The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment
Abstract
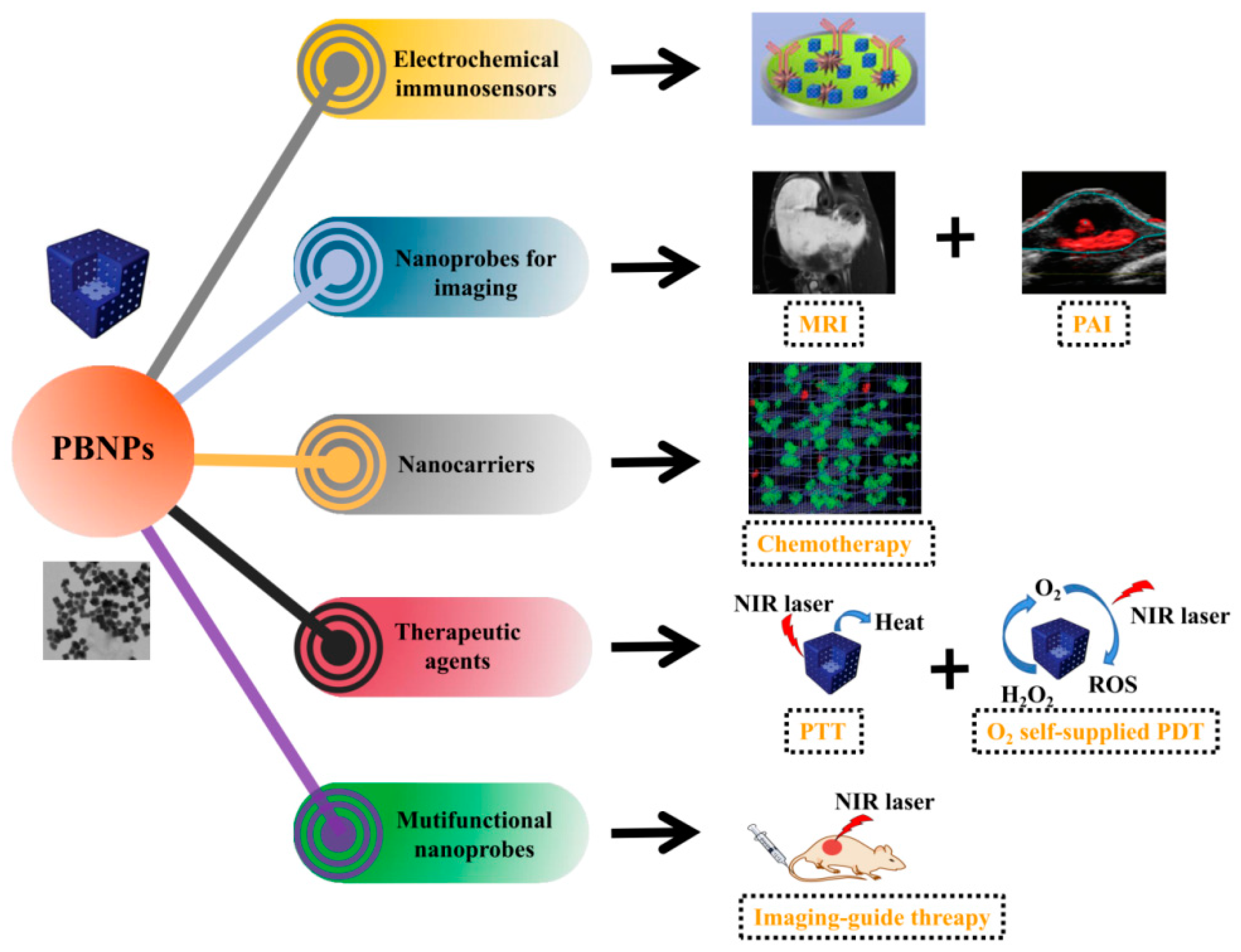
1. Introduction
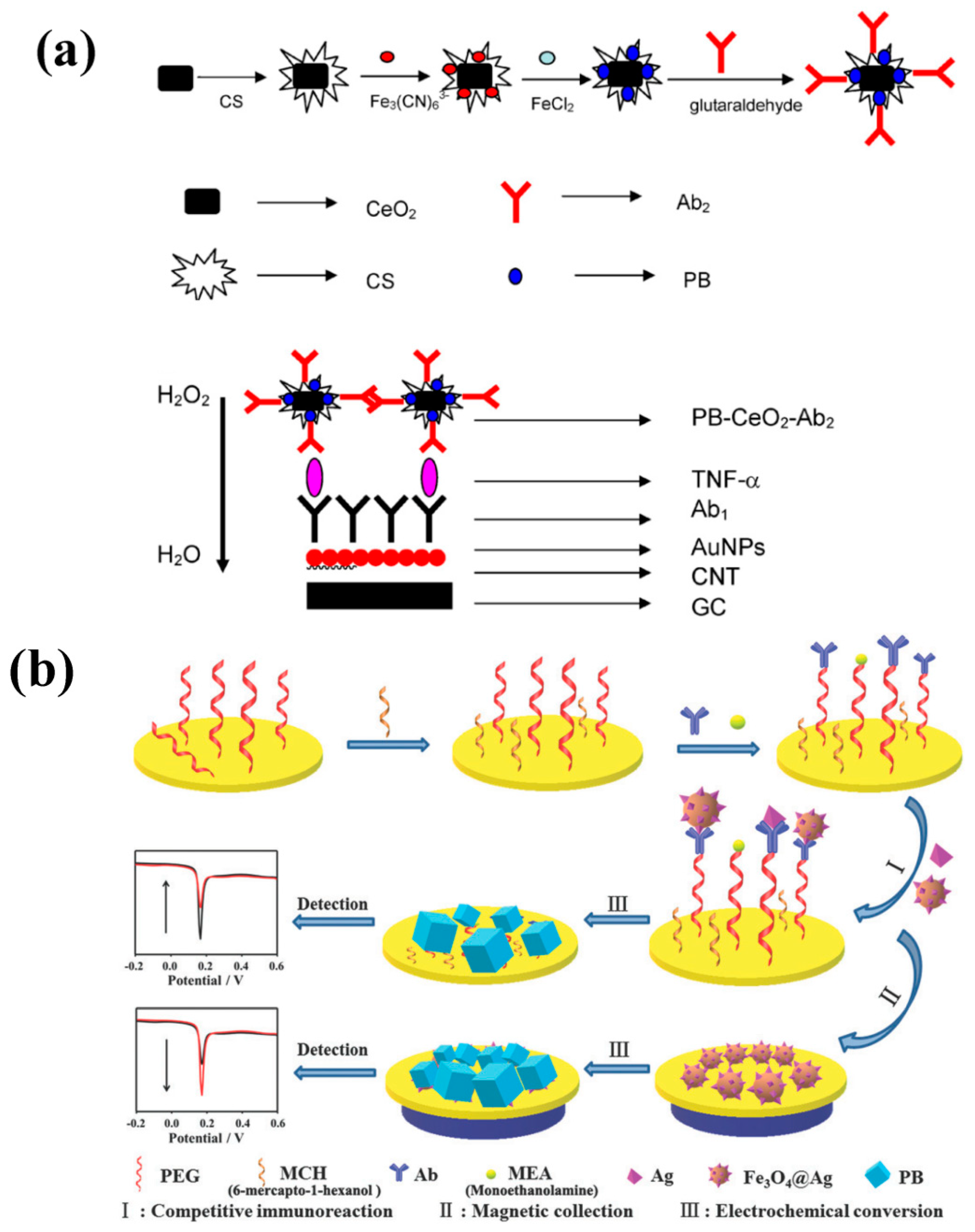
2. PBNPs for Tumor Diagnosis in Electrochemical Immunosensors
3. PBNPs for Tumor Diagnosis in Biological Imaging
3.1. Magnetic Resonance Imaging
3.2. Photoacoustic Imaging
4. PBNPs for Drug Delivery
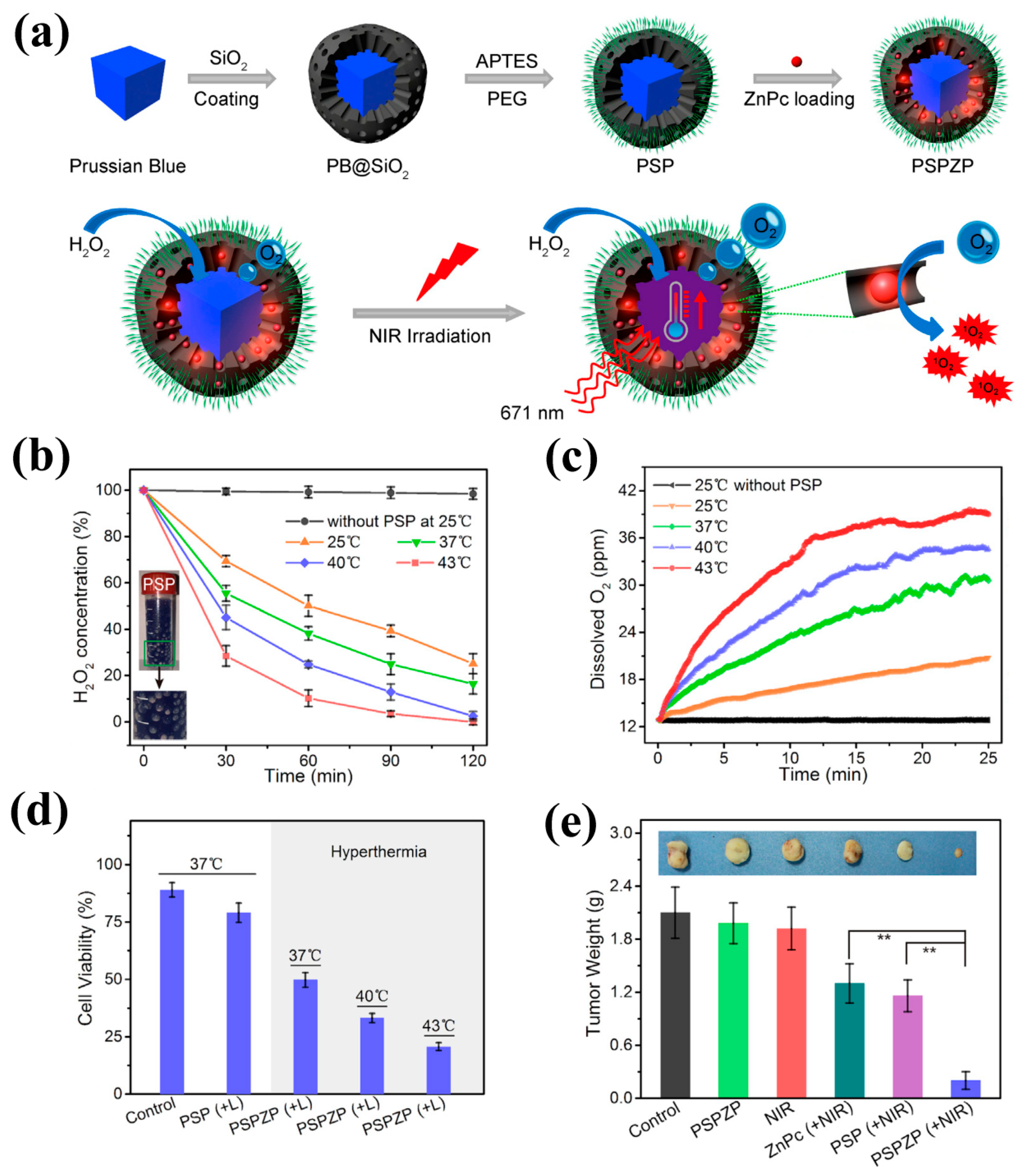
5. PBNPs for Tumor Photothermal and Photodynamic Therapy
6. PBNPs for Tumor Imaging-Guided Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, Q.; Merajver, S.D.; Li, J.Z. Cancer classification in the genomic era: Five contemporary problems. Hum. Genom. 2015, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Prablek, M.; Srinivasan, V.M.; Srivatsan, A.; Holdener, S.; Oneissi, M.; Heck, K.A.; Jalali, A.; Mandel, J.; Viswanathan, A.; Patel, A.J. Gastrointestinal stromal tumor with intracranial metastasis: Case presentation and systematic review of literature. BMC Cancer 2019, 19, 1119. [Google Scholar] [CrossRef] [PubMed]
- Emon, B.; Bauer, J.; Jain, Y.; Jung, B.; Saif, T. Biophysics of Tumor Microenvironment and Cancer Metastasis—A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef]
- Ullah, M.F.; Aatif, M. The footprints of cancer development: Cancer biomarkers. Cancer Treat. Rev. 2009, 35, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ali, S.; Philip, P.A.; Sarkar, F.H. Clinical advances in molecular biomarkers for cancer diagnosis and therapy. Int. J. Mol. Sci. 2013, 14, 14771–14784. [Google Scholar] [CrossRef]
- Peterson, R.D.; Wilund, K.R.; Cunningham, B.T.; Andrade, J.E. Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients. Sensors 2017, 17, 2203. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Z.; Yan, F.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trac. Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Griffith, W.P. Cyanide complexes of the transition metals. Q. Rev. Chem. Soc. 1962, 16, 188. [Google Scholar] [CrossRef]
- Adak, S.; Daemen, L.L.; Hartl, M.; Williams, D.; Summerhill, J.; Nakotte, H. Thermal expansion in 3d-metal Prussian Blue Analogs—A survey study. Solid State Chem. 2011, 184, 2854–2861. [Google Scholar] [CrossRef]
- Neff, V.D. Electrochemical Oxidation and Reduction of Thin Films of Prussian Blue. J. Electrochem. Soc. 1978, 125, 886. [Google Scholar] [CrossRef]
- Kong, B.; Tang, J.; Selomulya, C.; Li, W.; Wei, J.; Fang, Y.; Wang, Y.; Zheng, G.; Zhao, D. Oriented mesoporous nanopyramids as versatile plasmon-enhanced interfaces. J. Am. Chem Soc. 2014, 136, 6822–6825. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Liu, Y.; Jin, W. Recent progress in Prussian blue films: Methods used to control regular nanostructures for electrochemical biosensing applications. Biosens. Bioelectron. 2017, 96, 17–25. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Cheng, G.; Chou, S.L.; Liu, H.K.; Dou, S.X. Chemical Properties, Structural Properties, and Energy Storage Applications of Prussian Blue Analogues. Small 2019, 15, e1900470. [Google Scholar] [CrossRef] [PubMed]
- Matos-Peralta, Y.; Antuch, M. Review-Prussian Blue and Its Analogs as Appealing Materials for Electrochemical Sensing and Biosensing. J. Electrochem. Soc. 2020, 167, 037510. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Y.; Gu, N. Progress in Applications of Prussian Blue Nanoparticles in Biomedicine. Adv. Health Mater. 2018, 7, e1800347. [Google Scholar] [CrossRef] [PubMed]
- Duanghathaipornsuk, S.; Kanel, S.; Haushalter, E.F.; Ruetz, J.E.; Kim, D.S. Detection of Hydroxyl Radicals Using Cerium Oxide/Graphene Oxide Composite on Prussian Blue. Nanomaterials 2020, 10, 1136. [Google Scholar] [CrossRef]
- Chen, J.; Wei, L.; Mahmood, A.; Pei, Z.; Zhou, Z.; Chen, X.; Chen, Y. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Jerez-Masaquiza, M.D.; Fernandez, L.; Gonzalez, G.; Montero-Jimenez, M.; Espinoza-Montero, P.J. Electrochemical Sensor Based on Prussian Blue Electrochemically Deposited at ZrO2 Doped Carbon Nanotubes Glassy Carbon Modified Electrode. Nanomaterials 2020, 10, 1328. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Fu, W.; Chen, X.; Zhang, Q.; Dong, S.; Chen, H.; Zhang, S. A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors 2020, 20, 2924. [Google Scholar] [CrossRef]
- Li, T.; Si, Z.; Hu, L.; Qi, H.; Yang, M. Prussian Blue-functionalized ceria nanoparticles as label for ultrasensitive detection of tumor necrosis factor-α. Sens. Actuators B Chem. 2012, 171–172, 1060–1065. [Google Scholar] [CrossRef]
- Zargar, B.; Hatamie, A. Prussian blue nanoparticles: A simple and fast optical sensor for colorimetric detection of hydralazine in pharmaceutical samples. Anal. Methods 2014, 6, 5951. [Google Scholar] [CrossRef]
- Xu, W.; He, J.; Gao, L.; Zhang, J.; Hui, J.; Guo, Y.; Li, W.; Yu, C. A sensitive glucose biosensor based on the abundant immobilization of glucose oxidase on hollow Pt nanospheres assembled on graphene oxide-Prussian Blue–PTC-NH2 nanocomposite film. J. Electroanal. Chem. 2015, 741, 8–13. [Google Scholar] [CrossRef]
- Petropoulos, K.; Piermarini, S.; Bernardini, S.; Palleschi, G.; Moscone, D. Development of a disposable biosensor for lactate monitoring in saliva. Sens. Actuators B Chem. 2016, 237, 8–15. [Google Scholar] [CrossRef]
- Zhu, D.; Zhu, W.; Xin, J.; Tan, L.; Wang, X.; Pang, H.; Ma, H. Prussian blue nanocubes with an open framework structure coated with polyoxometalates as a highly sensitive platform for ascorbic acid detection in drinks/human urine. New J. Chem. 2019, 43, 9420–9429. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Zhu, Y.; He, X.; Xu, G.; Zhang, X. Prussian blue-Au nanocomposites actuated hemin/G-quadruplexes catalysis for amplified detection of DNA, Hg2+ and adenosine triphosphate. Analyst 2014, 139, 5297–5303. [Google Scholar] [CrossRef]
- Namgung, H.; Gwon, Y.J.; Kim, J.; Jang, G.; Pepper, S.E.; Ogden, M.D.; Whittle, K.R.; Harwood, L.M.; Lee, T.S. Synthesis of Prussian blue-embedded porous polymer for detection and removal of Cs ions. Polymer 2018, 158, 320–326. [Google Scholar] [CrossRef]
- Yamada, M.; Ohnishi, N.; Watanabe, M.; Hino, Y. Prussian blue nanoparticles protected by the water-soluble pi-conjugated polymer PEDOT-S: Synthesis and multiple-color pH-sensing with a redox reaction. Chem. Commun. 2009, 7203–7205. [Google Scholar] [CrossRef]
- Jo, G.; Lee, B.Y.; Kim, E.J.; Park, M.H.; Hyun, H. Indocyanine Green and Methyl-beta-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy. Biomedicines 2020, 8, 476. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Fu, R.; Su, Y.; Lin, X.; Jin, X.; Du, W.; Shan, X.; Huang, G. Biomimetic Upconversion Nanoparticles and Gold Nanoparticles for Novel Simultaneous Dual-Modal Imaging-Guided Photothermal Therapy of Cancer. Cancers 2020, 12, 3136. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Guerin, C.; Larionova, J. Prussian blue type nanoparticles for biomedical applications. Dalton Trans. 2016, 45, 17581–17587. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Soehnlen, E.S.; Hao, J.; Griswold, M.; Flask, C.; Fan, X.; Basilion, J.P.; Basu, S.; Huang, S.D. Dual purpose Prussian blue nanoparticles for cellular imaging and drug delivery: A new generation of T1-weighted MRI contrast and small molecule delivery agents. J. Mater. Chem. 2010, 20, 5251. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 antibody functionalized Prussian blue nanoparticles for targeted MR imaging and photothermal therapy of hepatocellular carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Perera, V.S.; Chen, G.; Cai, Q.; Huang, S.D. Nanoparticles of gadolinium-incorporated Prussian blue with PEG coating as an effective oral MRI contrast agent for gastrointestinal tract imaging. Analyst 2016, 141, 2016–2022. [Google Scholar] [CrossRef]
- Paul, G.; Prado, Y.; Dia, N.; Riviere, E.; Laurent, S.; Roch, M.; Elst, L.V.; Muller, R.N.; Sancey, L.; Perriat, P.; et al. MnII-containing coordination nanoparticles as highly efficient T1 contrast agents for magnetic resonance imaging. Chem. Commun. 2014, 50, 6740–6743. [Google Scholar] [CrossRef]
- Fetiveau, L.; Paul, G.; Nicolas-Boluda, A.; Volatron, J.; George, R.; Laurent, S.; Muller, R.; Sancey, L.; Mejanelle, P.; Gloter, A.; et al. Tailored ultra-small Prussian blue-based nanoparticles for MRI imaging and combined photothermal/photoacoustic theranostics. Chem. Commun. 2019, 55, 14844–14847. [Google Scholar] [CrossRef]
- Ali, L.M.A.; Mathlouthi, E.; Kajdan, M.; Daurat, M.; Long, J.; Sidi-Boulenouar, R.; Cardoso, M.; Goze-Bac, C.; Amdouni, N.; Guari, Y.; et al. Multifunctional manganese-doped Prussian blue nanoparticles for two-photon photothermal therapy and magnetic resonance imaging. Photodiagnosis Photodyn 2018, 22, 65–69. [Google Scholar] [CrossRef]
- Cai, X.; Gao, W.; Ma, M.; Wu, M.; Zhang, L.; Zheng, Y.; Chen, H.; Shi, J. A Prussian Blue-Based Core-Shell Hollow-Structured Mesoporous Nanoparticle as a Smart Theranostic Agent with Ultrahigh pH-Responsive Longitudinal Relaxivity. Adv. Mater. 2015, 27, 6382–6389. [Google Scholar] [CrossRef]
- Cheng, M.; Peng, W.; Hua, P.; Chen, Z.; Sheng, J.; Yang, J.; Wu, Y. In situ formation of pH-responsive Prussian blue for photoacoustic imaging and photothermal therapy of cancer. RSC Adv. 2017, 7, 18270–18276. [Google Scholar] [CrossRef]
- Dumani, D.S.; Cook, J.R.; Kubelick, K.P.; Luci, J.J.; Emelianov, S.Y. Photomagnetic Prussian blue nanocubes: Synthesis, characterization, and biomedical applications. Nanomedicine 2020, 24, 102138. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Cai, X.; Gao, W.; Tang, X.; Chen, Y.; Chen, J.; Chen, L.; Tian, Q.; Yang, S.; et al. Large-scale synthesis of monodisperse Prussian blue nanoparticles for cancer theranostics via an “in situ modification” strategy. Int. J. Nanomed. 2019, 14, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Kubelick, K.P.; Emelianov, S.Y. Prussian blue nanocubes as a multimodal contrast agent for image-guided stem cell therapy of the spinal cord. Photoacoustics 2020, 18, 100166. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, R.; Lv, J.; Wang, H.; Liu, Y.; Peng, Y.; Qian, Z.; Fu, G.; Nie, L. In Vivo Photoacoustic Imaging of Brain Injury and Rehabilitation by High-Efficient Near-Infrared Dye Labeled Mesenchymal Stem Cells with Enhanced Brain Barrier Permeability. Adv. Sci. 2018, 5, 1700277. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Fan, J.; Long, Y.; Xiao, F.; Daniyal, M.; Tong, C.; Xie, Q.; Jian, Y.; Li, B.; et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 2019, 217, 119301. [Google Scholar] [CrossRef]
- Xiao, F.; Fan, J.; Tong, C.; Xiao, C.; Wang, Z.; Liu, B.; Daniyal, M.; Wang, W. An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging. RSC Adv. 2019, 9, 27911–27926. [Google Scholar] [CrossRef]
- Gautam, M.; Poudel, K.; Yong, C.S.; Kim, J.O. Prussian blue nanoparticles: Synthesis, surface modification, and application in cancer treatment. Int. J. Pharm 2018, 549, 31–49. [Google Scholar] [CrossRef]
- Busquets, M.A.; Novella-Xicoy, A.; Guzman, V.; Estelrich, J. Facile Synthesis of Novel Prussian Blue-Lipid Nanocomplexes. Molecules 2019, 24, 4137. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, Y.; Zhang, X.; Zhu, L.; Hou, L.; Chang, J.; Zhang, Z. Engineering of an intelligent cascade nanoreactor for sequential improvement of microenvironment and enhanced tumor phototherapy. Appl. Mater. Today 2020, 18, 100494. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Wang, Q.; Wu, M.; Xu, B.; Liu, X.; Liu, J. Toxicological evaluation of Prussian blue nanoparticles after short exposure of mice. Hum. Exp. Toxicol. 2016, 35, 1123–1132. [Google Scholar] [CrossRef]
- Patra, C.R. Prussian blue nanoparticles and their analogues for application to cancer theranostics. Nanomedicine 2016, 11, 569–572. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef]
- Itaya, K. Electrochemistry of Prussian Blue Modified Electrodes: An Electrochemical Preparation Method. J. Electrochem. Soc. 1982, 129, 1498–1500. [Google Scholar] [CrossRef]
- Itaya, K.; Ataka, T.; Toshima, S. Spectroelectrochemistry and electrochemical preparation method of Prussian blue modified electrodes. J. Am. Chem. Soc. 1982, 104, 4767–4772. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. A High-Sensitive Glucose Amperometric Biosensor Based on Prussian Blue Modified Electrodes. Anal. Lett. 1994, 27, 2861–2869. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio)sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Dai, Y.; Cai, Y.; Zhao, Y.; Wu, D.; Liu, B.; Li, R.; Yang, M.; Wei, Q.; Du, B.; Li, H. Sensitive sandwich electrochemical immunosensor for alpha fetoprotein based on prussian blue modified hydroxyapatite. Biosens. Bioelectron. 2011, 28, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Wang, X.; Wang, C.; Li, C.; Wang, Z. Electrochemical Sensing of alpha-Fetoprotein Based on Molecularly Imprinted Polymerized Ionic Liquid Film on a Gold Nanoparticle Modified Electrode Surface. Sensors 2019, 19, 3218. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A. Electrochemical immunosensors for the detection of cytokine tumor necrosis factor alpha: A review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü.; Timur, S. Towards the electrochemical diagnosis of cancer: Nanomaterial-based immunosensors and cytosensors. RSC Adv. 2016, 6, 111831–111841. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Buffa, F.M.; Wilson, G.D. Multiple biomarker tissue microarrays: Bioinformatics and practical approaches. Cancer Metastasis Rev. 2008, 27, 481–494. [Google Scholar] [CrossRef]
- Lai, G.; Yan, F.; Ju, H. Dual Signal Amplification of Glucose Oxidase-Functionalized Nanocomposites as a Trace Label for Ultrasensitive Simultaneous Multiplexed Electrochemical Detection of Tumor Markers. Anal. Chem. 2009, 81, 9730–9736. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.C. Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview. Molecules 2015, 20, 14155–14190. [Google Scholar] [CrossRef]
- Lai, G.; Wang, L.; Wu, J.; Ju, H.; Yan, F. Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal. Chim. Acta 2012, 721, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.C.; Fan, X.; Zhang, R.; Wang, L.; Wang, W.; Luo, X. Low fouling and ultrasensitive electrochemical immunosensors with dual assay methods based on Fe3O4 magnetic nanoparticles. J. Mater. Chem. B 2019, 7, 5842–5847. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, G.; Liu, L.; Li, X.; Wei, Q.; Cao, W. Ultrasensitive competitive method-based electrochemiluminescence immunosensor for diethylstilbestrol detection based on Ru(bpy)32+ as luminophor encapsulated in metal-organic frameworks UiO-67. Biosens. Bioelectron. 2018, 110, 201–206. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoot, W.; Petrou, P.; Kakabakos, S. Lab-on-a-Membrane Foldable Devices for Duplex Drop-Volume Electrochemical Biosensing Using Quantum Dot Tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Ma, Z. Self-sacrificial label assisted electroactivity conversion of sensing interface for ultrasensitive electrochemical immunosensor. Biosens. Bioelectron. 2019, 140, 111355. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1-T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Gu, Z. Photoacoustic Drug Delivery. Sensors 2017, 17, 1400. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yuan, Z. Photoacoustic Tomography Imaging of the Adult Zebrafish by Using Unfocused and Focused High-Frequency Ultrasound Transducers. Appl. Sci. 2016, 6, 392. [Google Scholar] [CrossRef]
- Kratkiewicz, K.; Manwar, R.; Rajabi-Estarabadi, A.; Fakhoury, J.; Meiliute, J.; Daveluy, S.; Mehregan, D.; Avanaki, K.M. Photoacoustic/Ultrasound/Optical Coherence Tomography Evaluation of Melanoma Lesion and Healthy Skin in a Swine Model. Sensors 2019, 19, 2815. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Yang, H.; Jiang, H.; Ding, Y.; Xie, H. MEMS Ultrasound Transducers for Endoscopic Photoacoustic Imaging Applications. Micromachines 2020, 11, 928. [Google Scholar] [CrossRef]
- Tsang, V.T.C.; Li, X.; Wong, T.T.W. A Review of Endogenous and Exogenous Contrast Agents Used in Photoacoustic Tomography with Different Sensing Configurations. Sensors 2020, 20, 5595. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Deng, Z.; Jing, L.; Li, X.; Dai, Z.; Li, C.; Huang, M. Prussian blue nanoparticles operate as a contrast agent for enhanced photoacoustic imaging. Chem. Commun. 2013, 49, 11029–11031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhou, T.; Feng, X.; Shen, J.; Zhang, M.; Sun, Y. Enhanced Plasmon-Induced Resonance Energy Transfer (PIRET)-Mediated Photothermal and Photodynamic Therapy Guided by Photoacoustic and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2019, 11, 31615–31626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tian, G.; Zeng, L.; Song, X.; Bian, X.-W. Nanoscaled Metal-Organic Frameworks for Biosensing, Imaging, and Cancer Therapy. Adv. Health Mater. 2018, 7, 1800022. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Kang, M.; Song, Y.; He, L.; Zhou, N.; Wang, M.; Zhang, Z.; et al. PEGMA-modified bimetallic NiCo Prussian blue analogue doped with TbIII ions: Efficiently pH-responsive and controlled release system for anticancer drug. Chem. Eng. J. 2020, 389, 124468. [Google Scholar] [CrossRef]
- Li, Y.; Dang, J.; Liang, Q.; Yin, L. Thermal-Responsive Carbon Monoxide (CO) Delivery Expedites Metabolic Exhaustion of Cancer Cells toward Reversal of Chemotherapy Resistance. ACS Cent. Sci 2019, 5, 1044–1058. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Tang, J.; Zhou, L.; Wei, S. Ytterbium—Doped Prussian blue: Fabrication, photothermal performance and antibacterial activity. Inorganic Chem. Commun. 2020, 114, 107821. [Google Scholar] [CrossRef]
- Fu, G.; Liu, W.; Feng, S.; Yue, X. Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem. Commun. 2012, 48, 11567–11569. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, W.; Zhao, H.; Miao, X.; Guan, Y.; Cai, W.; Zeng, Z.; Fan, Q.; Tan, T.T.Y. Generating New Cross-Relaxation Pathways by Coating Prussian Blue on NaNdF4 To Fabricate Enhanced Photothermal Agents. Angew. Chem. 2019, 58, 8536–8540. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Li, F.; Han, X.; Chen, G. Prussian blue-coated lanthanide-doped core/shell/shell nanocrystals for NIR-II image-guided photothermal therapy. Nanoscale 2019, 11, 22079–22088. [Google Scholar] [CrossRef] [PubMed]
- Shou, P.; Yu, Z.; Wu, Y.; Feng, Q.; Zhou, B.; Xing, J.; Liu, C.; Tu, J.; Akakuru, O.U.; Ye, Z.; et al. Zn2+ Doped Ultrasmall Prussian Blue Nanotheranostic Agent for Breast Cancer Photothermal Therapy under MR Imaging Guidance. Adv. Health Mater. 2020, 9, e1900948. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.M.A.; Mathlouthi, E.; Cahu, M.; Sene, S.; Daurat, M.; Long, J.; Guari, Y.; Salles, F.; Chopineau, J.; Devoisselle, J.-M.; et al. Synergic effect of doxorubicin release and two-photon irradiation of Mn2+-doped Prussian blue nanoparticles on cancer therapy. RSC Adv. 2020, 10, 2646–2649. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Chen, H.; He, W.; Guo, Z. An H2O2-responsive nanocarrier for dual-release of platinum anticancer drugs and O2: Controlled release and enhanced cytotoxicity against cisplatin resistant cancer cells. Chem. Commun. 2014, 50, 9714–9717. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Ding, L.; Li, X.W.; Wu, Y.; Tang, J.H. Prussian blue-modified ferritin nanoparticles for effective tumor chemo-photothermal combination therapy via enhancing reactive oxygen species production. J. Biomater. Appl. 2019, 33, 1202–1213. [Google Scholar] [CrossRef]
- Yang, F.; Hu, S.; Zhang, Y.; Cai, X.; Huang, Y.; Wang, F.; Wen, S.; Teng, G.; Gu, N. A hydrogen peroxide-responsive O2 nanogenerator for ultrasound and magnetic-resonance dual modality imaging. Adv. Mater. 2012, 24, 5205–5211. [Google Scholar] [CrossRef]
- Wang, D.; Shi, R.; Zhou, J.; Shi, S.; Wu, H.; Xu, P.; Wang, H.; Xia, G.; Barnhart, T.E.; Cai, W.; et al. Photo-Enhanced Singlet Oxygen Generation of Prussian Blue-Based Nanocatalyst for Augmented Photodynamic Therapy. iScience 2018, 9, 14–26. [Google Scholar] [CrossRef]
- Odda, A.H.; Xu, Y.; Lin, J.; Wang, G.; Ullah, N.; Zeb, A.; Liang, K.; Wen, L.P.; Xu, A.W. Plasmonic MoO3-x nanoparticles incorporated in Prussian blue frameworks exhibit highly efficient dual photothermal/photodynamic therapy. J. Mater. Chem B 2019, 7, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Q.; Hou, M.; Gao, Y.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Light-activatable Chlorin e6 (Ce6)-imbedded erythrocyte membrane vesicles camouflaged Prussian blue nanoparticles for synergistic photothermal and photodynamic therapies of cancer. Biomater. Sci. 2018, 6, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Tian, W.; Wang, Q.; Zhao, Y.; Zhang, Y.L.; Tian, Y.; Tang, Y.X.; Wang, S.J.; Liu, Y.; Ni, Q.Q.; et al. Oxygen-Evolving Mesoporous Organosilica Coated Prussian Blue Nanoplatform for Highly Efficient Photodynamic Therapy of Tumors. Adv. Sci. 2018, 5, 1700847. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Gao, W.; Zhang, L.; Ma, M.; Liu, T.; Du, W.; Zheng, Y.; Chen, H.; Shi, J. Enabling Prussian Blue with Tunable Localized Surface Plasmon Resonances: Simultaneously Enhanced Dual-Mode Imaging and Tumor Photothermal Therapy. ACS Nano 2016, 10, 11115–11126. [Google Scholar] [CrossRef]
- Peng, J.; Dong, M.; Ran, B.; Li, W.; Hao, Y.; Yang, Q.; Tan, L.; Shi, K.; Qian, Z. “One-for-All”-Type, Biodegradable Prussian Blue/Manganese Dioxide Hybrid Nanocrystal for Trimodal Imaging-Guided Photothermal Therapy and Oxygen Regulation of Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 13875–13886. [Google Scholar] [CrossRef]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, M.Y.; Zhu, Q.; Chi, B.; Zeng, L.W.; Hu, J.M.; Shen, A.G. Monodispersed plasmonic Prussian blue nanoparticles for zero-background SERS/MRI-guided phototherapy. Nanoscale 2020, 12, 3292–3301. [Google Scholar] [CrossRef]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon Dots/Prussian Blue Satellite/Core Nanocomposites for Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef]
- Sahu, A.; Lee, J.H.; Lee, H.G.; Jeong, Y.Y.; Tae, G. Prussian blue/serum albumin/indocyanine green as a multifunctional nanotheranostic agent for bimodal imaging guided laser mediated combinatorial phototherapy. J. Control. Release 2016, 236, 90–99. [Google Scholar] [CrossRef]
- Zhang, N.; Cai, X.; Gao, W.; Wang, R.; Xu, C.; Yao, Y.; Hao, L.; Sheng, D.; Chen, H.; Wang, Z.; et al. A Multifunctional Theranostic Nanoagent for Dual-Mode Image-Guided HIFU/Chemo-Synergistic Cancer Therapy. Theranostics 2016, 6, 404–417. [Google Scholar] [CrossRef]
- Zhang, M.; Sheng, B.; Ashley, J.; Zheng, T.; Wang, W.; Zhang, Q.; Zhang, J.; Zhou, N.; Shen, J.; Sun, Y. Manganese ion chelated FeOCl@PB@PDA@BPQDs nanocomposites as a tumor microenvironment-mediated nanoplatform for enhanced tumor imaging and therapy. Sens. Actuators B Chem. 2020, 307, 127491. [Google Scholar] [CrossRef]
- Santha Moorthy, M.; Hoang, G.; Subramanian, B.; Bui, N.Q.; Panchanathan, M.; Mondal, S.; Thi Tuong, V.P.; Kim, H.; Oh, J. Prussian blue decorated mesoporous silica hybrid nanocarriers for photoacoustic imaging-guided synergistic chemo-photothermal combination therapy. J. Mater. Chem. B 2018, 6, 5220–5233. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, W.; Li, Y.; Jin, Y.; Jiang, L.; Liang, X.; Feng, S.; Dai, Z. Magnetic Prussian blue nanoparticles for targeted photothermal therapy under magnetic resonance imaging guidance. Bioconjugate Chem. 2014, 25, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef]
- Terreno, E.; Castelli, D.D.; Viale, A.; Aime, S. Challenges for Molecular Magnetic Resonance Imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Jia, X.; Cai, X.; Chen, Y.; Wang, S.; Xu, H.; Zhang, K.; Ma, M.; Wu, H.; Shi, J.; Chen, H. Perfluoropentane-encapsulated hollow mesoporous prussian blue nanocubes for activated ultrasound imaging and photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2015, 7, 4579–4588. [Google Scholar] [CrossRef]
- Faust, O.; Acharya, U.R.; Meiburger, K.M.; Molinari, F.; Koh, J.E.W.; Yeong, C.H.; Kongmebhol, P.; Ng, K.H. Comparative assessment of texture features for the identification of cancer in ultrasound images: A review. Biocybern. Biomed. Eng. 2018, 38, 275–296. [Google Scholar] [CrossRef]
- Wu, W.J.; Moon, W.K. Ultrasound breast tumor image computer-aided diagnosis with texture and morphological features. Acad. Radiol. 2008, 15, 873–880. [Google Scholar] [CrossRef]

| NPs | Approximate Size (nm) | Surface Coating | Shape | r1 (mM−1s−1) | r2 (mM−1s−1) | Field (T) | T (°C) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PBNPs | 25 | - | Cubes | 0.2 | 1.22 | 1.5 | - | [32] |
| 0.14 | 2.88 | 7 | ||||||
| AntiGPC3-PBNPs | 21 | Citrate | Cubes | 0.14 | 11.73 | 9.4 | 20 | [33] |
| Gd@PBNPs | 24 ± 9 | PEG | Cubes | 16.4 | 20.9 | 1.4 | 37 | [34] |
| Mn@PBNPs | 5.5 | Dextain | Sphere | 12.9 | - | 3 | 5 | [35] |
| 10 | 15 | 37 | ||||||
| Gd@PBNPs (GdFeFe) | 3.9 | Dextain | Sphere | 81 | - | 1.4 | 5 | [36] |
| 55 | 77 | 37 | ||||||
| Mn@PBNPs | 71/63 | - | Cubes | 2.58/5.3 | - | 4.7 | RT | [37] |
| Mn@HMPBNPs | 290 | PVP | Cubes | 3.0 | - | 7.0 | 25 | [38] |
| Name | Drug Release | Size (nm) | Targeting | Anti-Tumor Drug | Loading Efficiency (%) | Circulation Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| HA@RBC@PB@CS-6 | pH-/photo-responsive release | 140 | HA, RBC | CS-6 | - | 10 | [46] |
| PB@DOX@EM@FA NPs | pH-/photo-responsive release | 185 | FA, EM | DOX | 130.69 | 48 | [47] |
| NiCo-PBA@Tb3+@PEGMA@AS1411@DOX | pH-responsive release | 173 | As1411 | DOX | 77.2 | - | [86] |
| PAH@PAA@PEG@PB@CO@DOX | pH-/NIR light release | 128 | Passive targeting | DOX | 14.5 | - | [87] |
| Formulations | Treatment Approaches | Imaging | Targeting | Cell Lines | Laser Irradiation | Ref. |
|---|---|---|---|---|---|---|
| antiglypican-3-PBNPs | PTT | MR | Antiglypican-3 | HepG2; HL-7702 cells | 808 nm laser (2 W cm−2, 10 min) | [33] |
| PVP or dextran-coated Gd3+@PBNPs | PTT | MR; PA | - | CT26 cells | 808 nm laser (1 W cm−2, 5 min) | [36] |
| Mn2+-doped PBNPs | PTT | MR | - | MDA-MB-231 cells | Two-photon light at 808 nm (3.7 W, 10 min) | [37] |
| PVP-coated HMPB-Mn | Chemothermal therapy | MR | - | 4T1 cells | 808 nm laser (1 W cm−2, 5 min) | [38] |
| Au@PB@Cu2O@BPQDs/PAHNCs. | PTT/PDT | MR; PA; FL | - | HeLa cells | 650 nm laser (1.5 W cm−2, 5 min) | [83] |
| PBNPs | PTT | MR/PA | - | 4T1 cells | 808 nm laser (0.8 W cm−2, 5 min) | [41] |
| PB@DOX@EM@FANPs | Chemo-photothermal therapy | PA; FL | Folic acid | HeLa cells | 808 nm laser (0.8 W cm−2, 5 min) | [47] |
| PBNPs@Fe(CO)5@DOX | Chemo-photothermal-photodynamic therapy | US | - | MCF-7/ADR cells | 808 nm laser (0.5 W cm−2, 15 min) | [87] |
| NdNdF4@PBNPs | PTT | PA | - | HeLa cells | 808 nm laser (0.6 W cm−2, 10 min) | [91] |
| Zn2+@PBNPs | PTT | MR | - | 4T1 cells | 808 nm laser (1 W cm−2, 5 min) | [94] |
| PB@SiO2-PEG-ZnPc | PDT; PTT | PET; PA | - | 4T1 cells | 671 nm laser (0.4 W cm−2, 5 min) | [100] |
| Gd3+@PBNPs | PTT | PA; MR | - | 4T1 cells | 808 nm laser (0.58 W cm−2, 10 min) | [104] |
| MnO2@PBNPs | PTT | PA; T1/T2 weighted MRI | - | MCF-7 cells | 808 nm laser (2.5 W cm−2, 5 min) | [105] |
| Au@PB | PTT | CT; PA | - | HT-29 cells | 808 nm laser (1.5 W cm−2, 10 min) | [106] |
| Au@PB-HA | PDT; PTT | MR; SERS | Hyaluronic acid | 4T1 cells | 808 nm laser (2 W cm−2, 10 min) | [107] |
| CD-decorated PBNP | PTT | FL | - | C6 cells | 808 nm laser (0.8 W cm−2, 10 min) | [108] |
| PB-BSA-ICG | PDT; PTT | MR; FL | - | SCC7 cells | 808 nm laser (1 W cm−2, 10 min) | [109] |
| HMPBs-DOX/PFH | Chemo-HIFU therapy | PA; US | - | VX2 cells | - | [110] |
| FeOCl@PB@PDA@BPQDs | CDT; PDT; PTT | PA; MR; US | - | 4T1 cells | 650 nm laser (1.5 W cm−2, 5 min) | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wang, Q.; Cheng, C.; Lin, S.; Lin, T.; Liu, C.; Han, X. The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment. Sensors 2020, 20, 6905. https://doi.org/10.3390/s20236905
Gao X, Wang Q, Cheng C, Lin S, Lin T, Liu C, Han X. The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment. Sensors. 2020; 20(23):6905. https://doi.org/10.3390/s20236905
Chicago/Turabian StyleGao, Xiaoran, Qiaowen Wang, Cui Cheng, Shujin Lin, Ting Lin, Chun Liu, and Xiao Han. 2020. "The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment" Sensors 20, no. 23: 6905. https://doi.org/10.3390/s20236905
APA StyleGao, X., Wang, Q., Cheng, C., Lin, S., Lin, T., Liu, C., & Han, X. (2020). The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment. Sensors, 20(23), 6905. https://doi.org/10.3390/s20236905





