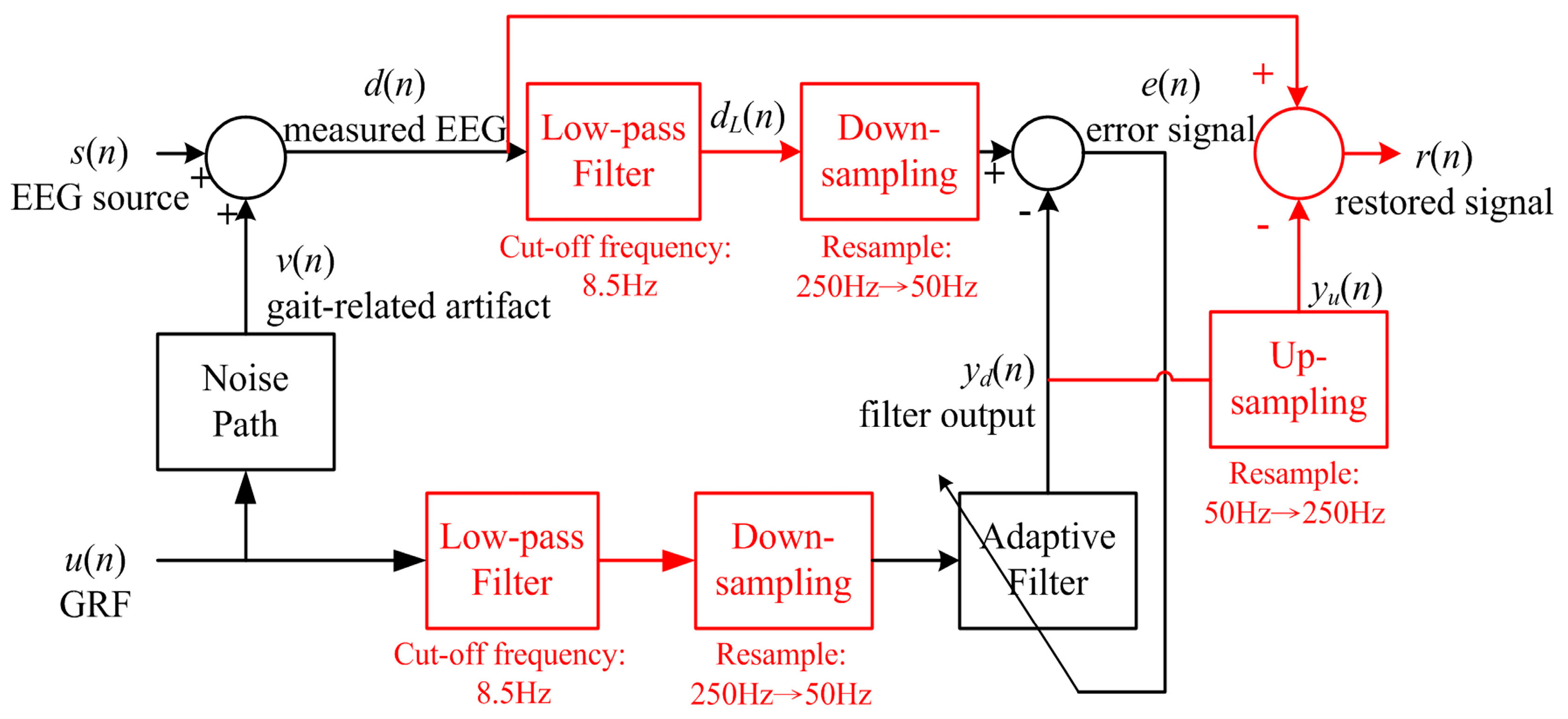
2.3.2. Least-Mean-Square Filter
2.3.3. ICA
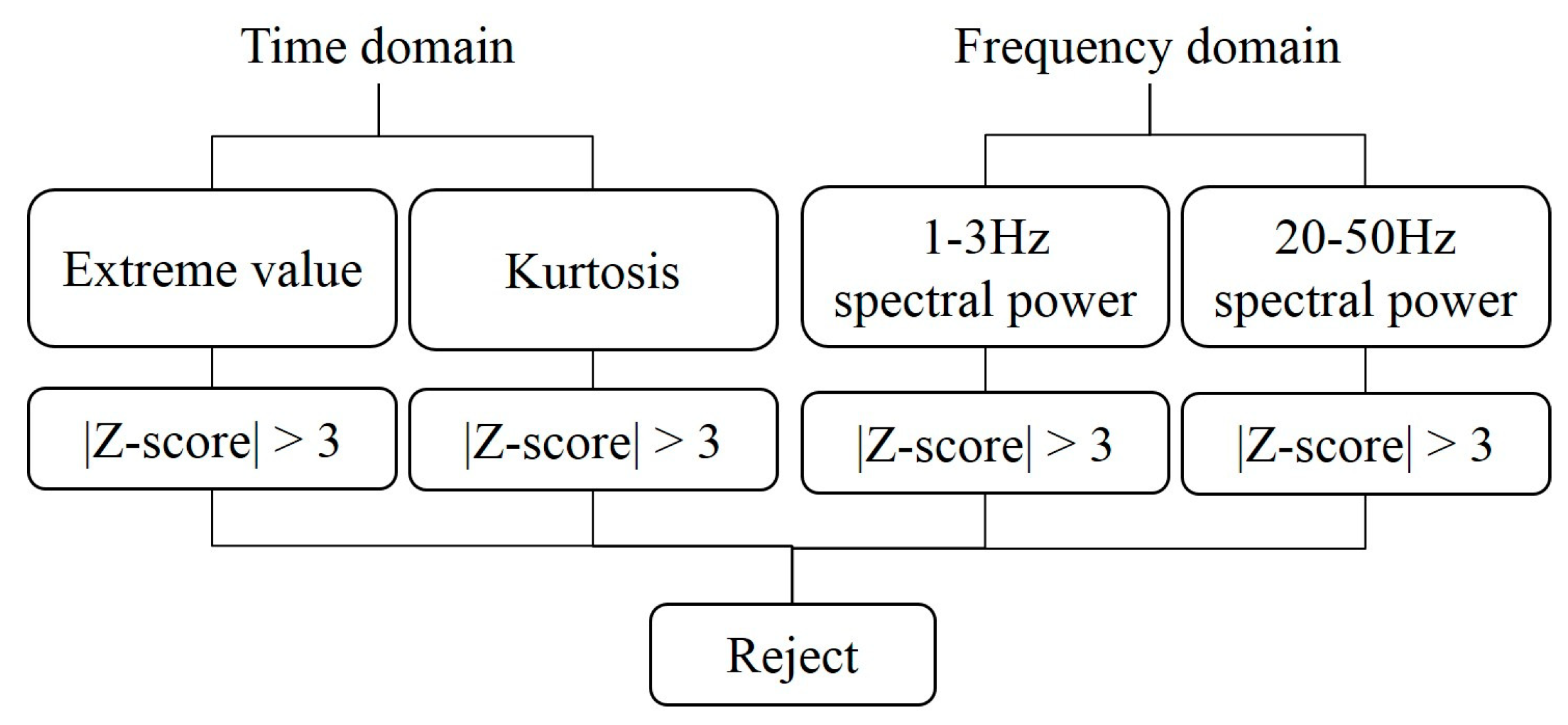
2.3.4. Statistical Outlier Removal
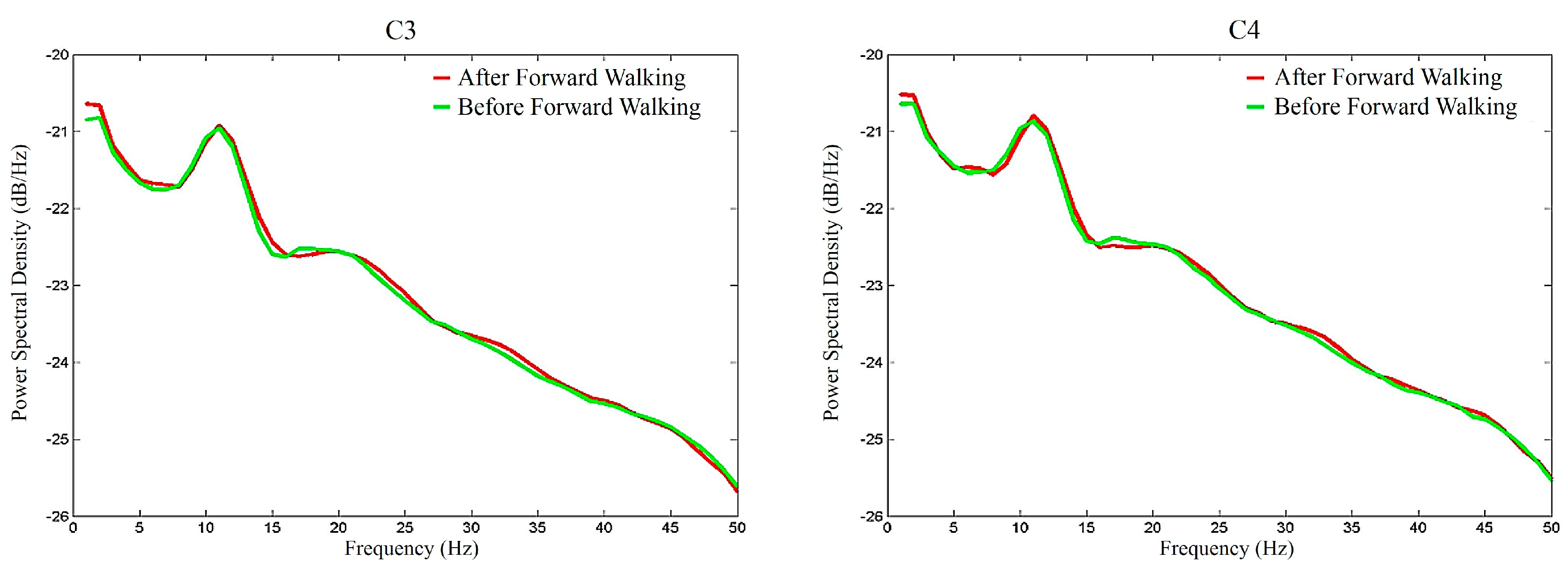
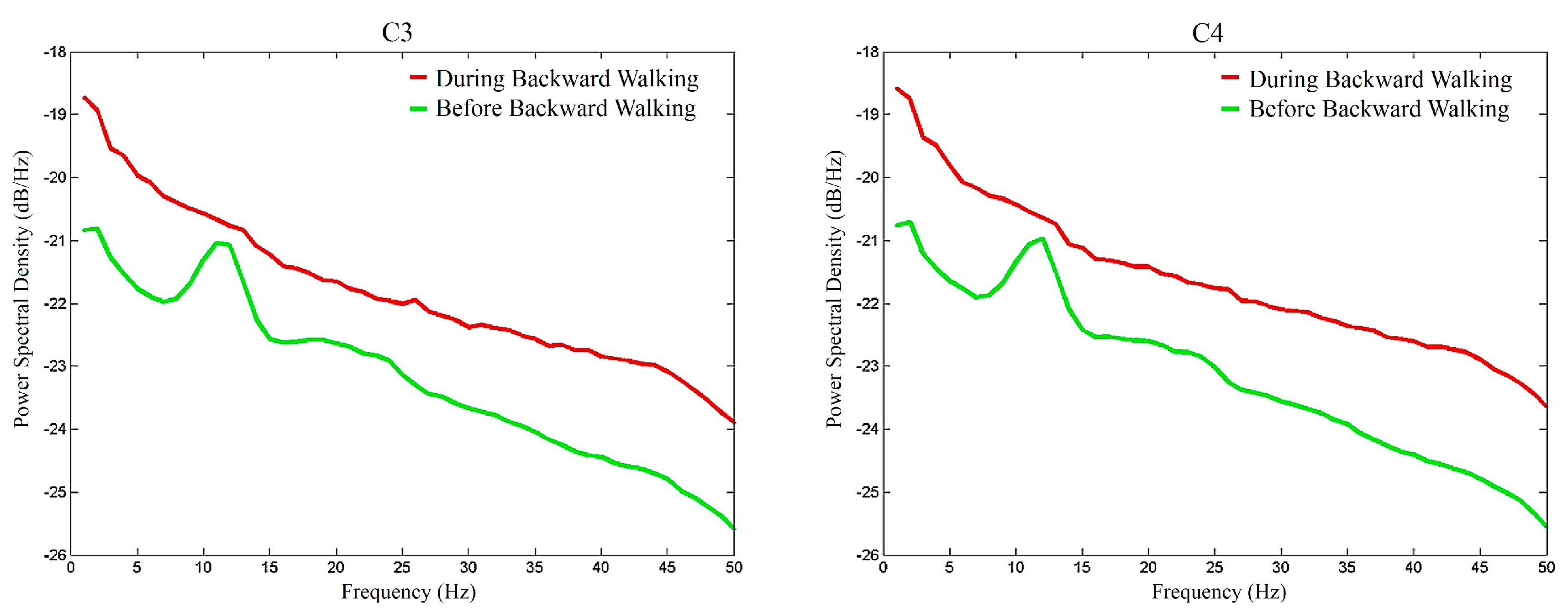
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Yuan, W.; An, R. Effectiveness of backward walking training on spatial-temporal gait characteristics: A systematic review and meta-analysis. Hum. Mov. Sci. 2018, 60, 57–71. [Google Scholar] [CrossRef]
- Balasukumaran, T.; Olivier, B.; Ntsiea, M.V. The effectiveness of backward walking as a treatment for people with gait impairments: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 171–182. [Google Scholar] [CrossRef]
- Elnahhas, A.M.; Elshennawy, S.; Aly, M.G. Effects of backward gait training on balance, gross motor function, and gait in children with cerebral palsy: A systematic review. Clin. Rehabil. 2019, 33, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; An, R. Effectiveness of backward walking training on balance performance: A systematic review and meta-analysis. Gait Posture 2019, 68, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Viviani, P.; Figliozzi, F.; Campione, G.C.; Lacquaniti, F. Detecting temporal reversals in human locomotion. Exp. Brain Res. 2011, 214, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; Desloovere, K.; Molenaers, G.; Swinnen, S.P.; Duysens, J. Interlimb coordination during forward and backward walking in primary school-aged children. PLoS ONE 2013, 8, e62747. [Google Scholar] [CrossRef]
- Grasso, R.; Bianchi, L.; Lacquaniti, F. Motor patterns for human gait: Backward versus forward locomotion. J. Neurophysiol. 1998, 80, 1868–1885. [Google Scholar] [CrossRef]
- Winter, D.A.; Pluck, N.; Yang, J.F. Backward walking: A simple reversal of forward walking? J. Motor Behav. 1989, 21, 291–305. [Google Scholar] [CrossRef]
- Flynn, T.W.; Connery, S.M.; Smutok, M.A.; Zeballos, R.J.; Weisman, I.M. Comparison of cardiopulmonary responses to forward and backward walking and running. Med. Sci. Sports Exerc. 1994, 26, 89–94. [Google Scholar] [CrossRef]
- Chaloupka, E.C.; Kang, J.; Mastrangelo, M.A.; Donnelly, M.S. Cardiorespiratory and metabolic responses during forward and backward walking. J. Orthop. Sports Phys. Ther. 1997, 25, 302–306. [Google Scholar] [CrossRef]
- Nadeau, S.; Amblard, B.; Mesure, S.; Bourbonnais, D. Head and trunk stabilization strategies during forward and backward walking in healthy adults. Gait Posture 2003, 18, 134–142. [Google Scholar] [CrossRef]
- Zelenin, P.V.; Deliagina, T.G.; Orlovsky, G.N.; Karayannidou, A.; Stout, E.E.; Sirota, M.G.; Beloozerova, I.N. Activity of motor cortex neurons during backward locomotion. J. Neurophysiol. 2011, 105, 2698–2714. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage 2012, 59, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.; Jeng, C.; Yuan, R.-Y. Comparisons of forward and backward gait between poorer and better attention capabilities in early Parkinson’s disease. Gait Posture 2012, 36, 367–371. [Google Scholar] [CrossRef]
- Soda, N.; Ueki, T.; Aoki, T. Three-dimensional motion analysis of the ankle during backward walking. J. Phys. Ther. Sci. 2013, 25, 747–749. [Google Scholar] [CrossRef][Green Version]
- Kachanathu, S.J.; Hafez, A.R.; Zakaria, A.R. Efficacy of backward versus forward walking on hamstring strain rehabilitation. Int. J. Ther. Rehabil. Res. 2013, 2, 8–14. [Google Scholar] [CrossRef]
- Kachanathu, S.J.; Alenazi, A.M.; Algarni, A.D.; Hafez, A.R.; Hameed, U.A.; Nuhmani, S.; Alghamdi, M.S.; Melam, G. Effect of forward and backward locomotion training on anaerobic performance and anthropometrical composition. J. Phys. Ther. Sci. 2014, 26, 1879–1882. [Google Scholar] [CrossRef]
- Cha, H.-G.; Kim, T.-H.; Kim, M.-K. Therapeutic efficacy of walking backward and forward on a slope in normal adults. J. Phys. Ther. Sci. 2016, 28, 1901–1903. [Google Scholar] [CrossRef]
- Drew, T.; Marigold, D.S. Taking the next step: Cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Huang, M.X.; Harrington, D.L.; Paulson, K.M.; Weisend, M.P.; Lee, R.R. Temporal dynamics of ipsilateral and contralateral motor activity during voluntary finger movement. Hum. Brain Mapp. 2004, 23, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Becker, K.M.; Heinrichs-Graham, E.; Wilson, T.W. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J. Cognit. Neurosci. 1999, 11, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Solodkin, A.; Hlustik, P.; Chen, E.E.; Small, S.L. Fine modulation in network activation during motor execution and motor imagery. Cereb. Cortex 2004, 14, 1246–1255. [Google Scholar] [CrossRef]
- Hawkins, K.A.; Fox, E.J.; Daly, J.J.; Rose, D.K.; Christou, E.A.; McGuirk, T.E.; Otzel, D.M.; Butera, K.A.; Chatterjee, S.A.; Clark, D.J. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum. Mov. Sci. 2018, 59, 46–55. [Google Scholar] [CrossRef]
- Milla, K.; Bakhshipour, E.; Bodt, B.; Getchell, N. Does movement matter? Prefrontal cortex activity during 2D vs. 3D performance of the Tower of Hanoi Puzzle. Front. Hum. Neurosci. 2019, 13, 156. [Google Scholar] [CrossRef]
- Storzer, L.; Butz, M.; Hirschmann, J.; Abbasi, O.; Gratkowski, M.; Saupe, D.; Alfons Schnitzler, A.; Dalal, S.S. Bicycling and walking are associated with different cortical oscillatory dynamics. Front. Hum. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kasuga, S.; Takasaki, K.; Mizuno, K.; Liu, M.; Ushiba, J. Ipsilateral EEG mu rhythm reflects the excitability of uncrossed pathways projecting to shoulder muscles. J. Neuroeng. Rehabil. 2017, 14, 85. [Google Scholar] [CrossRef]
- Islam, M.K.; Rastegarnia, A.; Yang, Z. Methods for artifact detection and removal from scalp EEG: A review. Neurophysiol. Clin. 2016, 46, 287–305. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.B.; Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef]
- Yin, S.; Liu, Y.; Ding, M. Amplitude of sensorimotor Mu rhythm is correlated with BOLD from multiple brain regions: A simultaneous EEG-fMRI study. Front. Hum. Neurosci. 2016, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Thürer, B.; Stockinger, C.; Putze, F.; Schultz, T.; Stein, T. Mechanisms within the parietal cortex correlate with the benefits of random practice in motor adaptation. Front. Hum. Neurosci. 2017, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Brümmer, V.; Schneider, S.; Strüder, H.; Askew, C. Primary motor cortex activity is elevated with incremental exercise intensity. Neuroscience 2011, 181, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Gramann, K.; Gwin, J.T.; Bigdely-Shamlo, N.; Ferris, D.P.; Makeig, S. Visual evoked responses during standing and walking. Front. Hum. Neurosci. 2010, 4, 202. [Google Scholar] [CrossRef]
- Whitmer, D.; Worrell, G.; Stead, M.; Lee, I.K.; Makeig, S. Utility of independent component analysis for interpretation of intracranial EEG. Front. Hum. Neurosci. 2010, 4, 184. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Snyder, K.L.; Kline, J.E.; Huang, H.J.; Ferris, D.P. Independent component analysis of gait-related movement artifact recorded using EEG electrodes during treadmill walking. Front. Hum. Neurosci. 2015, 9, 639. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Restricted vision increases sensorimotor cortex involvement in human walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of movement artifact from high-density EEG recorded during walking and running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Dual-electrode motion artifact cancellation for mobile electroencephalography. J. Neural Eng. 2018, 15, 056024. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Human electrocortical dynamics while stepping over obstacles. Sci. Rep. 2019, 9, 4693. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Faster gait speeds reduce alpha and beta EEG spectral power from human sensorimotor cortex. IEEE Trans. Biomed. Eng. 2019, 67, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.E.; Huang, H.J.; Snyder, K.L.; Ferris, D.P. Isolating gait-related movement artifacts in electroencephalography during human walking. J. Neural Eng. 2015, 12, 046022. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef]
- Başar, E. A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int. J. Psychophysiol. 2012, 86, 1–24. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C.; Krausz, G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. 2000, 111, 1873–1879. [Google Scholar] [CrossRef]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Kropotov, J.D. Quantitative EEG, Event-Related Potentials and Neurotherapy; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Fox, N.A.; Bakermans-Kranenburg, M.J.; Yoo, K.H.; Bowman, L.C.; Cannon, E.N.; Vanderwert, R.E.; Ferrari, P.F.; Van IJzendoorn, M.H. Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychol. Bull. 2016, 142, 291–313. [Google Scholar] [CrossRef]
- Makino, H.; Hwang, E.J.; Hedrick, N.G.; Komiyama, T. Circuit mechanisms of sensorimotor learning. Neuron 2016, 92, 705–721. [Google Scholar] [CrossRef]
- Ostry, D.J.; Gribble, P.L. Sensory plasticity in human motor learning. Trends. Neurosci. 2016, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Baumert, M. Intra- and Inter-subject Variability in EEG-Based Sensorimotor Brain Computer Interface: A Review. Front. Comput. Neurosci. 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. NeuroImage 2015, 112, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, C.; Zarka, D.; Hoellinger, T.; Leroy, A.; Dan, B.; Chéron, G. Oscillations in the human brain during walking execution, imagination and observation. Neuropsychologia 2015, 79, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Makeig, S.; Gola, M.; Neuper, C.; Müller-Putz, G. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J. Neurosci. 2016, 36, 2212–2226. [Google Scholar] [CrossRef]
- Démas, J.; Bourguignon, M.; Périvier, M.; De Tiège, X.; Dinomais, M.; Van Bogaert, P. Mu rhythm: State of the art with special focus on cerebral palsy. Ann. Phys. Rehabil. Med. 2019, 63, 439–446. [Google Scholar] [CrossRef]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Fumuro, T.; Matsuhashi, M.; Miyazaki, T.; Inouchi, M.; Hitomi, T.; Matsumoto, R.; Takahashi, R.; Fukuyama, H.; Ikeda, A. Alpha-band desynchronization in human parietal area during reach planning. Clin. Neurophysiol. 2015, 126, 756–762. [Google Scholar] [CrossRef]
- Denis, D.; Rowe, R.; Williams, A.M.; Milne, E. The role of cortical sensorimotor oscillations in action anticipation. NeuroImage 2017, 146, 1102–1114. [Google Scholar] [CrossRef]
- Hudac, C.M.; Stessman, H.A.; DesChamps, T.D.; Kresse, A.; Faja, S.; Neuhaus, E.; Webb, S.J.; Eichler, E.E.; Bernier, R.A. Exploring the heterogeneity of neural social indices for genetically distinct etiologies of autism. J. Neurodev. Disord. 2017, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, L.; Ghio, M.; Lange, J.; Bellebaum, C. Event-related desynchronization of mu and beta oscillations during the processing of novel tool names. Brain Lang. 2018, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Brunner, C.; Schlögl, A.; Da Silva, F.L. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage 2006, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Kwon, G.; Kim, D.; Kim, Y.; Kim, S.; Kim, L. Assessment of cognitive engagement in stroke patients from single-trial EEG during motor rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-Y.; Guo, L.-Y.; Song, R.; Nagurka, M.L.; Sung, J.-L.; Yen, C.-W. Developing a Low-Cost Force Treadmill via Dynamic Modeling. J. Healthc. Eng. 2017, 2017, 9875471. [Google Scholar] [CrossRef]
- Rashid, U.; Niazi, I.K.; Signal, N.; Taylor, D. An EEG experimental study evaluating the performance of Texas instruments ADS1299. Sensors 2018, 18, 3721. [Google Scholar] [CrossRef]
- Morley, A.; Hill, L.; Kaditis, A.G. 10–20 System EEG Placement. Available online: https://www.ers-education.org/lrmedia/2016/pdf/298830.pdf (accessed on 10 October 2020).
- Thomas, M.A.; Fast, A. One step forward and two steps back: The dangers of walking backwards in therapy. Am. J. Phys. Med. Rehabil. 2000, 79, 459–461. [Google Scholar] [CrossRef]
- Widrow, B.; Hoff, M.E. Adaptive switching circuits. In IRE WESCON Convention Record; IRE: New York, NY, USA, 1960; pp. 96–104. [Google Scholar]
- Dixit, S.; Nagaria, D. LMS Adaptive Filters for Noise Cancellation: A Review. Int. J. Electr. Comput. Eng. 2017, 7, 2520–2529. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Meth. 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Comani, S.; Velluto, L.; Schinaia, L.; Cerroni, G.; Serio, A.; Buzzelli, S.; Sorbi, S.; Guarnieri, B. Monitoring neuro-motor recovery from stroke with high-resolution EEG, robotics and virtual reality: A proof of concept. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1106–1116. [Google Scholar] [CrossRef]
- Vukelić, M.; Gharabaghi, A. Oscillatory entrainment of the motor cortical network during motor imagery is modulated by the feedback modality. NeuroImage 2015, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, A.; Chen, Q.; Liu, Y.; Zou, L.; McKeown, M.J. Simultaneous ocular and muscle artifact removal from EEG data by exploiting diverse statistics. Comput. Biol. Med. 2017, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Freyer, F.; Reinacher, M.; Nolte, G.; Dinse, H.R.; Ritter, P. Repetitive tactile stimulation changes resting-state functional connectivity—Implications for treatment of sensorimotor decline. Front. Hum. Neurosci. 2012, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Sollfrank, T.; Hart, D.; Goodsell, R.; Foster, J.; Tan, T. 3D visualization of movements can amplify motor cortex activation during subsequent motor imagery. Front. Hum. Neurosci. 2015, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Sestito, M.; Harel, A.; Nador, J.; Flach, J. Investigating neural sensorimotor mechanisms underlying flight expertise in pilots: Preliminary data from an EEG study. Front. Hum. Neurosci. 2018, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Takeuchi, N.; Izumi, S.-I. Rehabilitation with Poststroke Motor Recovery: A Review with a Focus on Neural Plasticity. Stroke Res.Treat. 2013, 2013, 128641. [Google Scholar] [CrossRef]
- Ros, T.; Munneke, M.A.; Ruge, D.; Gruzelier, J.H.; Rothwell, J.C. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur. J. Neurosci. 2010, 31, 770–778. [Google Scholar] [CrossRef]
- Ros, T.; Munneke, M.A.; Parkinson, L.; Gruzelier, J.H. Neurofeedback facilitation of implicit motor learning. Biol. Psychol. 2014, 95, 54–58. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A. Brain–machine interface in chronic stroke rehabilitation: A controlled study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef]
- Kober, S.E.; Schweiger, D.; Reichert, J.L.; Neuper, C.; Wood, G. Upper alpha based neurofeedback training in chronic stroke: Brain plasticity processes and cognitive effects. Appl. Psychophys. Biofeedback 2017, 42, 69–83. [Google Scholar] [CrossRef] [PubMed]





| Walking Periods | Channels | Frequency Bands | ||
|---|---|---|---|---|
| μ0 | μ1 | μ2 | ||
| During vs. Before | C3 | 0.006 | 0.136 | 0.003 |
| C4 | * | 0.016 | * | |
| After vs. Before | C3 | 0.040 | 0.020 | 0.123 |
| C4 | 0.076 | 0.006 | 0.310 | |
| Walking Periods | Channels | Frequency Bands | ||
|---|---|---|---|---|
| μ0 | μ1 | μ2 | ||
| During vs. Before | C3 | * | 0.032 | * |
| C4 | * | 0.063 | * | |
| After vs. Before | C3 | 0.003 | 0.005 | 0.006 |
| C4 | * | 0.001 | 0.003 | |
| Walking Periods | Channels | Frequency Bands | ||
|---|---|---|---|---|
| μ0 | μ1 | μ2 | ||
| During | C3 | 0.052 | 0.117 | 0.022 |
| C4 | 0.089 | 0.370 | 0.012 | |
| After | C3 | 0.016 | 0.218 | 0.013 |
| C4 | 0.009 | 0.471 | 0.004 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, N.-H.; Liu, C.-H.; Lee, P.; Guo, L.-Y.; Sung, J.-L.; Yen, C.-W.; Liaw, L.-J. Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does. Sensors 2020, 20, 7250. https://doi.org/10.3390/s20247250
Lin N-H, Liu C-H, Lee P, Guo L-Y, Sung J-L, Yen C-W, Liaw L-J. Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does. Sensors. 2020; 20(24):7250. https://doi.org/10.3390/s20247250
Chicago/Turabian StyleLin, Nan-Hung, Chin-Hsuan Liu, Posen Lee, Lan-Yuen Guo, Jia-Li Sung, Chen-Wen Yen, and Lih-Jiun Liaw. 2020. "Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does" Sensors 20, no. 24: 7250. https://doi.org/10.3390/s20247250
APA StyleLin, N.-H., Liu, C.-H., Lee, P., Guo, L.-Y., Sung, J.-L., Yen, C.-W., & Liaw, L.-J. (2020). Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does. Sensors, 20(24), 7250. https://doi.org/10.3390/s20247250






