Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People
Abstract
1. Introduction
2. Methods
2.1. Ethical Aspects
2.2. Subjects
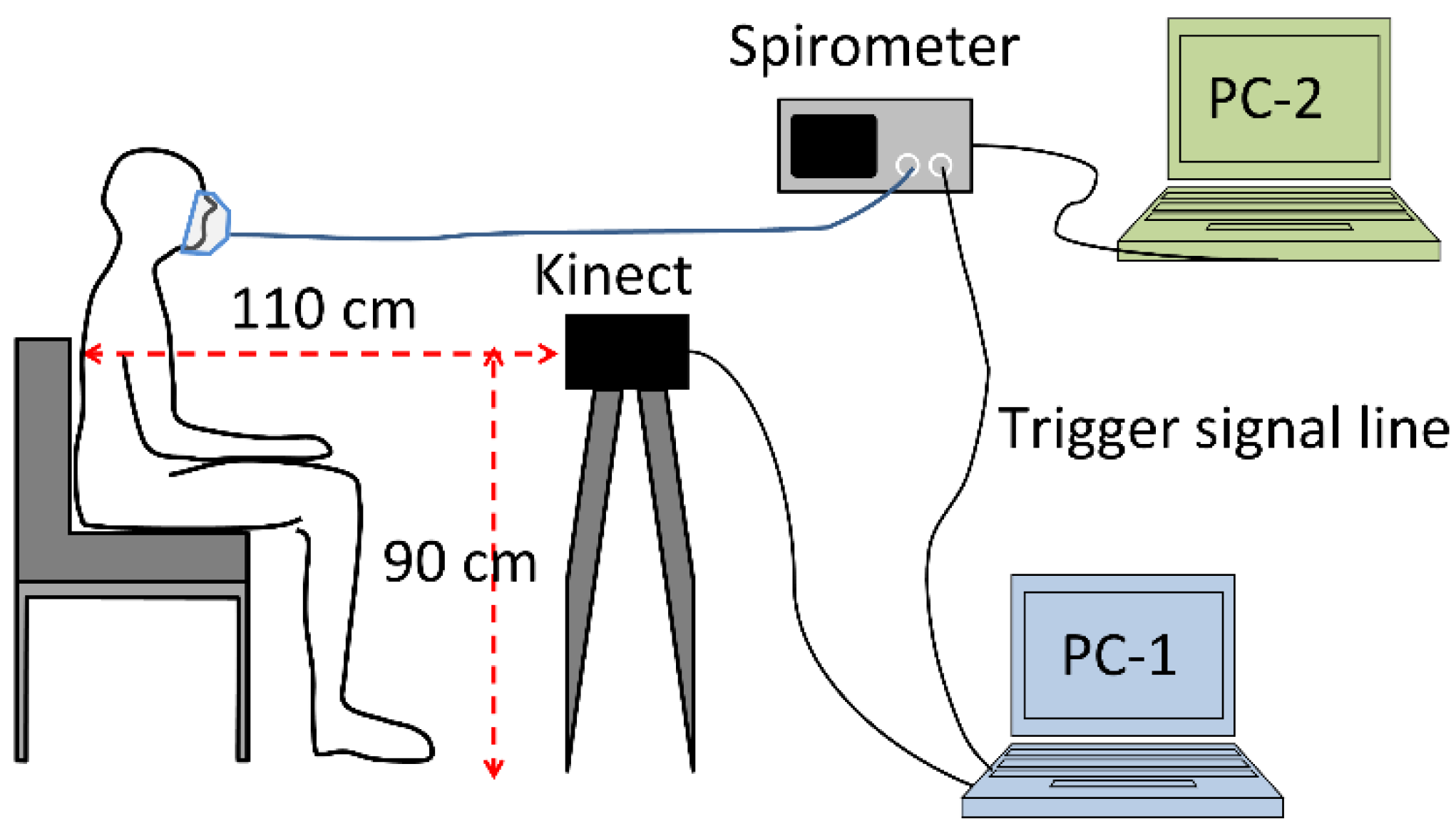
2.3. Experimental Setup and Measurement Procedure
2.4. Estimation Method
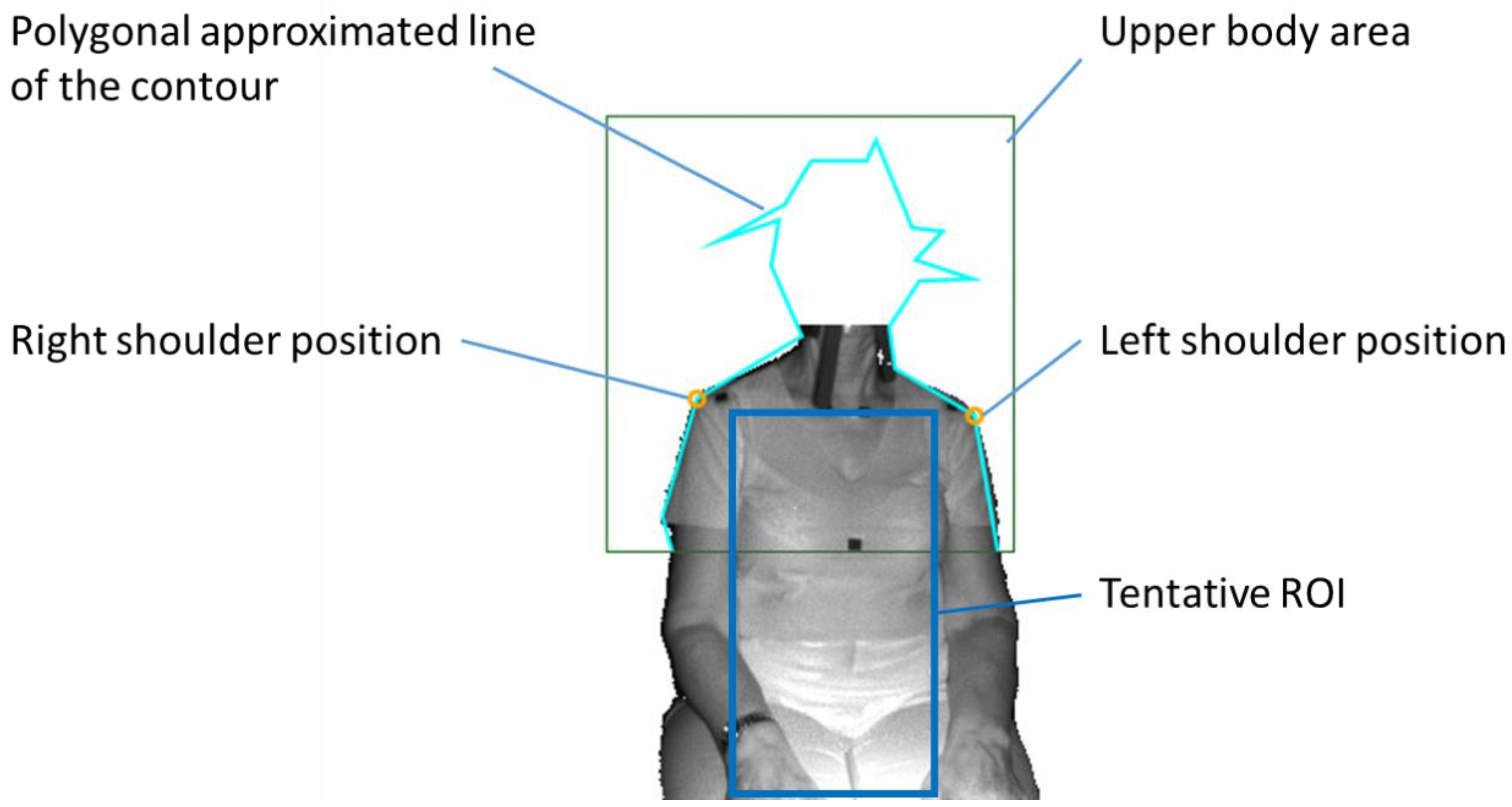
- The detection of the upper body area: Haar cascade classifier in OpenCV [15] was used.
- The detection of the position of both shoulders: Since the subject was sitting on a chair and had his/her arms lowered, the shoulder positions could easily be detected as the vertices in the polygonal approximated line of the contour of the upper body. We did not use the body tracking function in the Kinect for Windows SDK (Kinect SDK) [16] and also did not use a pose estimation library such as OpenPose [17]. This is because Kinect SDK is sometimes unstable when a subject is sitting, or because the pose estimation library requires a powerful computer with a graphics processing unit (GPU).
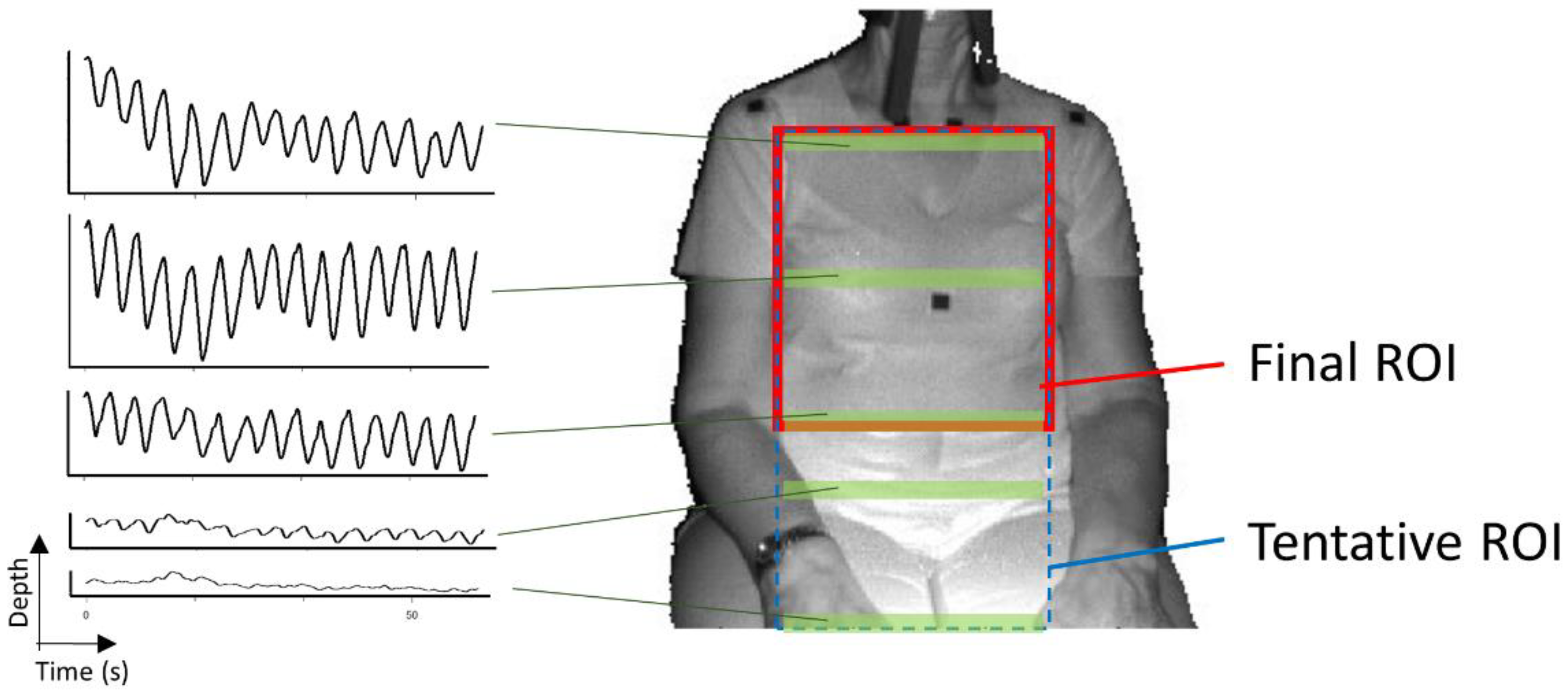
- The determination of the tentative ROI: An example of a tentative ROI is shown as the blue rectangle area in Figure 2. The top of the ROI was set at the lowest position of the two shoulders. The left and right ends of the ROI were at the position corresponding to the inside the approximate arm’s width from both shoulders. The bottom position of the ROI was tentatively decided as the lower end of the image. Here, we employed a unit area of 19 × 19 pixels to reduce the random error of the depth measurements. Since there was a strong correlation among the depth values of close pixels [18], the size of the unit was decided by trial and error. The units that covered the tentative ROI area were slid every 10 pixels to acquire the respiratory signal. The obtained depth signals in each unit area were sampled at a rate of 30 Hz and were averaged in the area.
- The determination of the final ROI: Since there is a difference in the contribution of the motion of the thoracic compartment or the abdomen to TV among sex and ages [19], we developed a unique algorithm to obtain the proper position of the bottom of the ROI. The algorithm was as follows:
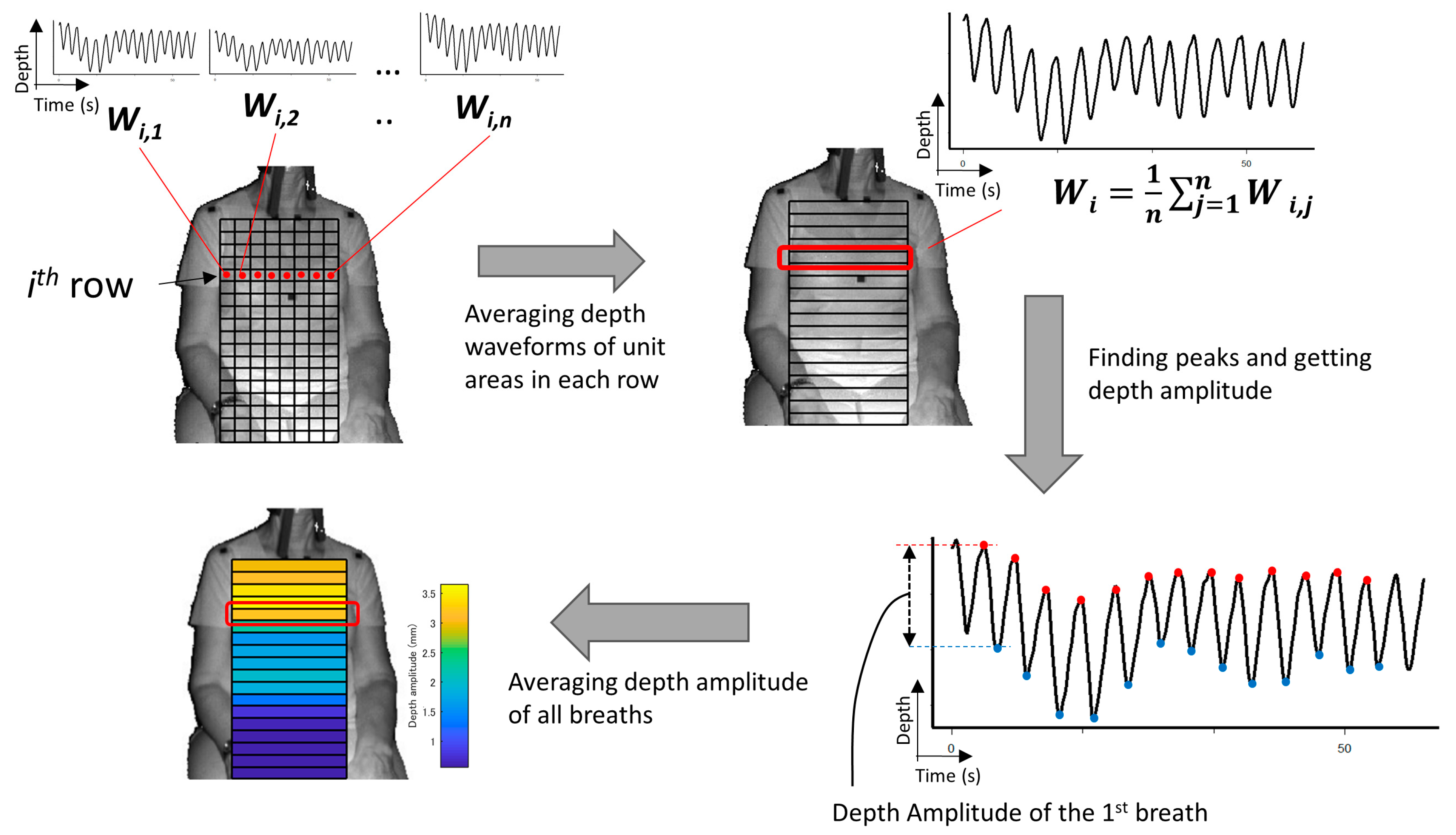
- (A)
- The depth waveform of each row (Wi) was calculated by averaging all of the depth waveforms (Wi,1, Wi,2, …, Wi,n) of the unit areas in each row. The depth amplitude of each row was calculated by averaging the depth amplitude of each breath in waveform Wi (Figure 3).
- (B)
- The amplitude ratio of each row was calculated from the sum of the depth amplitude of all rows.
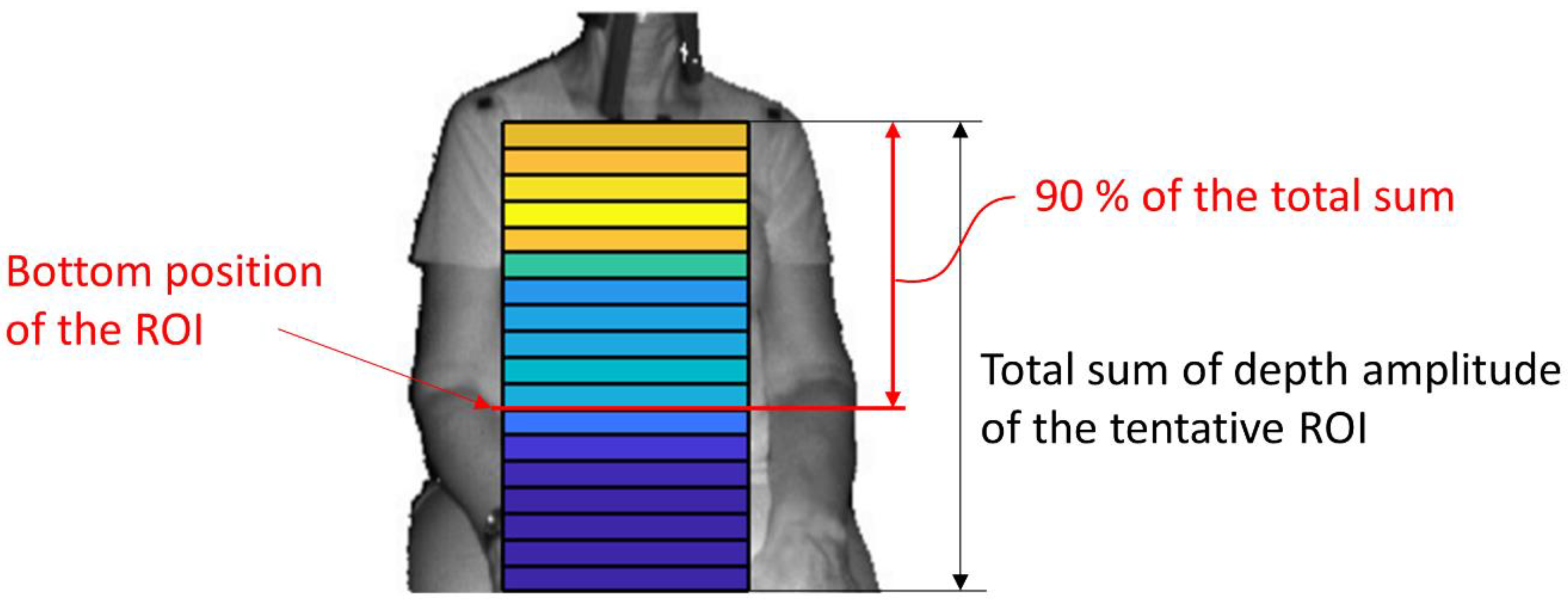
- (C)
- The amplitude ratio was integrated from the top of the ROI, and the position where it reached 90% was defined as the bottom of the ROI (Figure 4). A value of 90% was decided by trial and error.
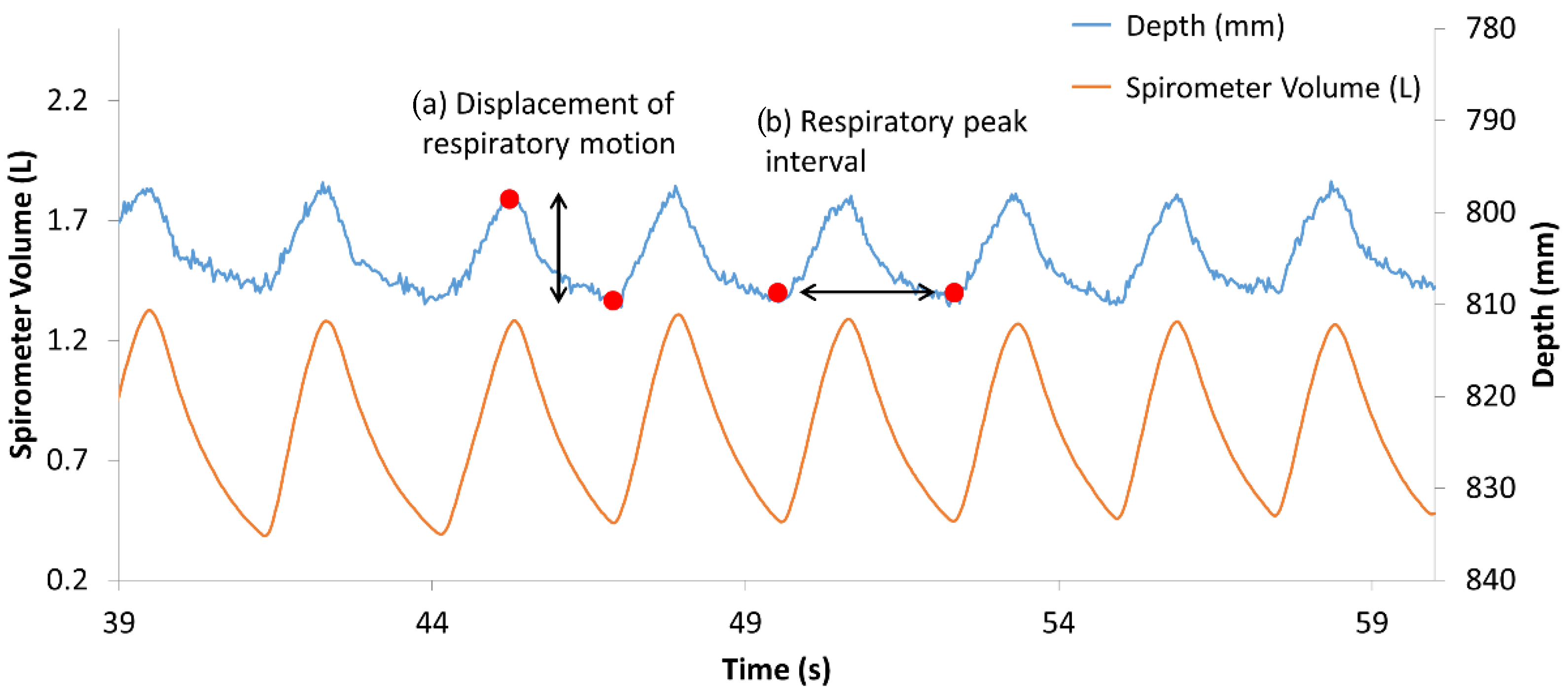
2.5. Statistical Analysis
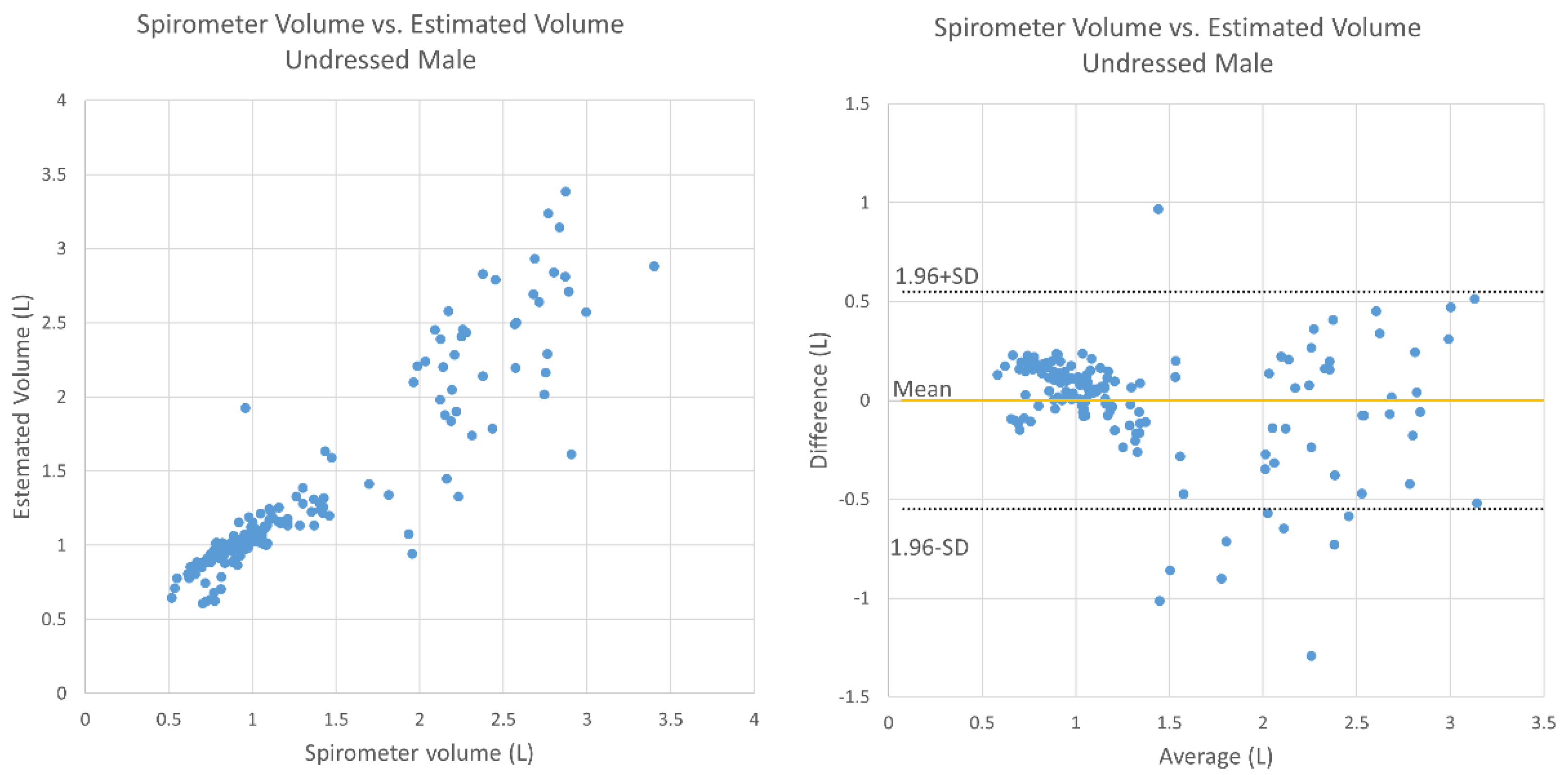
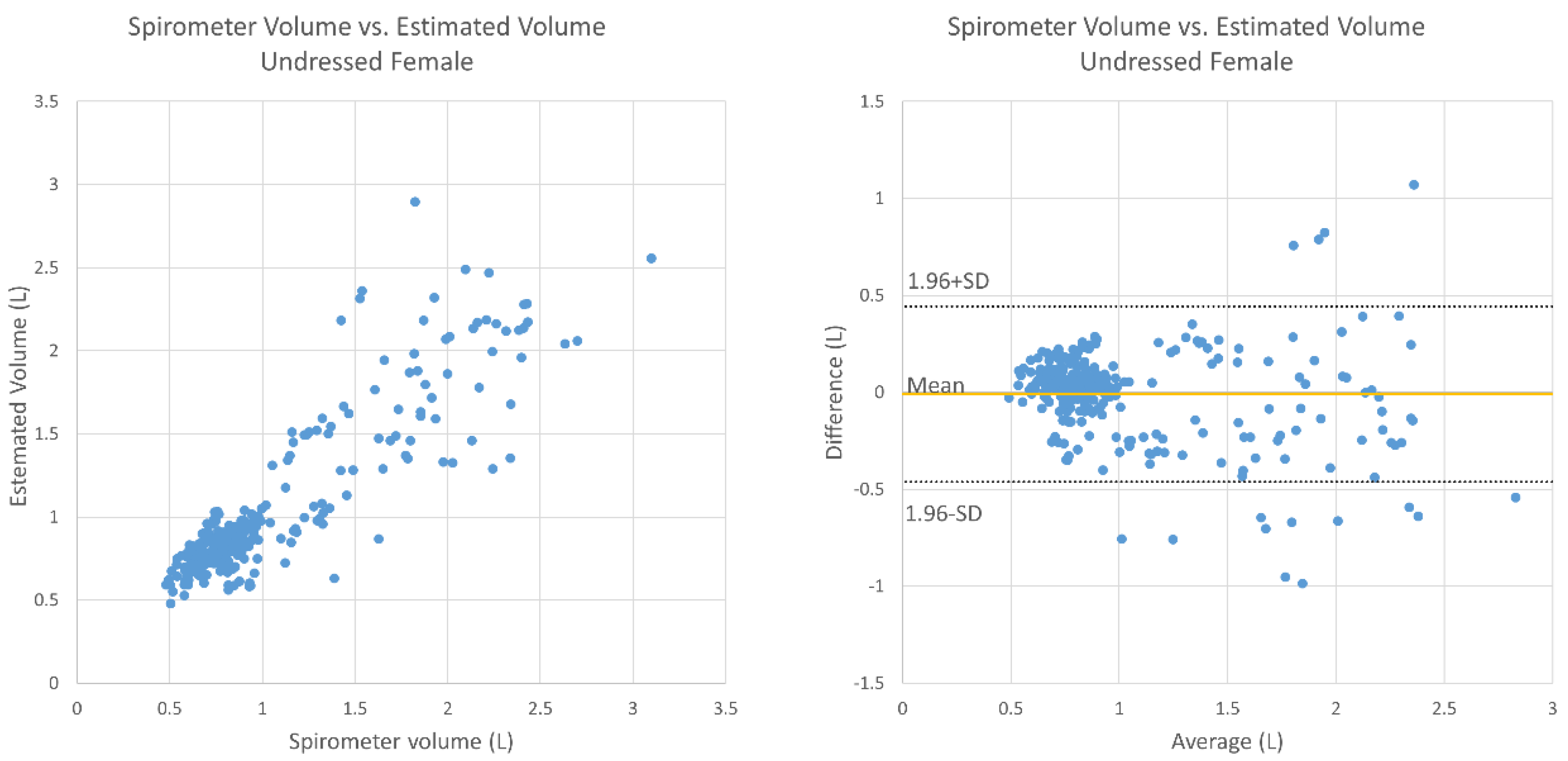
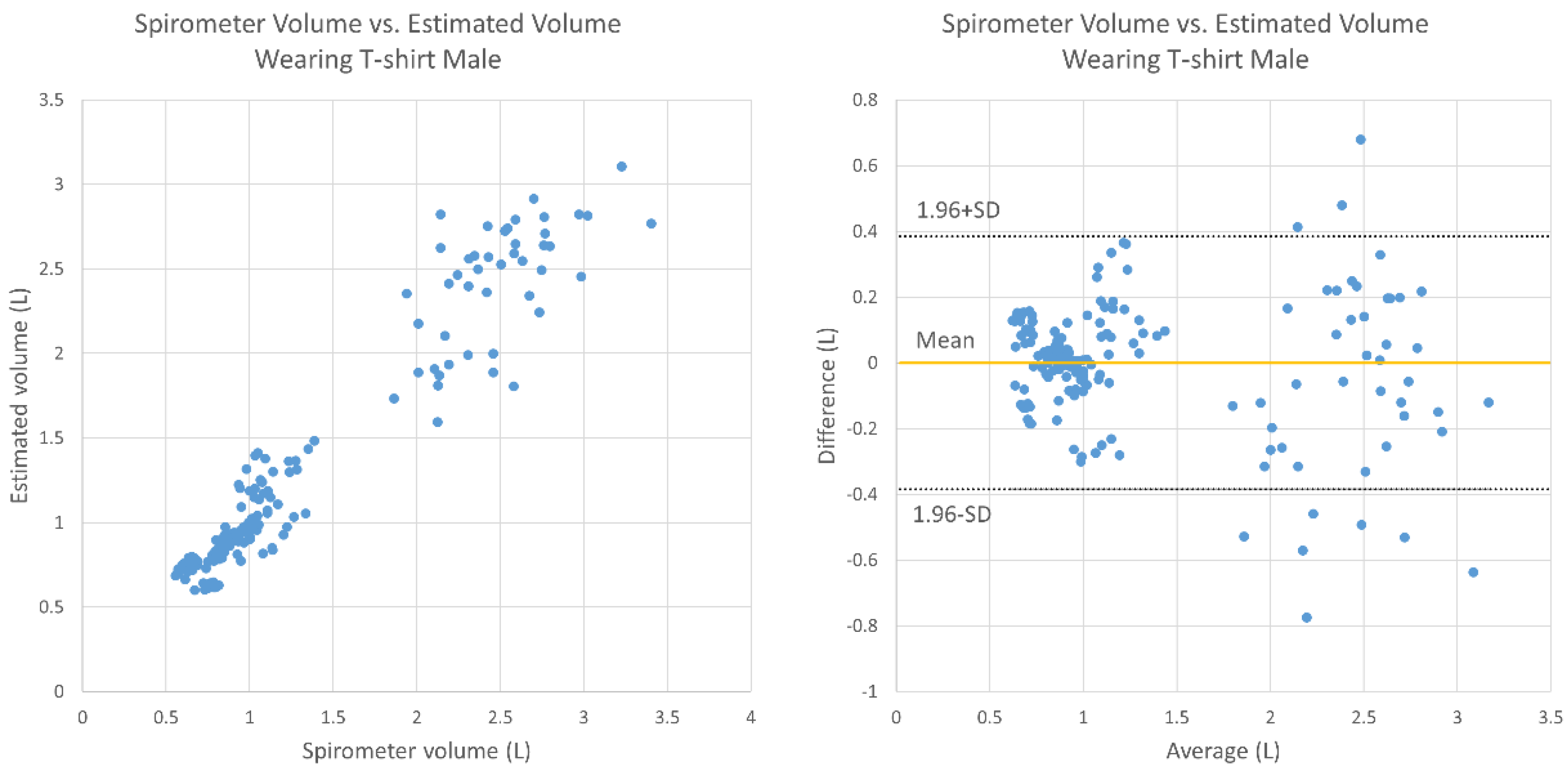
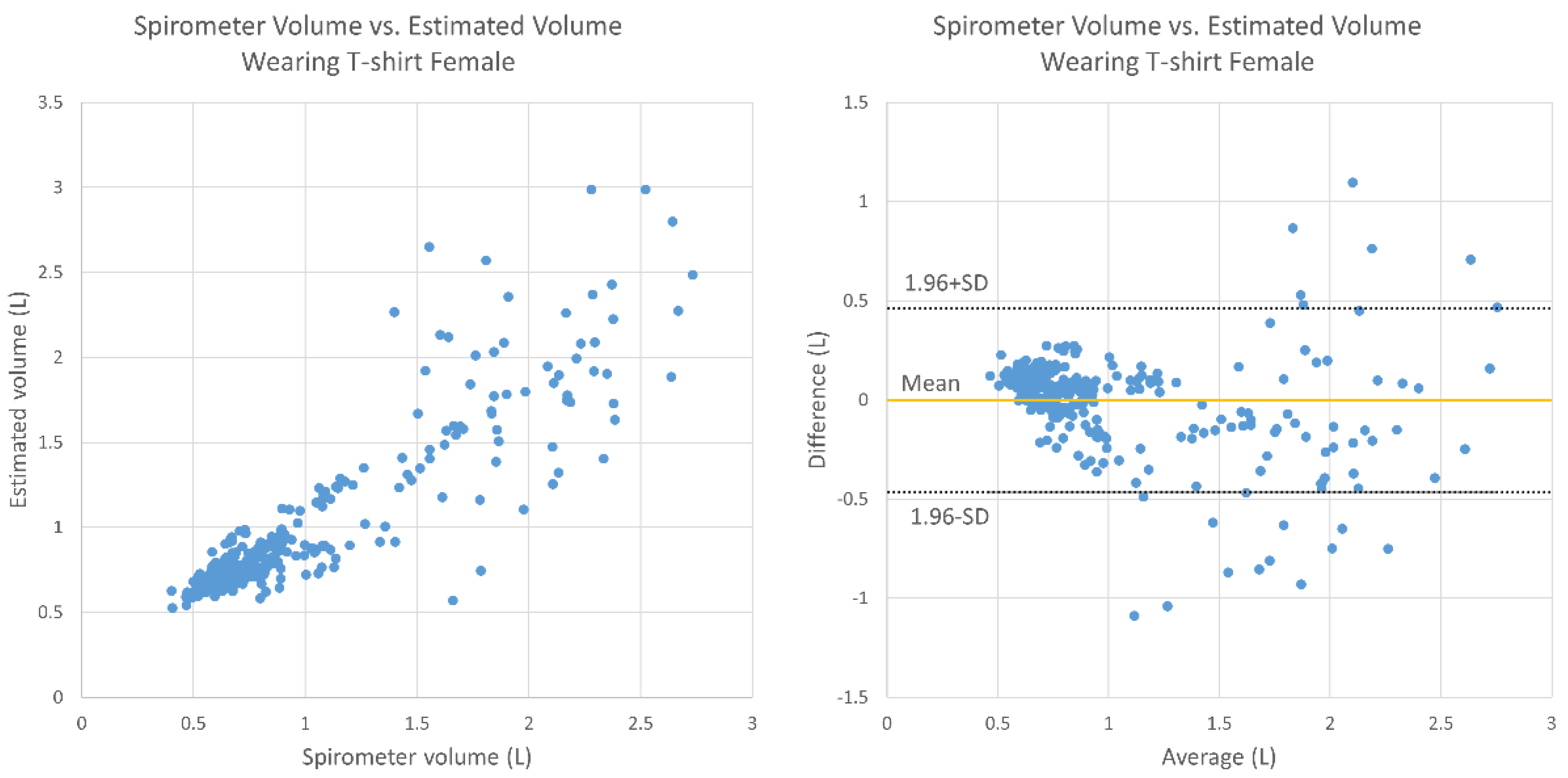
3. Results
4. Discussion
- The deep breathing outlier data could not be omitted since we set the minimum number of deep breaths as five in the protocol. This might have affected the accuracy of the TV estimation since there was relatively large motion during the deep breathing.
- There was only one set of trials for each subject, meaning that we could not confirm the reproducibility of the measurements for one person, which is important for tracking the change of HF pathology. Thus, future research is needed.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 12 November 2020).
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Bernocchi, P.; Vitacca, M.; La Rovere, M.T.; Volterrani, M.; Galli, T.; Baratti, D.; Paneroni, M.; Campolongo, G.; Sposato, B.; Scalvini, S. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: A randomized controlled trial. Age Ageing 2018, 47, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Riegel, B.; Dickson, V.V.; Cameron, J.; Johnson, J.C.; Bunker, S.; Page, K.; Worrall-Carter, L. Symptom recognition in elders with heart failure. J. Nurs. Scholarsh. 2010, 42, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.S.; Collins, S.P.; Sauser, K.; Andrei, A.-C.; Storrow, A.B.; Hollander, J.E.; Tavares, M.; Spinar, J.; Macarie, C.; Raev, D.; et al. Assessment of dyspnea early in acute heart failure: Patient characteristics and response differences between likert and visual analog scales. Acad. Emerg. Med. 2014, 21, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M. Abnormalities in cardiopulmonary exercise testing ventilatory parameters in heart failure: Pathophysiology and clinical usefulness. Curr. Heart Fail. Rep. 2014, 11, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Cleland, J.G.; Weatherley, B.D.; Dittrich, H.C.; Givertz, M.M.; Massie, B.M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Voors, A.A.; et al. Dyspnoea in patients with acute heart failure: An analysis of its clinical course, determinants, and relationship to 60-day outcomes in the PROTECT pilot study. Eur. J. Heart Fail. 2010, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Capucci, A.; Molon, G.; Gold, M.R.; Zhang, Y.; Sweeney, R.; Averina, V.; Boehmer, J.P. Rapid shallow breathing worsens prior to heart failure decompensation. J. Card. Fail. 2014, 20, S14. [Google Scholar] [CrossRef]
- Reyes, B.A.; Reljin, N.; Kong, Y.; Nam, Y.; Ha, S.; Chon, K.H. Employing an incentive spirometer to calibrate tidal volumes estimated from a smartphone camera. Sensors 2016, 16, 397. [Google Scholar] [CrossRef]
- Seppanen, T.M.; Kananen, J.; Noponen, K.; Alho, O.-P.; Seppanen, T. Accurate measurement of respiratory airflow waveforms using depth data. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 2015, 7857–7860. [Google Scholar] [CrossRef]
- Massagram, W.; Lubecke, V.M.; Boric-Lubecke, O. Microwave non-invasive sensing of respiratory tidal volume. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 4832–4835. [Google Scholar]
- Mizobe, Y.; Aoki, H.; Koshiji, K. Basic study on relationship between respiratory flow volume and volume change of thorax surface. In Proceedings of the 2007 6th International Special Topic Conference on Information Technology Applications in Biomedicine, Tokyo, Japan, 8–11 November 2007; IEEE: Piscataway, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Massaroni, C.; Lopes, D.S.; Lo Presti, D.; Schena, E.; Silvestri, S. Contactless Monitoring of Breathing Patterns and Respiratory Rate at the Pit of the Neck: A Single Camera Approach. Available online: https://www.hindawi.com/journals/js/2018/4567213/ (accessed on 12 November 2020).
- Aoki, H.; Nakamura, H. Non-contact respiration measurement during exercise tolerance test by using kinect sensor. Sports 2018, 6, 23. [Google Scholar] [CrossRef]
- Opencv/Opencv. Available online: https://github.com/opencv/opencv (accessed on 12 November 2020).
- Rahman, M. Body & face tracking. In Beginning Microsoft Kinect for Windows SDK 2.0: Motion and Depth Sensing for Natural User Interfaces; Rahman, M., Ed.; Apress: Berkeley, CA, USA, 2017; pp. 105–160. ISBN 978-1-4842-2316-1. [Google Scholar]
- CMU-Perceptual-Computing-Lab/openpose. Available online: https://github.com/CMU-Perceptual-Computing-Lab/openpose (accessed on 12 November 2020).
- Okubo, A.; Abe, M.; Kawamata, M.; Tokuda, K. Measuring the Vibration of the Thorax Using Depth Images Obtained by Kinect Sensor and Respiration Rate Estimation by an Adaptive Filter. In Proceedings of the 31st SIP SYMPOSIUM, Osaka, Japan, 8–11 November 2016; Volume B4-4, pp. 267–272. Available online: https://www.jstage.jst.go.jp/article/ieejeiss/138/7/138_927/_article/-char/ja/ (accessed on 12 November 2020).
- Mendes, L.P.D.S.; Vieira, D.S.R.; Gabriel, L.S.; Ribeiro-Samora, G.A.; Dornelas De Andrade, A.; Brandão, D.C.; Goes, M.C.; Fregonezi, G.A.F.; Britto, R.R.; Parreira, V.F. Influence of posture, sex, and age on breathing pattern and chest wall motion in healthy subjects. Braz. J. Phys. Ther. 2020, 24, 240–248. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Aoki, H.; Nakamura, H.; Fumoto, K.; Nakahara, K.; Teraoka, M. Basic study on non-contact respiration measurement during exercise tolerance test by using Kinect sensor. In Proceedings of the 2015 IEEE/SICE International Symposium on System Integration (SII), Nagoya, Japan, 11–13 December 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 217–222. [Google Scholar]
- Uccheddu, F.; Ghionzoli, M.; Volpe, Y.; Servi, M.; Furferi, R.; Governi, L.; Facchini, F.; Lo Piccolo, R.; McGreevy, K.S.; Martin, A.; et al. A novel objective approach to the external measurement of pectus excavatum severity by means of an optical device. Ann. Thorac. Surg. 2018, 106, 221–227. [Google Scholar] [CrossRef] [PubMed]
| Healthy Elderly People | |
|---|---|
| ➢ Inclusion Criteria: | |
| Age of 65–75 years | |
| No chronic cough | |
| No chronic sputum | |
| No exertional dyspnea | |
| ➢ Exclusion Criteria: | |
| Previous neurologic, musculoskeletal, or mental illnesses | |
| Lack of ability to cooperate | |
| Silicon allergy | |
| Surgical history or currently under treatment for cardiopulmonary diseases | |
| Aurinasal disease | |
| Symptoms of nasal congestion (cannot breathe in the nose) | |
| Suspected digestive organ diseases, liver diseases, renal diseases, cardiovascular diseases, blood diseases, endocrine diseases, or malignant neoplasms, or a history of any such conditions | |
| Depressive symptoms or a depression diagnosis | |
| Bronchial asthma | |
| Diffuse panbronchiolitis | |
| Congenital sinus bronchial syndrome | |
| Bronchiolitis obliterans | |
| Bronchial ectasia | |
| Lung tuberculosis | |
| Pneumoconiosis | |
| Pulmonary lymphangioleiomyomatosis | |
| Cold-like symptoms on the day of the examination | |
| Male (n = 14) | Female (n = 25) | |||
|---|---|---|---|---|
| Avg. | SD | Avg. | SD | |
| Age (years) | 69.9 | 2.85 | 68.6 | 8.93 |
| Height (cm) | 179.3 | 6.01 | 166.7 | 4.06 |
| Weight (kg) | 87.2 | 12.10 | 71.1 | 15.34 |
| Chest (cm) | 103.5 | 10.20 | 87.4 | 13.37 |
| Abdomen (cm) | 105.6 | 11.83 | 93.4 | 16.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imano, W.; Kameyama, K.; Hollingdal, M.; Refsgaard, J.; Larsen, K.; Topp, C.; Kronborg, S.H.; Gade, J.D.; Dinesen, B. Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People. Sensors 2020, 20, 6901. https://doi.org/10.3390/s20236901
Imano W, Kameyama K, Hollingdal M, Refsgaard J, Larsen K, Topp C, Kronborg SH, Gade JD, Dinesen B. Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People. Sensors. 2020; 20(23):6901. https://doi.org/10.3390/s20236901
Chicago/Turabian StyleImano, Wakana, Kenichi Kameyama, Malene Hollingdal, Jens Refsgaard, Knud Larsen, Cecilie Topp, Sissel Højsted Kronborg, Josefine Dam Gade, and Birthe Dinesen. 2020. "Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People" Sensors 20, no. 23: 6901. https://doi.org/10.3390/s20236901
APA StyleImano, W., Kameyama, K., Hollingdal, M., Refsgaard, J., Larsen, K., Topp, C., Kronborg, S. H., Gade, J. D., & Dinesen, B. (2020). Non-Contact Respiratory Measurement Using a Depth Camera for Elderly People. Sensors, 20(23), 6901. https://doi.org/10.3390/s20236901






