Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms
Abstract
1. Introduction
2. Background
2.1. Smart Wearables in the Healthcare Domain
2.2. Heart Rate Variability as a Health Indicator
2.3. Covid-19 and Cardiovascular Health
2.4. Motivation and Contribution
3. Materials and Methods
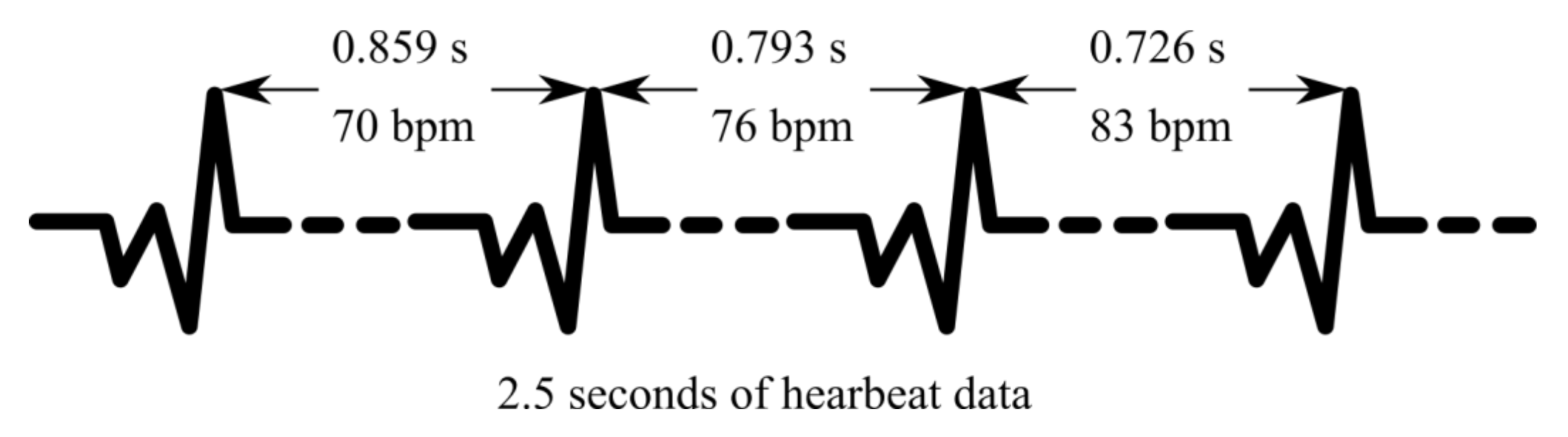
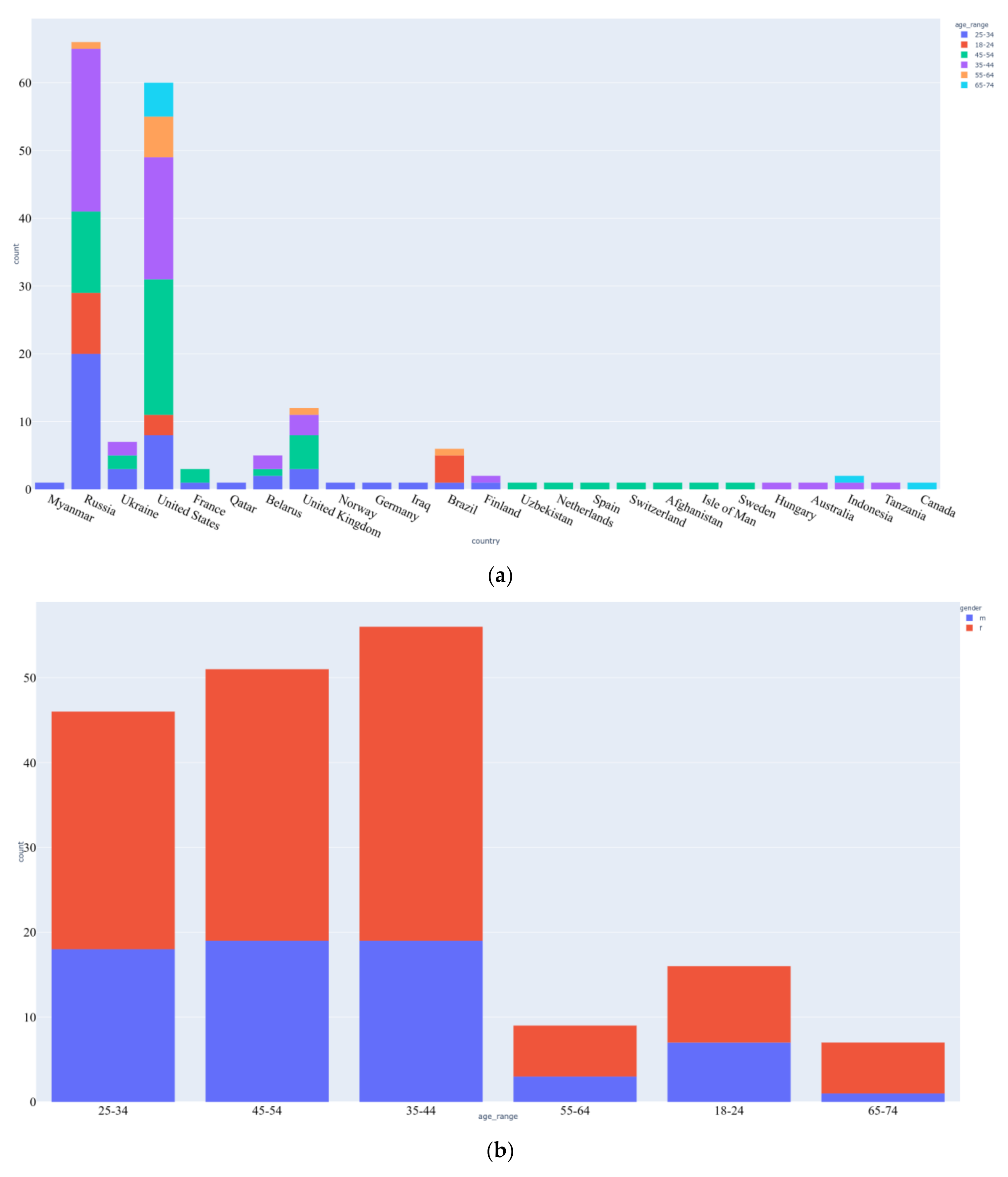
3.1. Data Acquisition
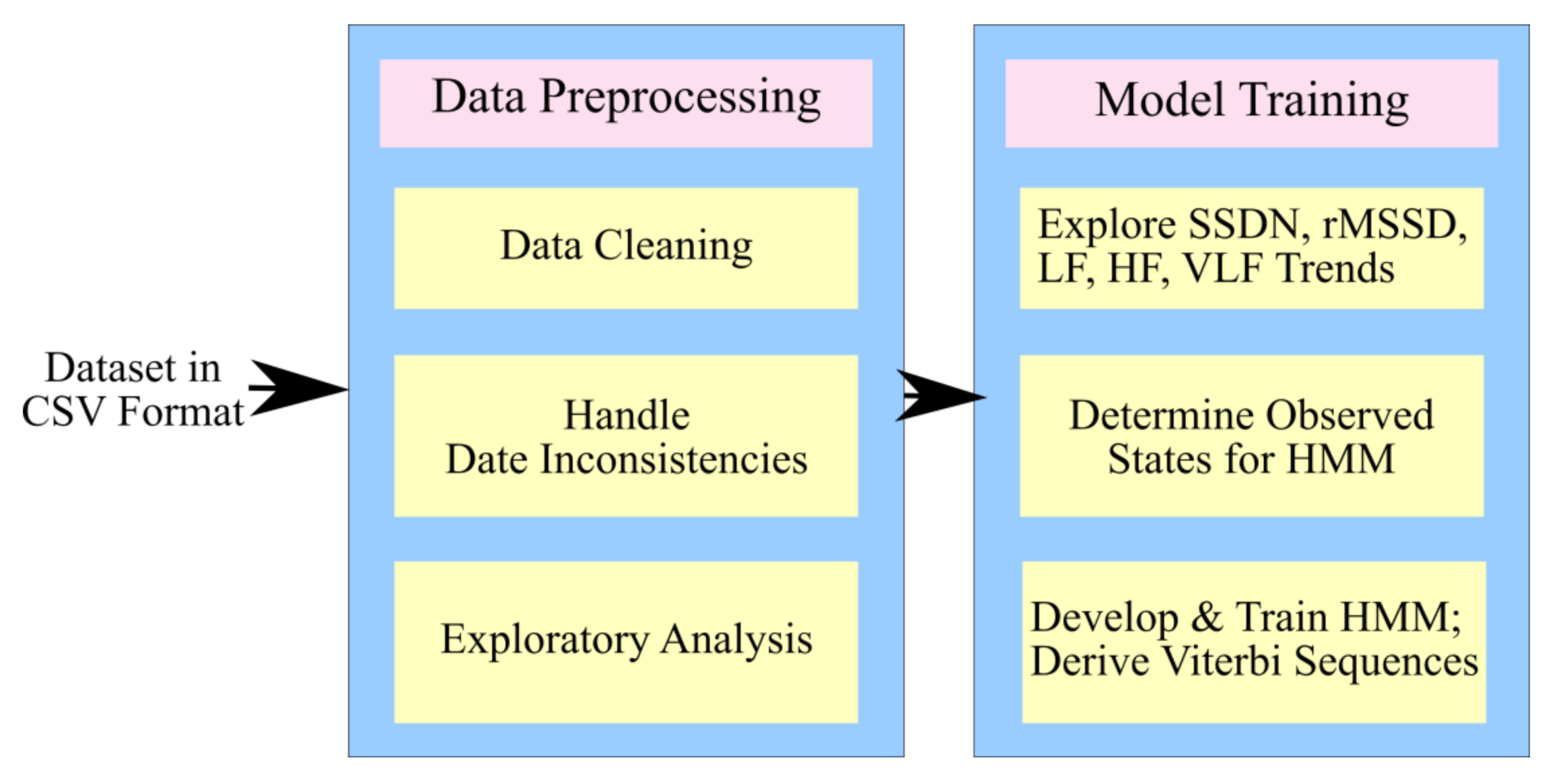
3.2. Process Design
3.2.1. The Proposed Architecture
3.2.2. Hidden Markov Model for HRV Trends and SARS-Cov-2 Symptoms
3.3. Preprocessing and Exploratory Analysis
3.4. The SARS-Cov-2 Detection Prototype
4. Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gubbi, J.; Buyya, R.; Marusic, S.; Palaniswami, M. Internet of Things (IoT): A vision, architectural elements, and future directions. Future Gener. Comput. Syst. 2013, 29, 1645–1660. [Google Scholar] [CrossRef]
- Mazhelis, O.; Tyrvainen, P. A framework for evaluating Internet-of-Things platforms: Application provider viewpoint. In Proceedings of the 2014 IEEE World Forum on Internet of Things, WF-IoT 2014, Seoul, Korea, 6–8 March 2014; pp. 147–152. [Google Scholar]
- Tanwar, G.; Chauhan, R.; Singh, D. User Privacy in Smart Systems: Recent Findings and Countermeasures. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Dian, F.J.; Vahidnia, R.; Rahmati, A. Wearables and the Internet of Things (IoT), Applications, Opportunities, and Challenges: A Survey. IEEE Access 2020, 8, 69200–69211. [Google Scholar] [CrossRef]
- Colantonio, S.; Coppini, G.; Giorgi, D.; Morales, M.A.; Pascali, M.A. Computer Vision for Ambient Assisted Living: Monitoring Systems for Personalized Healthcare and Wellness That Are Robust in the Real World and Accepted by Users, Carers, and Society. In Computer Vision for Assistive Healthcare; Academic Press: Orvieto, Italy, 2018; pp. 147–182. ISBN 9780128134450. [Google Scholar]
- Xu, K.; Lu, Y.; Takei, K. Multifunctional Skin-Inspired Flexible Sensor Systems for Wearable Electronics. Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar] [CrossRef]
- Xue, Y. A review on intelligent wearables: Uses and risks. Hum. Behav. Emerg. Technol. 2019, 1, 287–294. [Google Scholar] [CrossRef]
- Luo, L.; She, X.; Cao, J.; Zhang, Y.; Li, Y.; Song, P.X.K. Detection and Prediction of Ovulation from Body Temperature Measured by an In-Ear Wearable Thermometer. IEEE Trans. Biomed. Eng. 2020, 67, 512–522. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.; Bryson, D. Smart Clothes and Wearable Technology; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- González-Landero, F.; García-Magariño, I.; Lacuesta, R.; Lloret, J. Green Communication for Tracking Heart Rate with Smartbands. Sensors 2018, 18, 2652. [Google Scholar] [CrossRef]
- Santos-Gago, J.M.; Ramos-Merino, M.; Vallarades-Rodriguez, S.; Álvarez-Sabucedo, L.M.; Fernández-Iglesias, M.J.; García-Soidán, J.L. Innovative use of wrist-worn wearable devices in the sports domain: A systematic review. Electronics 2019, 8, 1257. [Google Scholar] [CrossRef]
- Kamiŝalić, A.; Fister, I.; Turkanović, M.; Karakatiĉ, S. Sensors and functionalities of non-invasive wrist-wearable devices: A review. Sensors 2018, 18, 1714. [Google Scholar] [CrossRef]
- Randazzo, V.; Ferretti, J.; Pasero, E. A wearable smart device to monitor multiple vital parameters—VITAL ECG. Electronics 2020, 9, 300. [Google Scholar] [CrossRef]
- Rukasha, T.; Woolley, S.I.; Kyriacou, T.; Collins, T. Evaluation of wearable electronics for epilepsy: A systematic review. Electronics 2020, 9, 968. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart rate variability: An old metric with new meaning in the era of using mhealth technologies for health and exercise training guidance. Part one: Physiology and methods. Arrhythmia Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, G.; Chauhan, R.; Singh, D. An Interactive Machine Learning-Driven Compromise History Dashboard of IoT Vendors. Multimed. Tools Appl. 2020, in press. [Google Scholar]
- Chauhan, R.; Kaur, H.; Chang, V. Advancement and applicability of classifiers for variant exponential model to optimize the accuracy for deep learning. J. Ambient Intell. Hum. Comput. 2020. [Google Scholar] [CrossRef]
- Chauhan, R.; Kaur, H. A Feature Based Reduction technique on Large Scale Databases. Int. J. Data Anal. Tech. Strateg. 2017, 9, 207–221. [Google Scholar] [CrossRef]
- Guzzetti, S. Heart rate variability. Ital. Heart J. Suppl. 2001, 90, 102–105. [Google Scholar]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Fahrudin, T.; Wijaya, D.R.; Agung, A.A.G. COVID-19 Confirmed Case Correlation Analysis Based on Spearman and Kendall Correlation. In Proceedings of the 2020 International Conference on Data Science and Its Applications (ICoDSA), Las Vegas, NV, USA, 27–30 July 2020; pp. 1–4. [Google Scholar]
- Welltory Help-How to Use Welltory for Research. Available online: https://support.welltory.com/article/show/62787-how-to-use-welltory-for-research (accessed on 25 October 2020).
- Ferreira, A.G.; Fernandes, D.; Branco, S.; Monteiro, J.L.; Cabral, J.; Catarino, A.P.; Rocha, A.M. A smart wearable system for sudden infant death syndrome monitoring. In Proceedings of the IEEE International Conference on Industrial Technology, Taipei, Taiwan, 14–17 March 2016; pp. 1920–1925. [Google Scholar]
- Wang, Z.; Burpee, H.; Tiridi, H.; Gagne, J.; Frasco, G.; Majidi, R.; Yu, C.H.; Kiapour, A. Smart adaptive boot for ankle instability treatment. In Proceedings of the 2016 IEEE MIT Undergraduate Research Technology Conference, URTC 2016, New York, NY, USA, 4 November 2016; pp. 1–4. [Google Scholar]
- Liaqat, S.; Dashtipour, K.; Arshad, K.; Ramzan, N. Noninvasive skin hydration level detection using machine learning. Electronics 2020, 9, 1086. [Google Scholar] [CrossRef]
- Vidya, V.; Poornachandran, P.; Sujadevi, V.G.; Dharmana, M.M. Suppressing Parkinson’s diseases induced involuntary movements using wearables. In Proceedings of the 2017 IEEE International Conference on Technological Advancements in Power and Energy: Exploring Energy Solutions for an Intelligent Power Grid, TAP Energy 2017, Istanbul, Turkey, 19–22 June 2017; pp. 1–4. [Google Scholar]
- Ge, Z.; Prasad, P.W.C.; Costadopoulos, N.; Alsadoon, A.; Singh, A.K.; Elchouemi, A. Evaluating the accuracy of wearable heart rate monitors. In Proceedings of the 2016 International Conference on Advances in Computing, Communication and Automation, Dehradun, India, 8–9 April 2016. [Google Scholar]
- Yang, G.; Deng, J.; Pang, G.; Zhang, H.; Li, J.; Deng, B.; Pang, Z.; Xu, J.; Jiang, M.; Liljeberg, P.; et al. An IoT-Enabled Stroke Rehabilitation System Based on Smart Wearable Armband and Machine Learning. IEEE J. Transl. Eng. Health Med. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lall, P.; Zhang, H.; Lall, R. Flexible wearable biometric band and smartphone application for prevention of sudden causes of death. In Proceedings of the Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2018; pp. 1790–1797. [Google Scholar]
- Steinmetzer, T.; Maasch, M.; Bönninger, I.; Travieso, C.M. Analysis and classification of motor dysfunctions in arm swing in parkinson’s disease. Electronics 2019, 8, 1471. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef]
- Kim, J.; Shin, M. Utilizing HRV-derived respiration measures for driver drowsiness detection. Electronics 2019, 8, 669. [Google Scholar] [CrossRef]
- Loungani, R.S.; Rehorn, M.R.; Newby, L.K.; Katz, J.N.; Klem, I.; Mentz, R.J.; Jones, W.S.; Vemulapalli, S.; Kelsey, A.M.; Blazing, M.A.; et al. A care pathway for the cardiovascular complications of COVID-19: Insights from an institutional response. Am. Heart J. 2020, 225, 3–9. [Google Scholar] [CrossRef]
- Ma, L.; Song, K.; Huang, Y. Coronavirus Disease-2019 (COVID-19) and Cardiovascular Complications. J. Cardiothorac. Vasc. Anesth. 2020. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, H.; Huang, M.; Yang, Y. Lower mortality of COVID-19 by early recognition and intervention: Experience from Jiangsu Province. Ann. Intensive Care 2020, 10, 33. [Google Scholar] [CrossRef]
- Stojanovic, R.; Skraba, A.; Lutovac, B. A Headset Like Wearable Device to Track COVID-19 Symptoms. In Proceedings of the 2020 9th Mediterranean Conference on Embedded Computing, MECO 2020, Budva, Montenegro, 8–11 June 2020. [Google Scholar]
- GitHub-Welltory/hrv-covid19: COVID-19 and Wearables Open Data Research. Available online: https://github.com/Welltory/hrv-covid19 (accessed on 25 October 2020).
- Stamp, M.; Stamp, M. A Revealing Introduction to Hidden Markov Models. In Introduction to Machine Learning with Applications in Information Security; Taylor & Francis Group: London, UK, 2018. [Google Scholar]
- Mor, B.; Garhwal, S.; Kumar, A. A Systematic Review of Hidden Markov Models and Their Applications. Arch. Comput. Methods Eng. 2020, 27, 923–937. [Google Scholar] [CrossRef]
- Baum-Welch Algorithm. In Encyclopedia of Machine Learning and Data Mining; Springer: Berlin/Heidelberg, Germany, 2017; p. 99.
- Forney, G.D. The Viterbi Algorithm. Proc. IEEE 1973, 61, 3. [Google Scholar] [CrossRef]
- GitHub-Maximtrp/Mchmm: Markov Chains and Hidden Markov Models in Python. Available online: https://github.com/maximtrp/mchmm (accessed on 25 October 2020).
- Ellson, J.; Ellson, J.; Gansner, E.R.; Koutsofios, E.; North, S.C.; Woodhull, G. Graphviz and dynagraph–static and dynamic graph drawing tools. GRAPH Draw. Softw. 2003, 127–148. [Google Scholar]
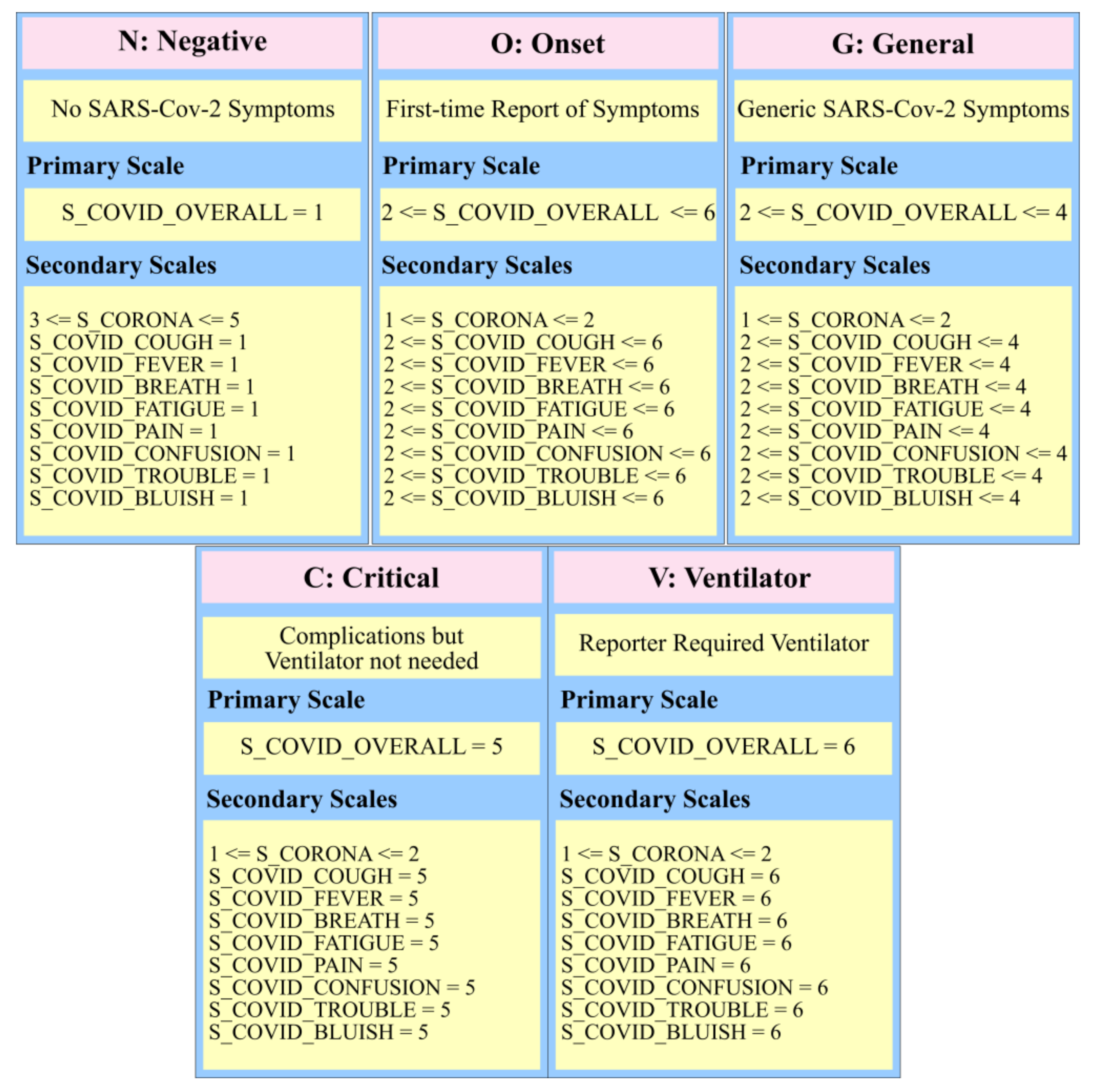
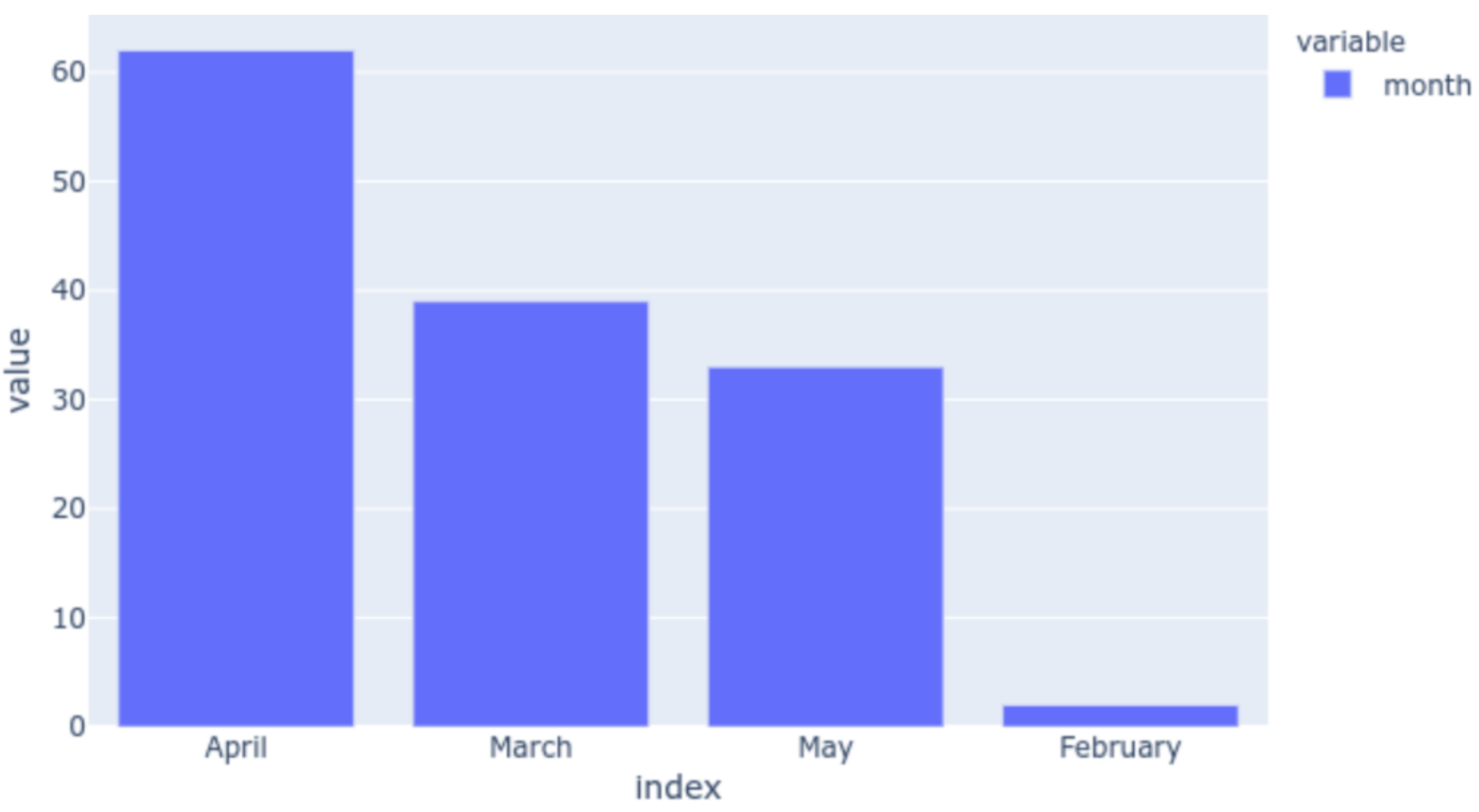
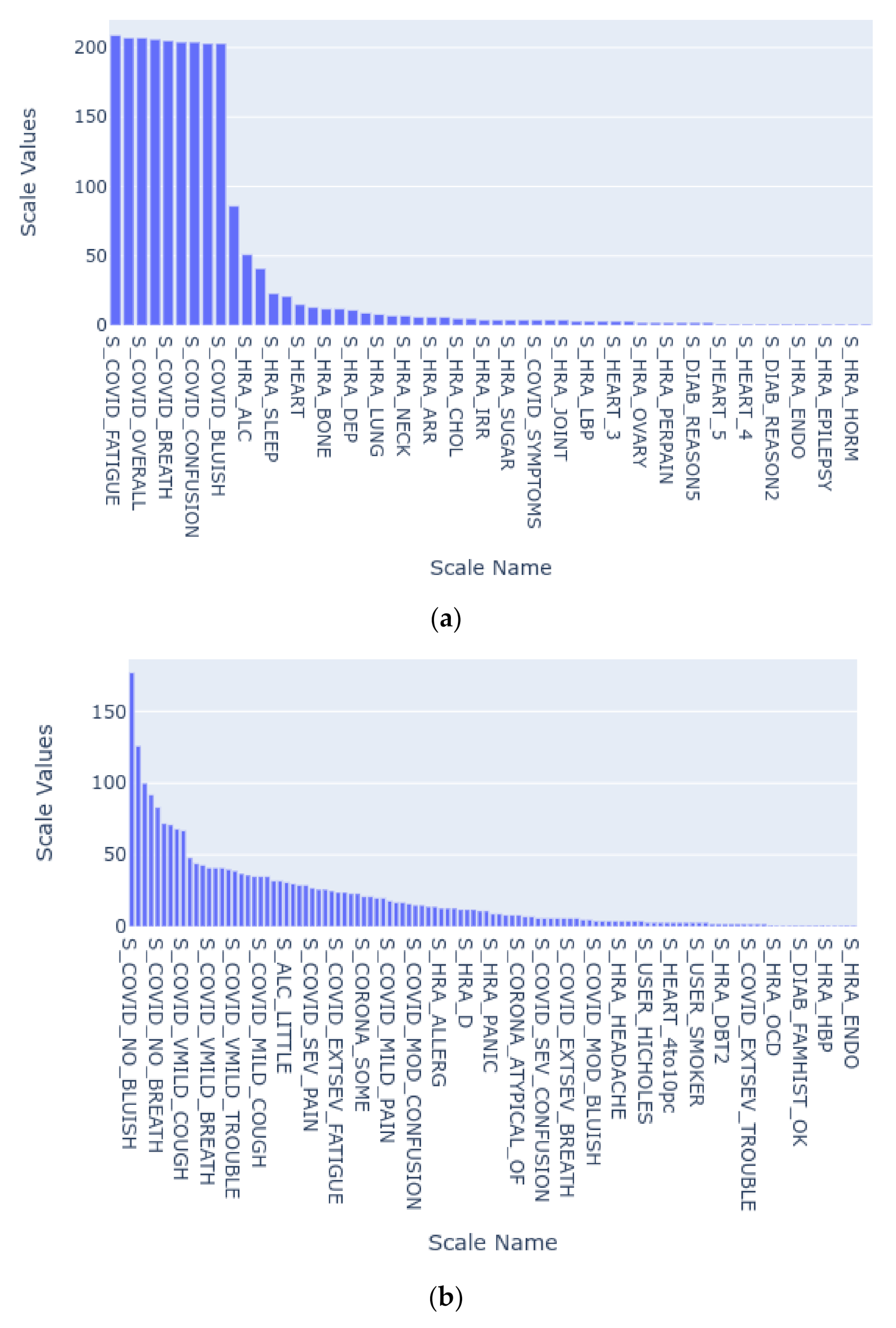
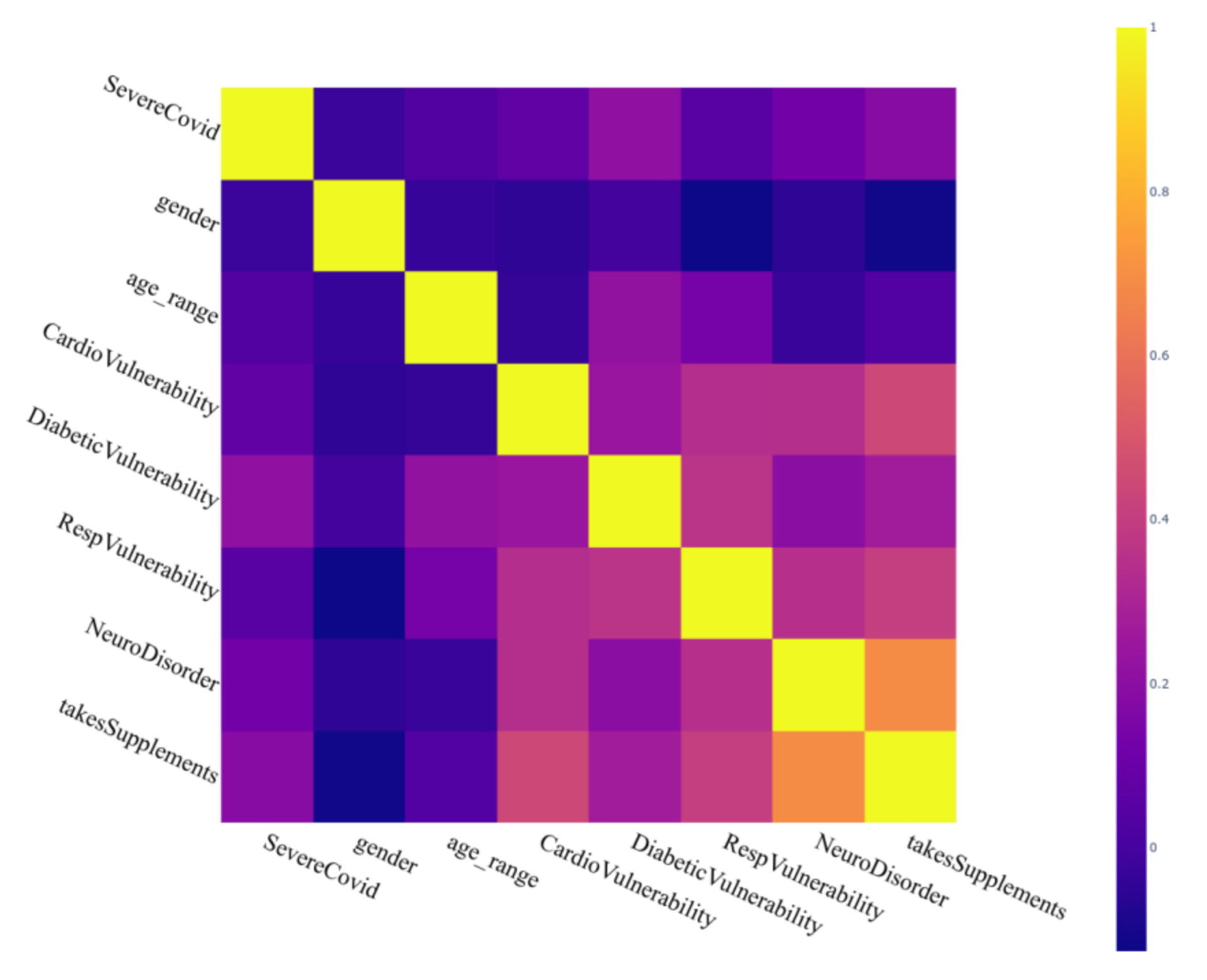
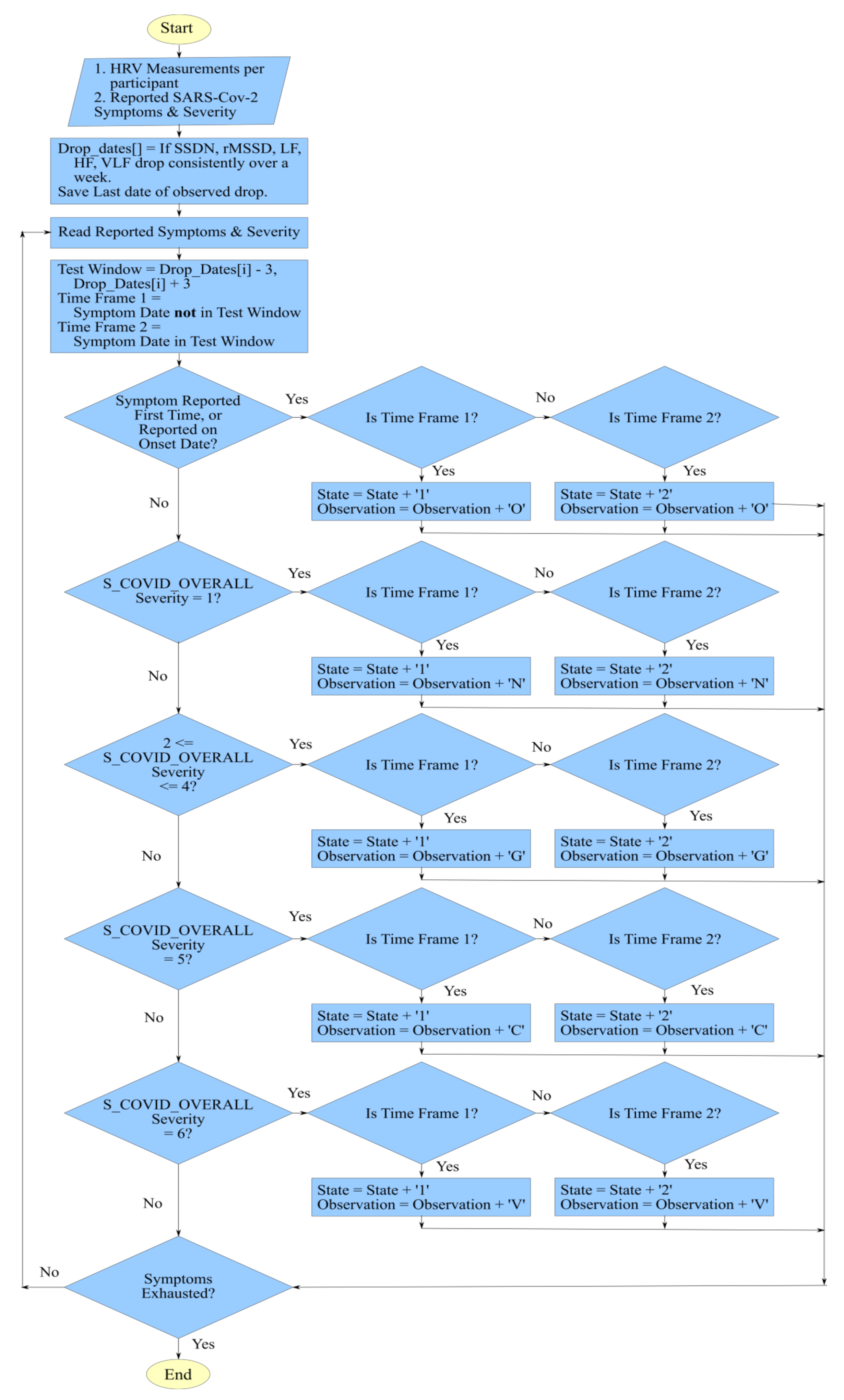
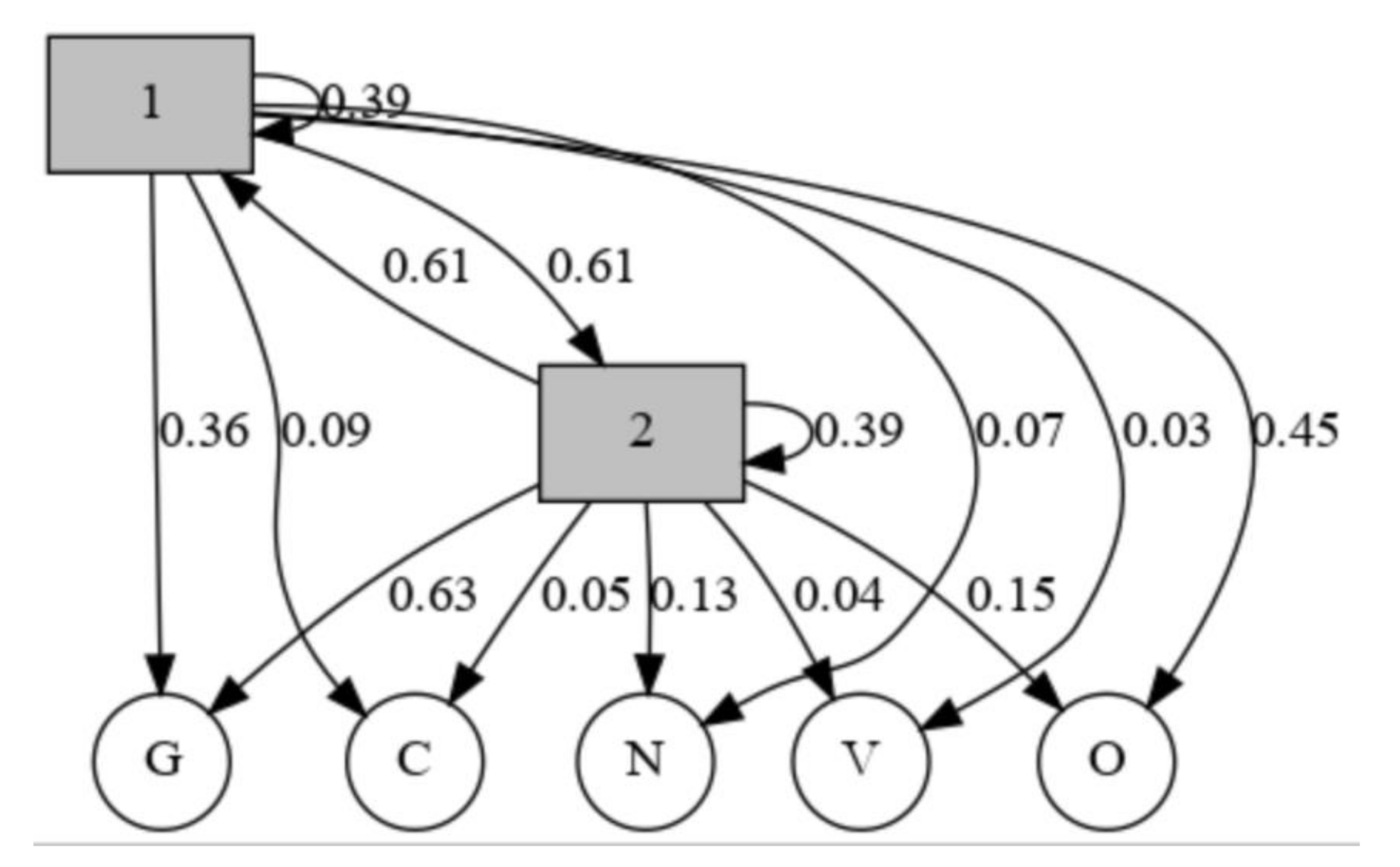
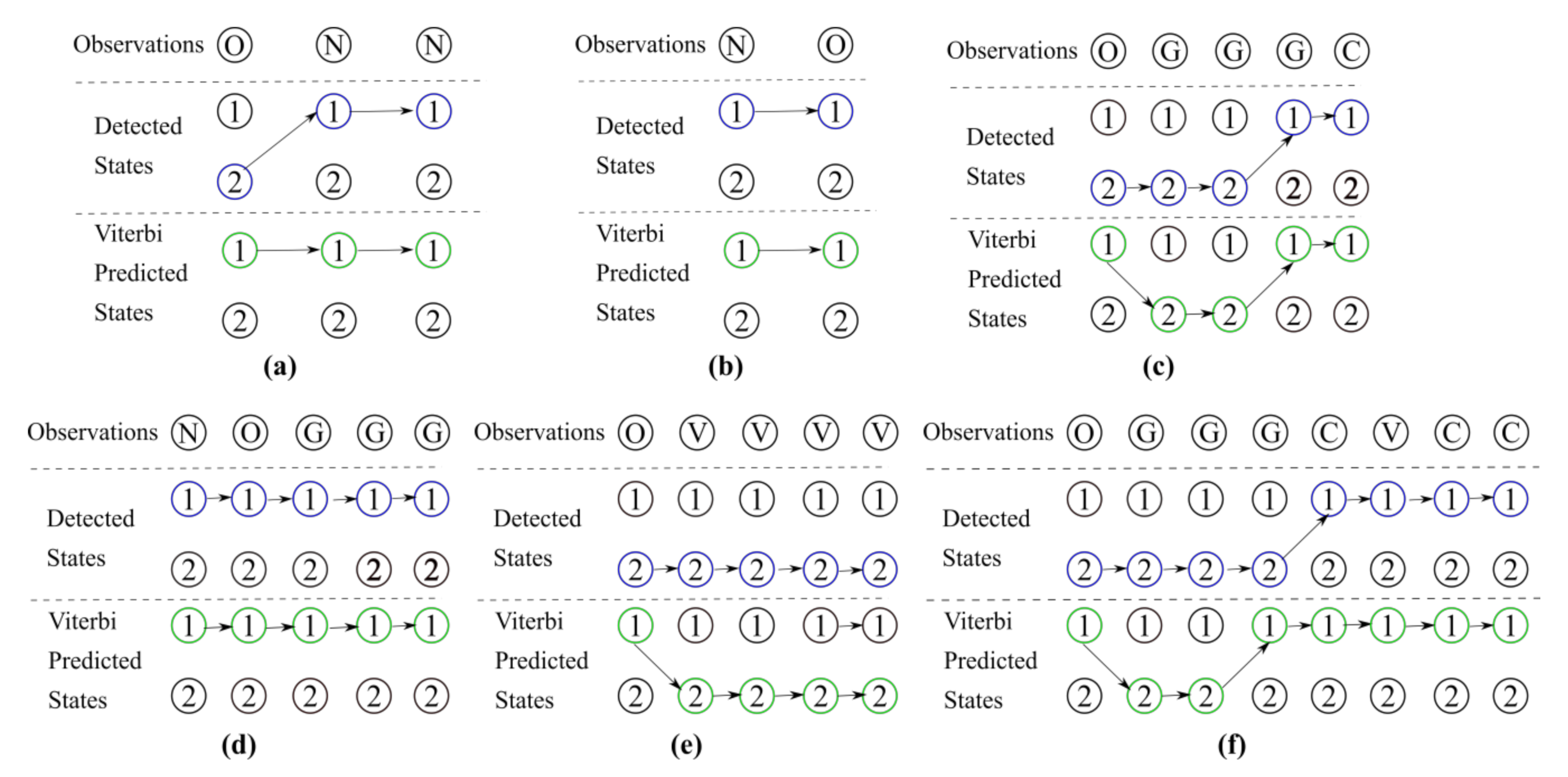
| Scale Name | Description |
|---|---|
| S_COVID_OVERALL | Overall State |
| S_COVID_SYMPTOMS | How long the user had been experiencing the symptoms |
| S_COVID_COUGH | The intensity of coughing |
| S_COVID_FEVER | The intensity of fever |
| S_COVID_BREATH | The intensity of shortness of breath |
| S_COVID_FATIGUE | The intensity of fatigue |
| S_COVID_PAIN | The intensity of pain or pressure in the chest |
| S_COVID_CONFUSION | The intensity of confusion |
| S_COVID_TROUBLE | The intensity of trouble in breathing |
| S_COVID_BLUISH | The intensity of bluish face or lips |
| S_CORONA | An assessment of reported symptoms to show how likely the reporter has Covid-19 |
| 1 | 2 | |
|---|---|---|
| 1 | 0.39021 | 0.61178 |
| 2 | 0.61178 | 0.39021 |
| N | O | G | C | V | |
|---|---|---|---|---|---|
| 1 | 0.065553 | 0.45182 | 0.362460 | 0.093164 | 0.027004 |
| 2 | 0.131894 | 0.14845 | 0.633617 | 0.050245 | 0.035794 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varma, G.; Chauhan, R.; Singh, M.; Singh, D. Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms. Sensors 2020, 20, 7068. https://doi.org/10.3390/s20247068
Varma G, Chauhan R, Singh M, Singh D. Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms. Sensors. 2020; 20(24):7068. https://doi.org/10.3390/s20247068
Chicago/Turabian StyleVarma, Gatha, Ritu Chauhan, Madhusudan Singh, and Dhananjay Singh. 2020. "Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms" Sensors 20, no. 24: 7068. https://doi.org/10.3390/s20247068
APA StyleVarma, G., Chauhan, R., Singh, M., & Singh, D. (2020). Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms. Sensors, 20(24), 7068. https://doi.org/10.3390/s20247068








