Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
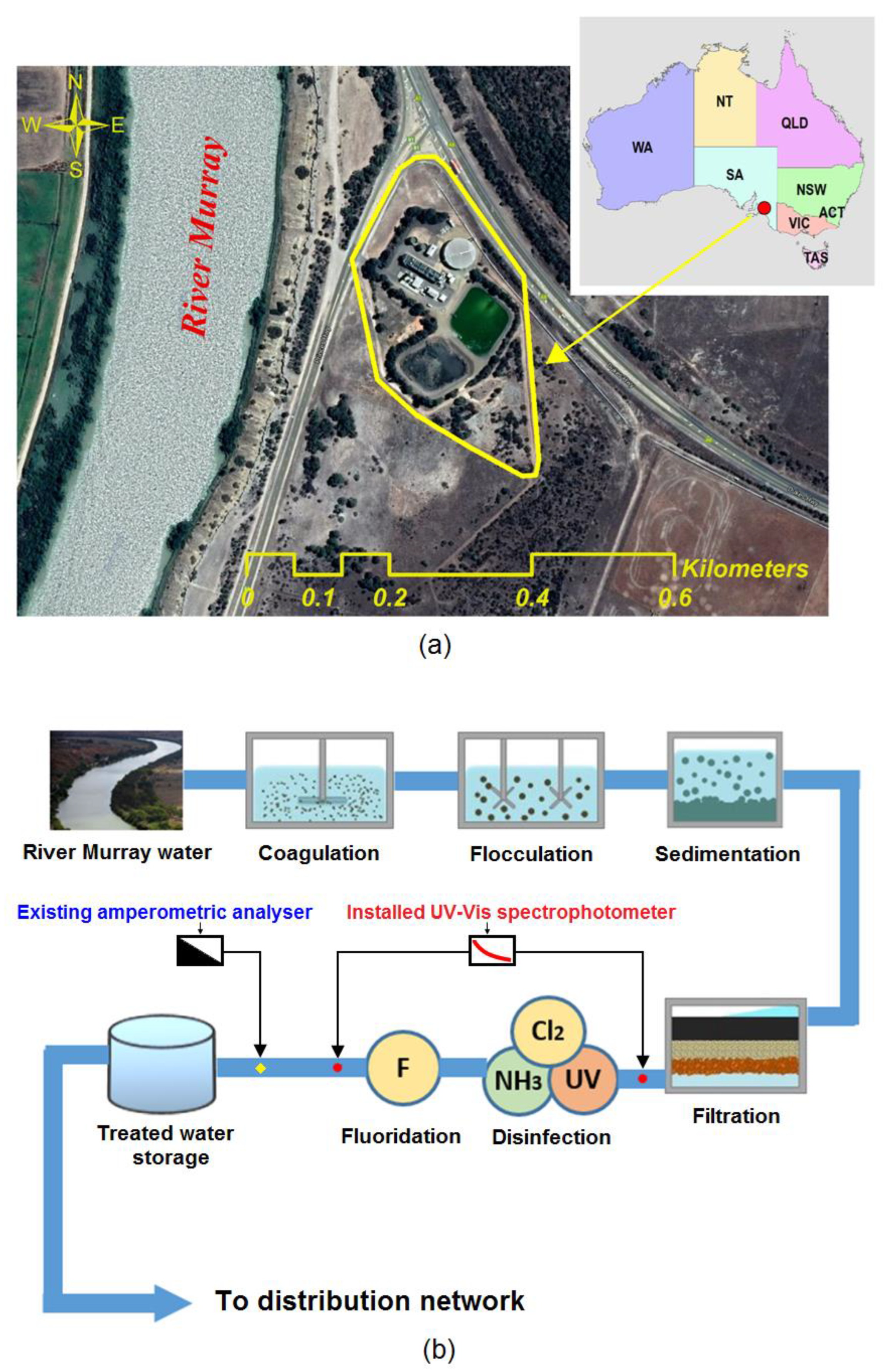
2.1. Study Area
2.2. UV-Vis Spectrophotometric Device
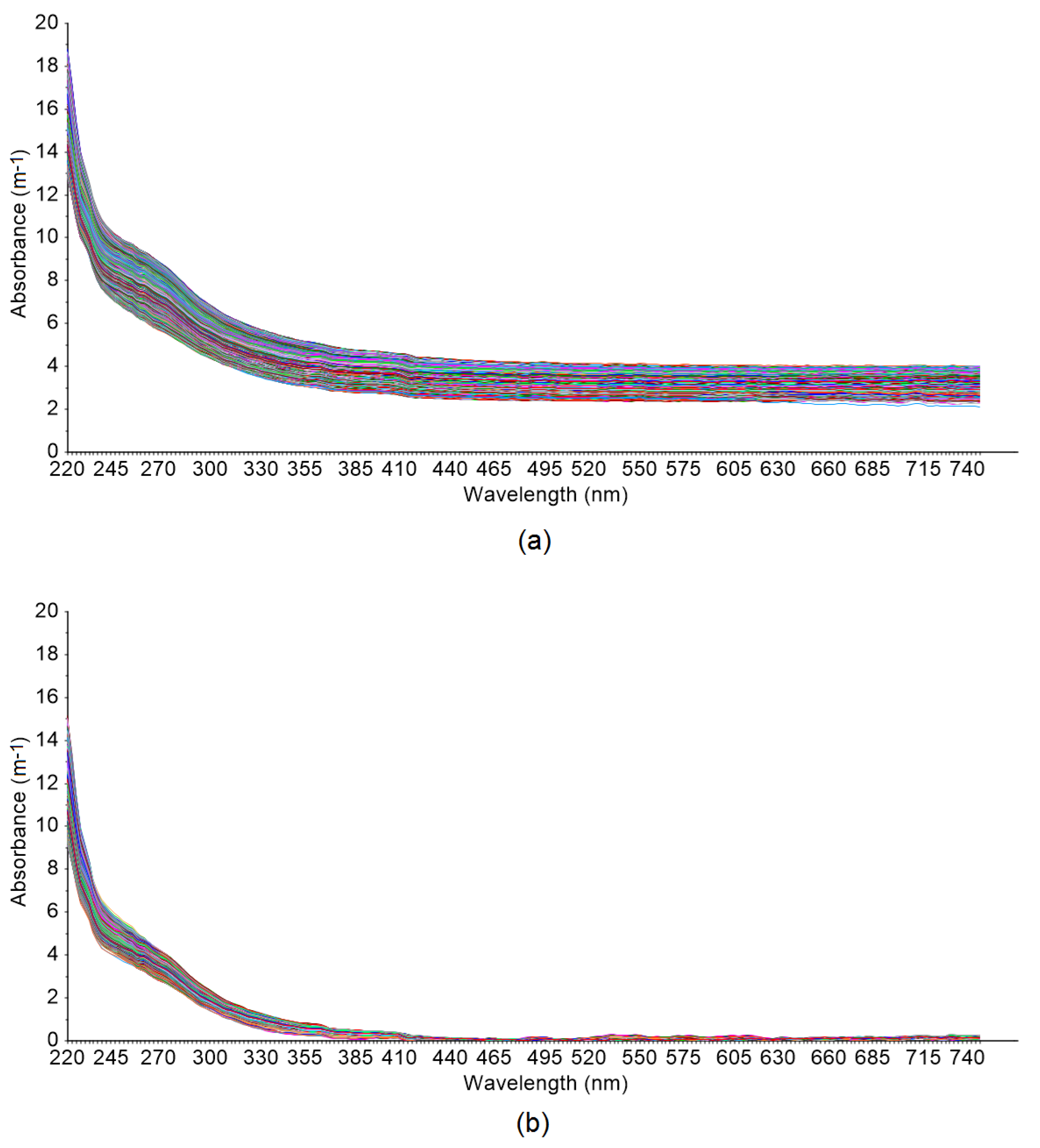
2.3. Particle Interference on UV-Vis Spectrum and Compensation
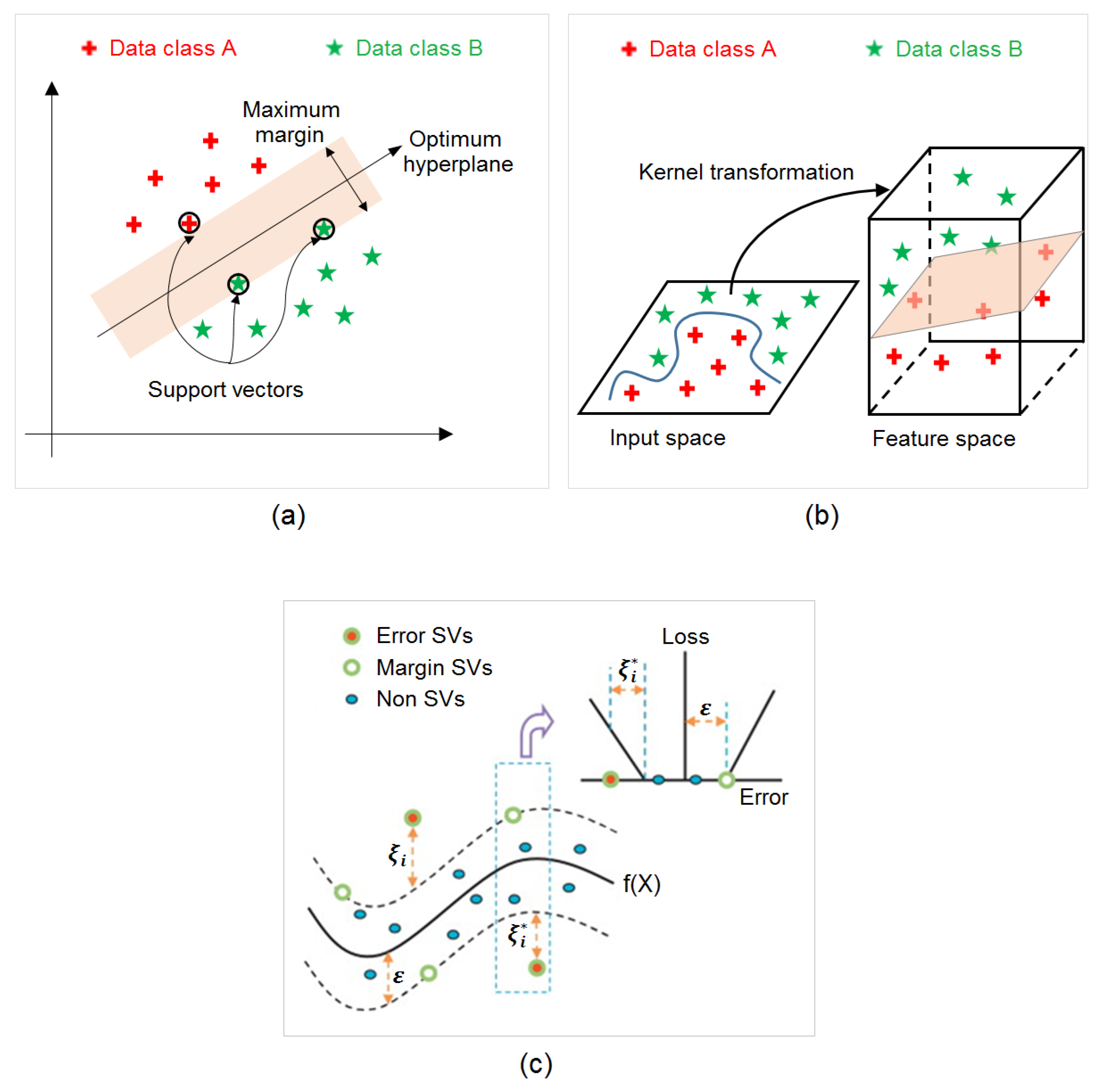
2.4. Support Vector Regression
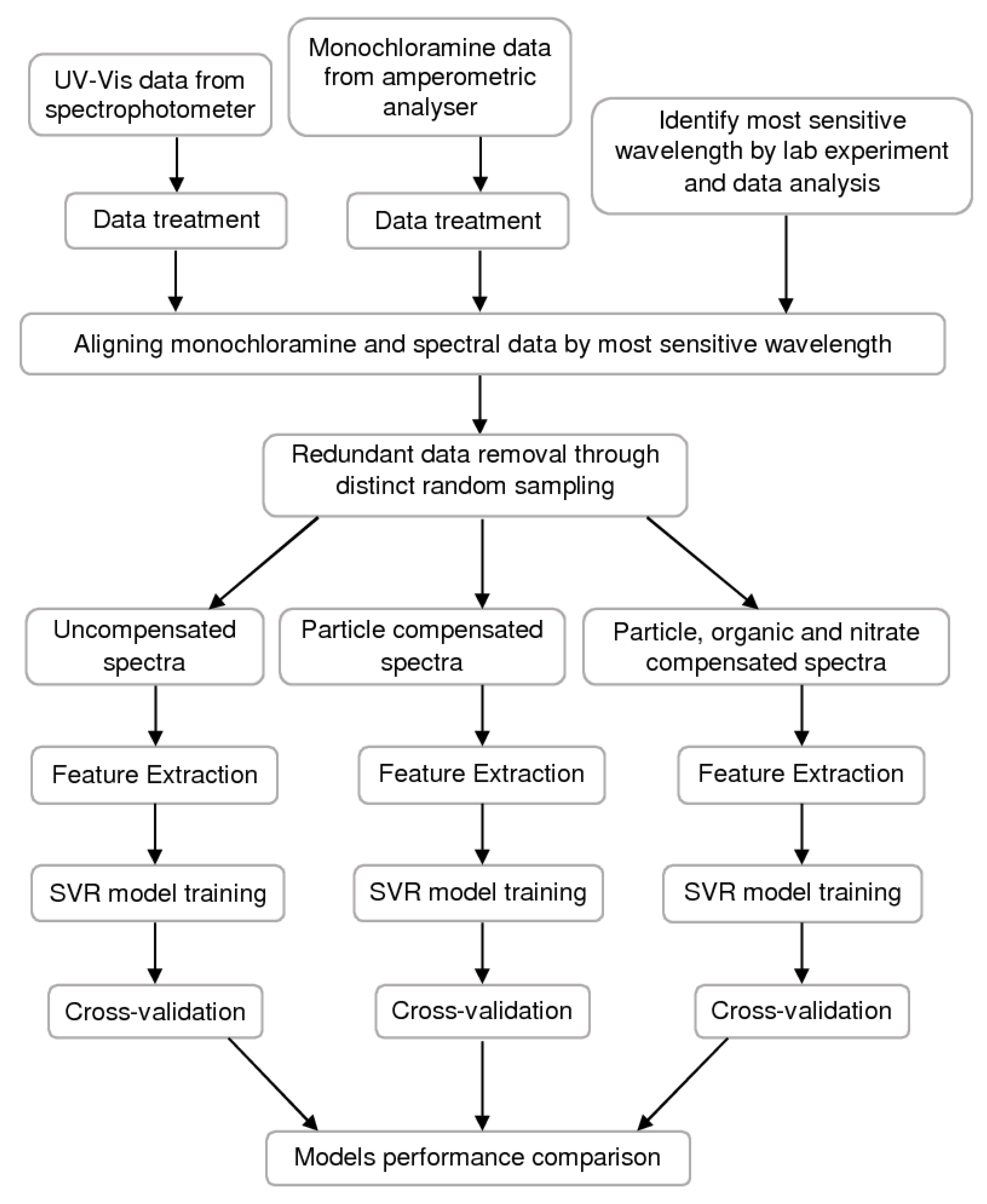
2.5. Methodology
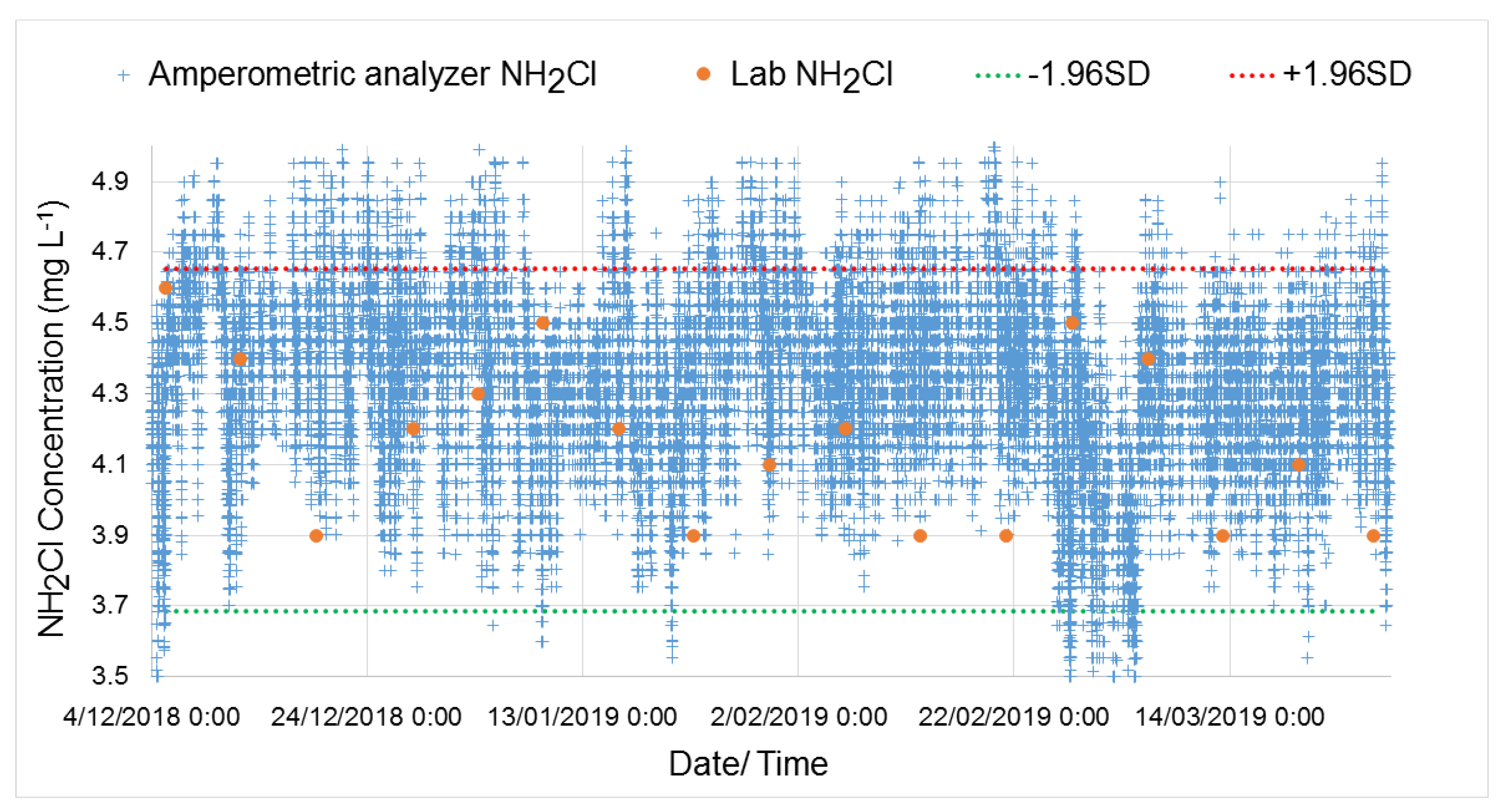
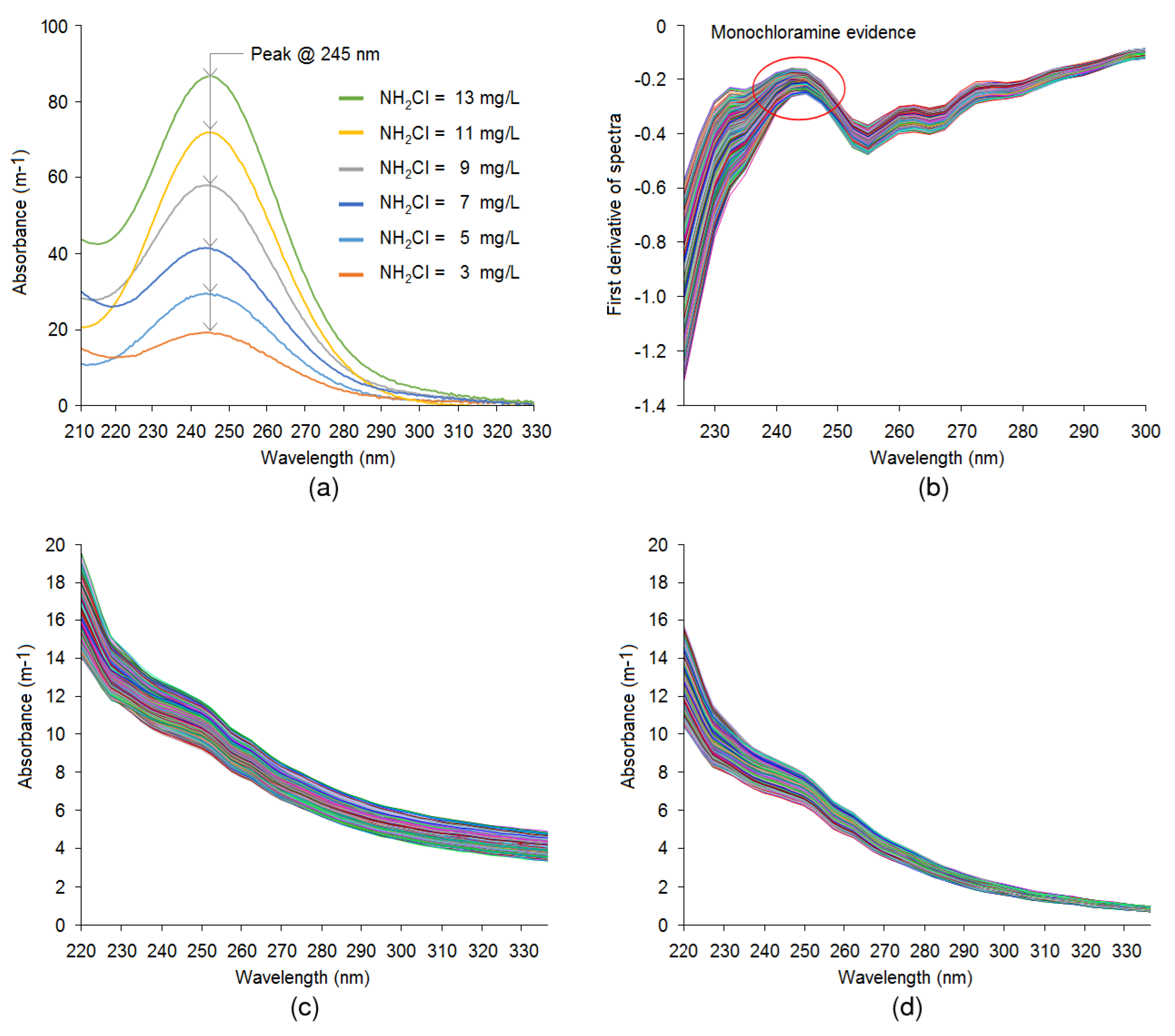
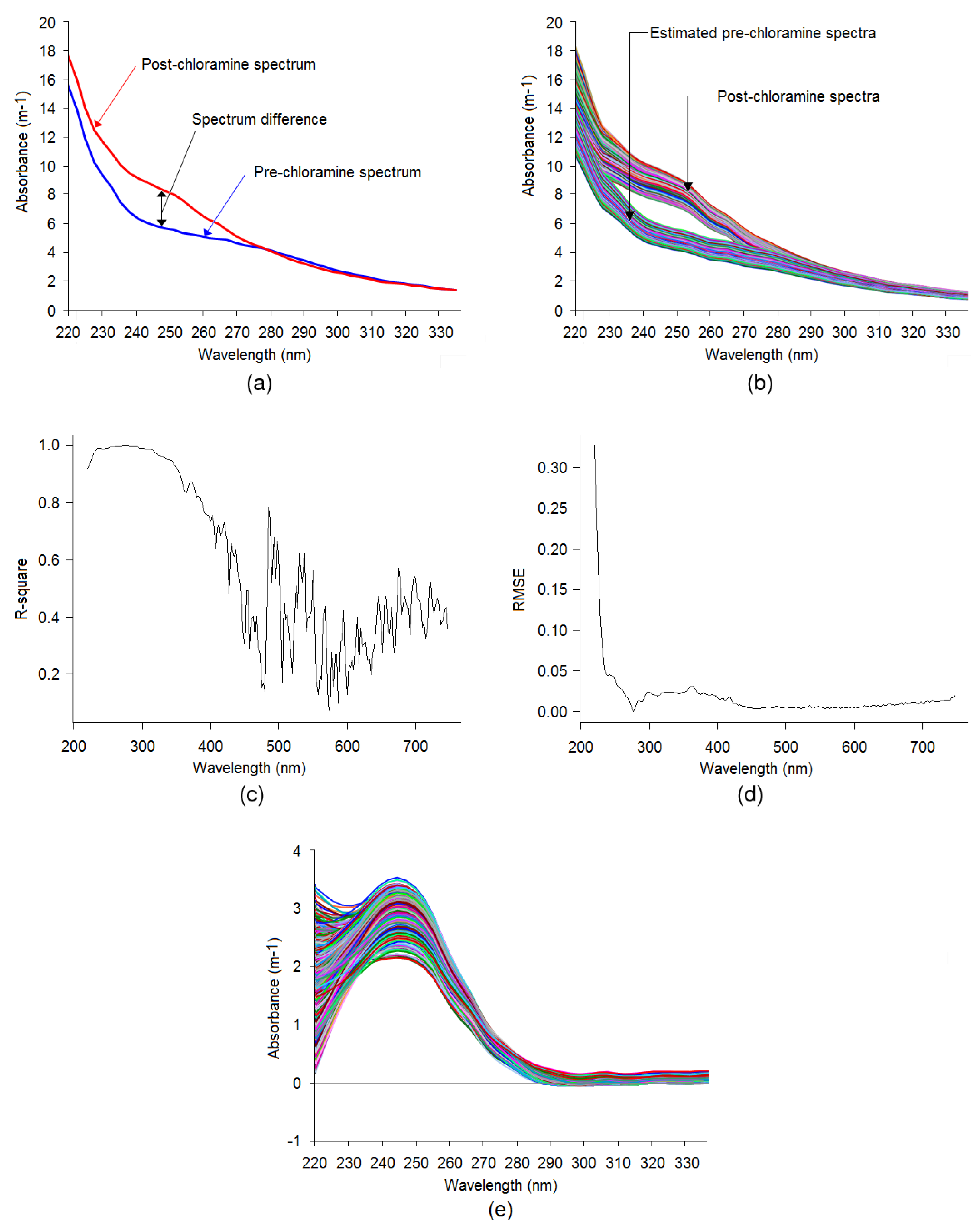
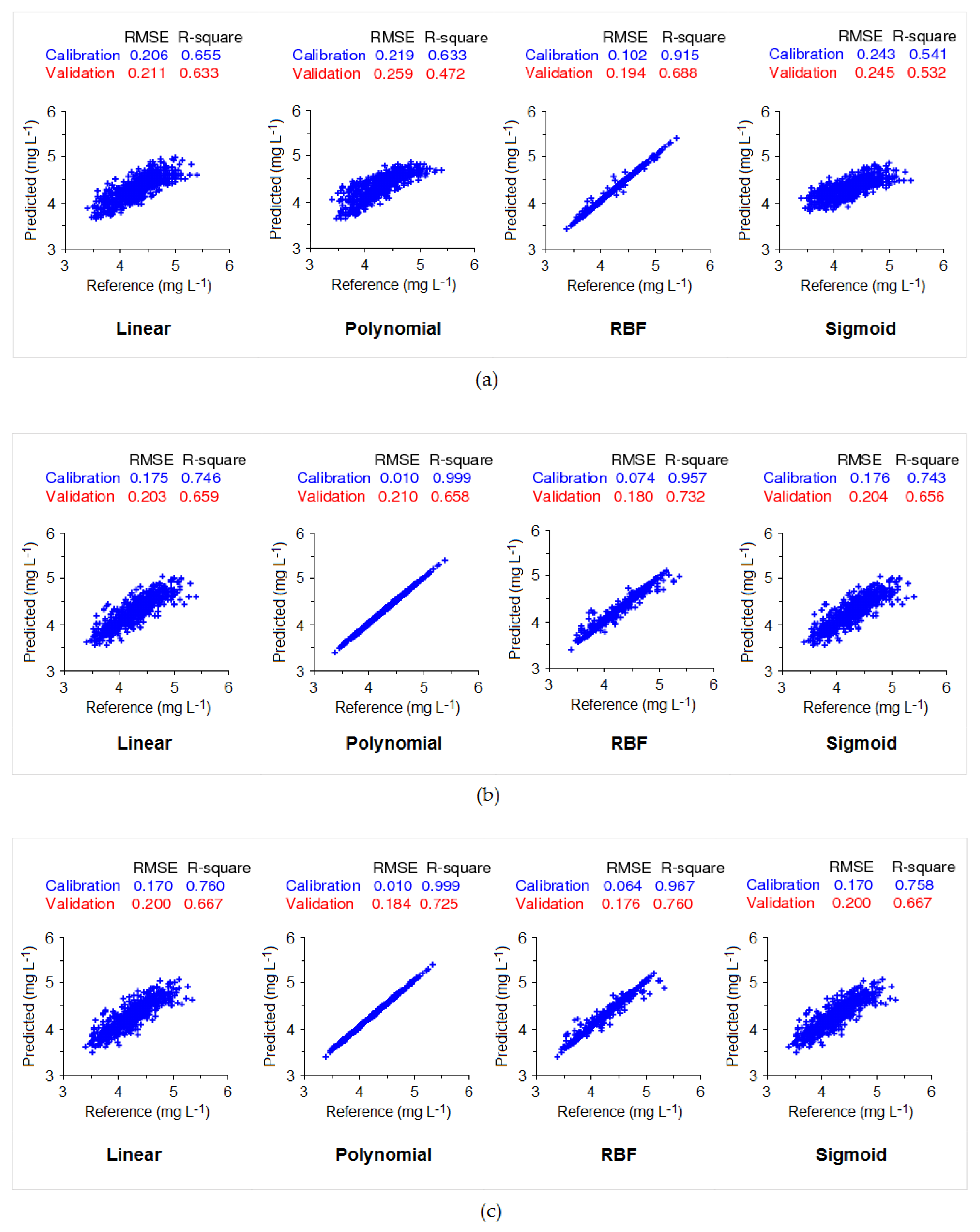
3. Results
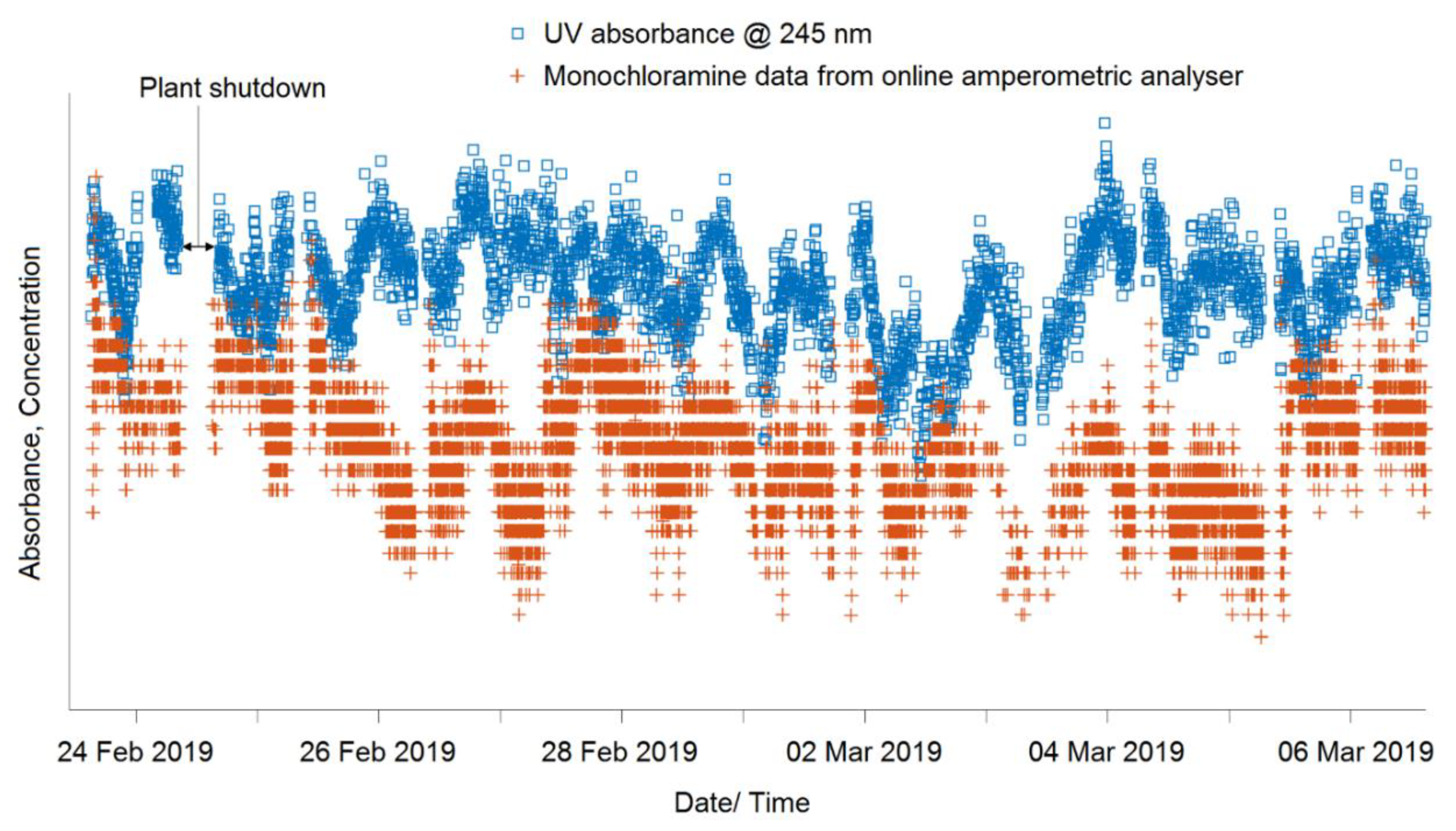
3.1. Monochloramine Peak Absorbance Wavelength Detection and Particle Compensation
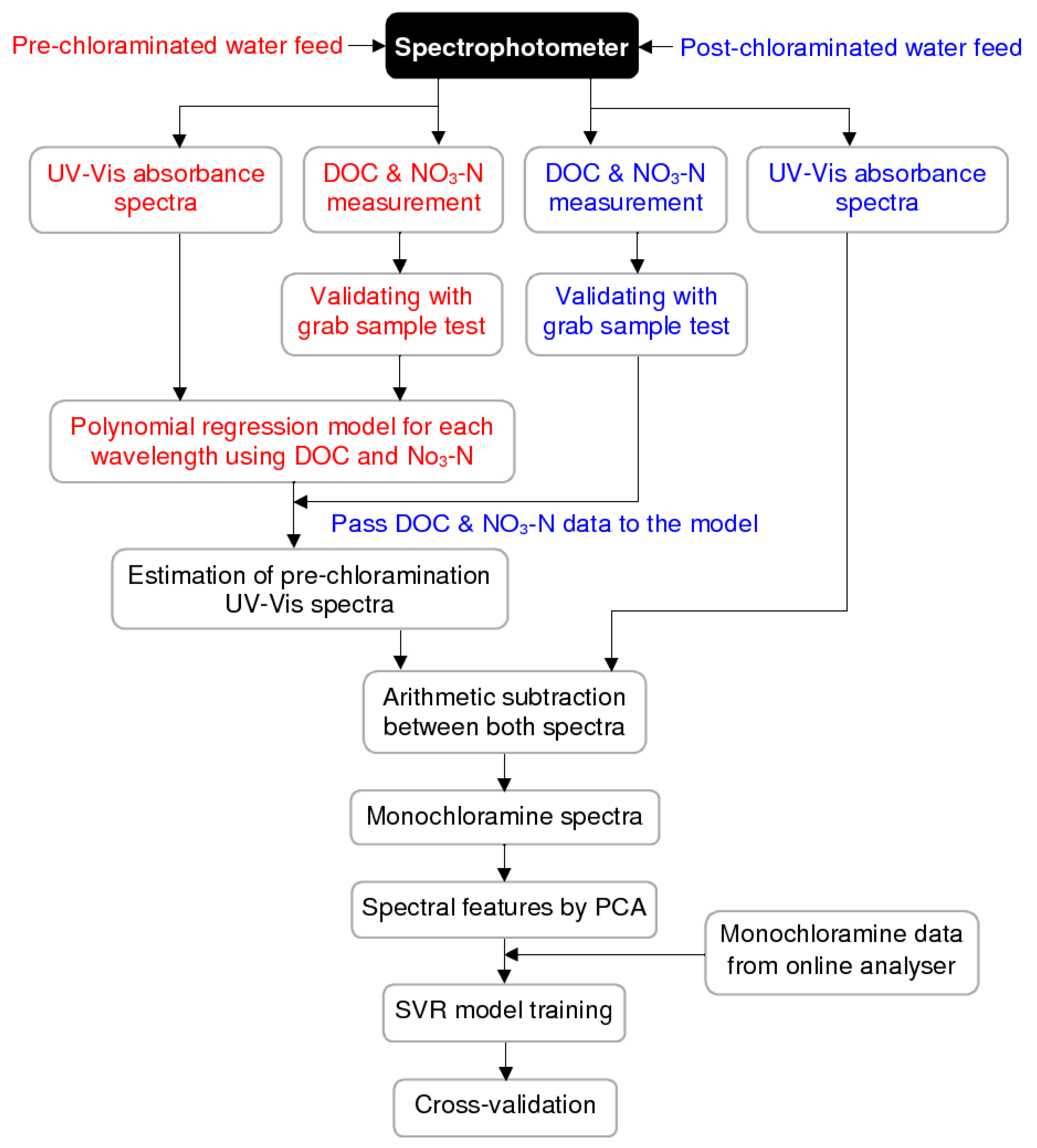
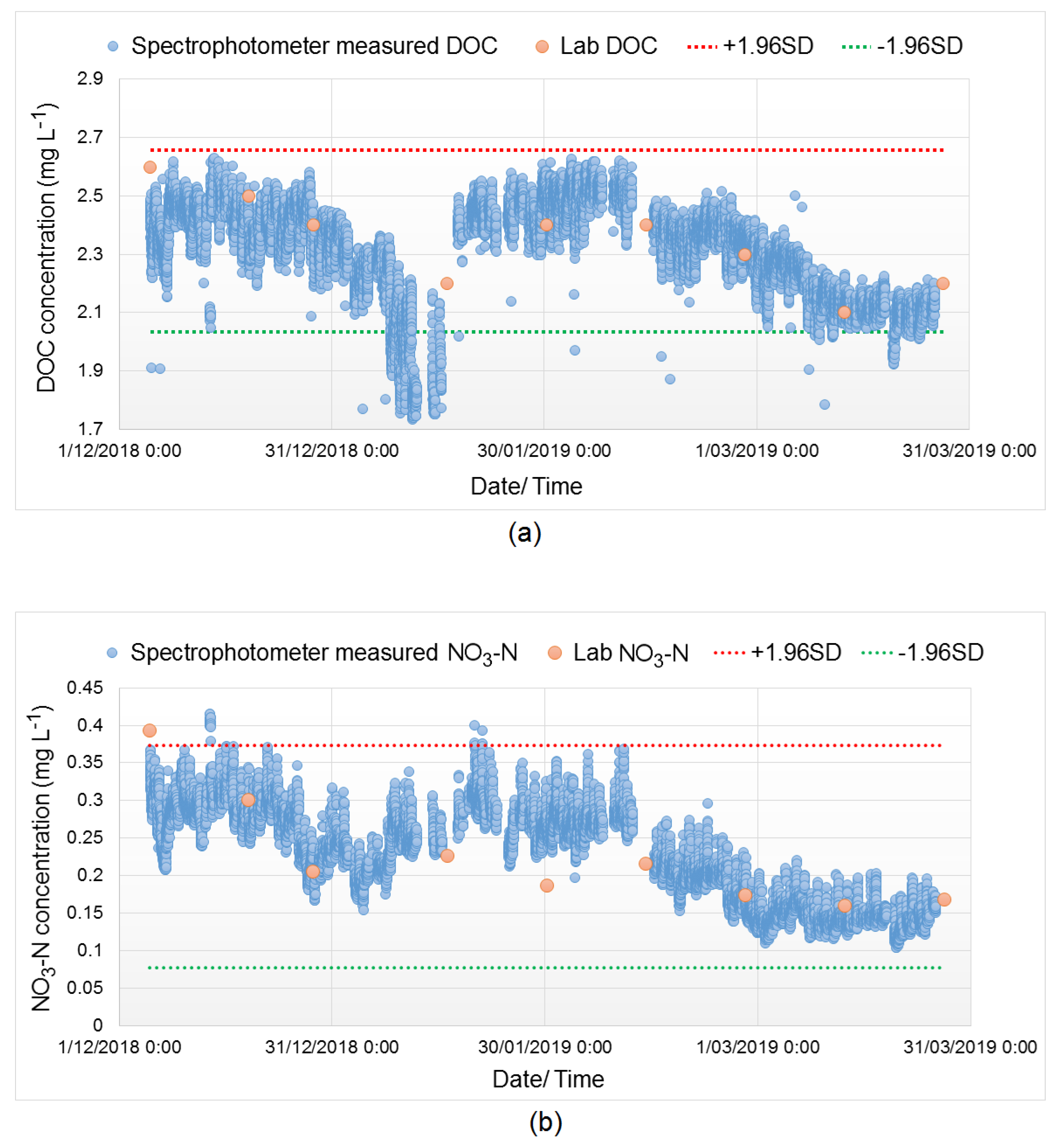
3.2. Spectral Compensation for Organic and Nitrate
3.3. SVR Model Fitting
4. Discussion
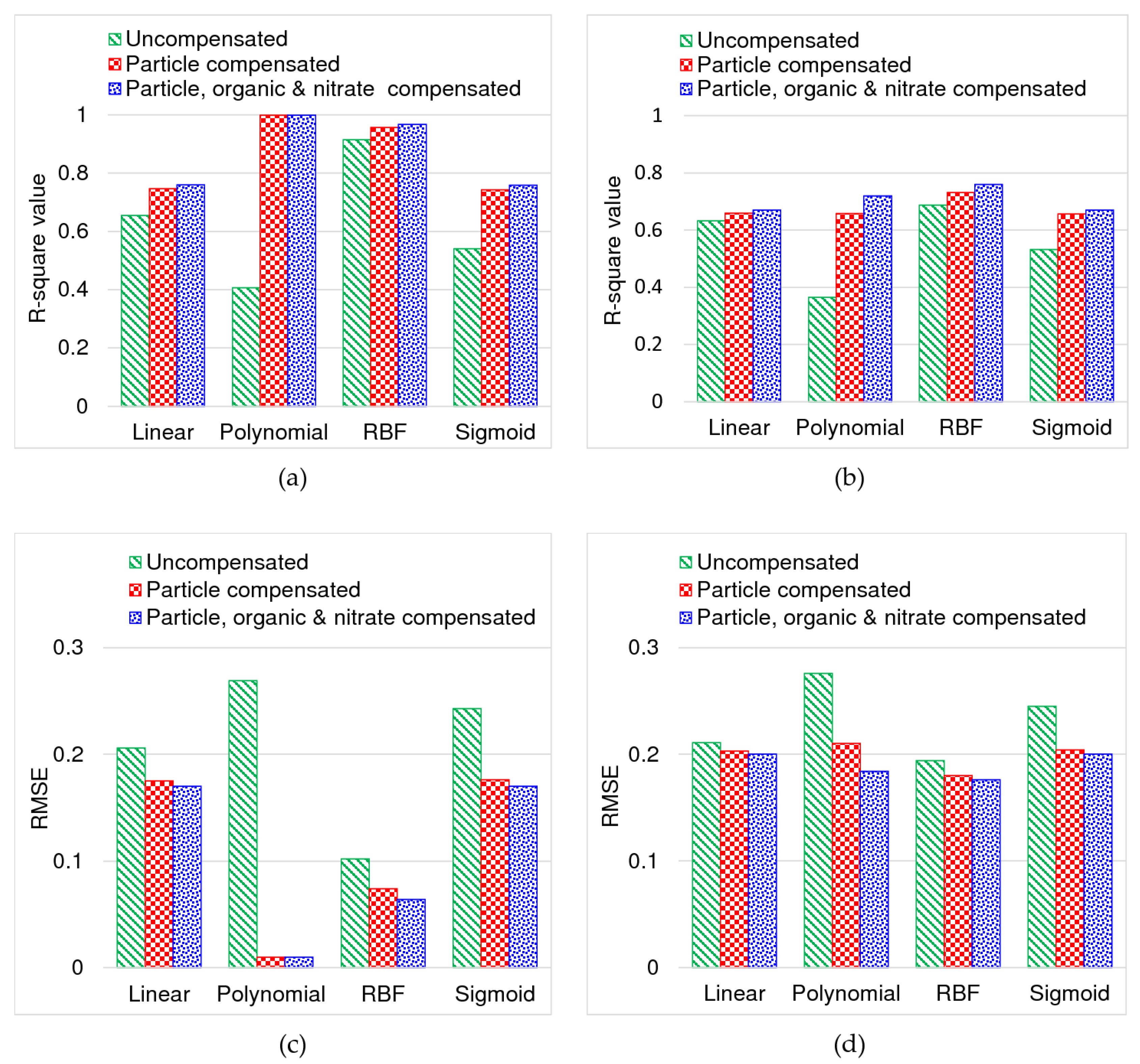
4.1. Comparison of Model Performance
4.2. Limitations of the Research
5. Conclusions
- Machine learning with UV-Vis spectrometry can be used in online detection of monochloramine residual;
- The choice of the kernel function has a high impact in modelling performance, particularly, RBF kernel has better accuracy for non-linear mapping of spectral data; and
- Particle compensation and the newly introduced organic and nitrate compensation improves modelling accuracy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Wavelength (nm) | R-Square | RMSE | Wavelength (nm) | R-Square | RMSE | Wavelength (nm) | R-Square | RMSE |
|---|---|---|---|---|---|---|---|---|
| 220 | 0.9164 | 0.3275 | 397.5 | 0.7522 | 0.0186 | 575 | 0.0707 | 0.0048 |
| 222.5 | 0.9284 | 0.2633 | 400 | 0.7359 | 0.0173 | 577.5 | 0.2760 | 0.0054 |
| 225 | 0.9487 | 0.1831 | 402.5 | 0.7541 | 0.0156 | 580 | 0.1558 | 0.0041 |
| 227.5 | 0.9661 | 0.1244 | 405 | 0.7327 | 0.0150 | 582.5 | 0.2685 | 0.0051 |
| 230 | 0.9735 | 0.0976 | 407.5 | 0.6383 | 0.0167 | 585 | 0.2684 | 0.0056 |
| 232.5 | 0.9819 | 0.0720 | 410 | 0.7127 | 0.0142 | 587.5 | 0.1004 | 0.0052 |
| 235 | 0.9887 | 0.0516 | 412.5 | 0.7237 | 0.0144 | 590 | 0.3123 | 0.0052 |
| 237.5 | 0.9900 | 0.0452 | 415 | 0.6850 | 0.0158 | 592.5 | 0.3177 | 0.0058 |
| 240 | 0.9892 | 0.0446 | 417.5 | 0.7042 | 0.0182 | 595 | 0.4229 | 0.0052 |
| 242.5 | 0.9881 | 0.0449 | 420 | 0.7294 | 0.0148 | 597.5 | 0.2581 | 0.0054 |
| 245 | 0.9882 | 0.0440 | 422.5 | 0.6889 | 0.0109 | 600 | 0.1275 | 0.0049 |
| 247.5 | 0.9889 | 0.0423 | 425 | 0.6698 | 0.0104 | 602.5 | 0.2366 | 0.0054 |
| 250 | 0.9904 | 0.0396 | 427.5 | 0.4827 | 0.0106 | 605 | 0.2209 | 0.0045 |
| 252.5 | 0.9932 | 0.0339 | 430 | 0.6553 | 0.0077 | 607.5 | 0.2487 | 0.0052 |
| 255 | 0.9947 | 0.0303 | 432.5 | 0.6268 | 0.0089 | 610 | 0.2179 | 0.0059 |
| 257.5 | 0.9954 | 0.0284 | 435 | 0.6094 | 0.0080 | 612.5 | 0.3086 | 0.0059 |
| 260 | 0.9960 | 0.0268 | 437.5 | 0.6328 | 0.0066 | 615 | 0.3980 | 0.0073 |
| 262.5 | 0.9966 | 0.0250 | 440 | 0.5436 | 0.0064 | 617.5 | 0.2363 | 0.0056 |
| 265 | 0.9976 | 0.0212 | 442.5 | 0.5335 | 0.0054 | 620 | 0.3605 | 0.0063 |
| 267.5 | 0.9985 | 0.0168 | 445 | 0.4764 | 0.0044 | 622.5 | 0.2977 | 0.0056 |
| 270 | 0.9989 | 0.0141 | 447.5 | 0.3530 | 0.0050 | 625 | 0.3081 | 0.0063 |
| 272.5 | 0.9995 | 0.0097 | 450 | 0.2955 | 0.0053 | 627.5 | 0.3099 | 0.0075 |
| 275 | 0.9998 | 0.0052 | 452.5 | 0.4921 | 0.0039 | 630 | 0.2489 | 0.0074 |
| 277.5 | 1.0000 | 0.0004 | 455 | 0.4920 | 0.0041 | 632.5 | 0.2515 | 0.0071 |
| 280 | 0.9997 | 0.0065 | 457.5 | 0.2891 | 0.0039 | 635 | 0.1987 | 0.0081 |
| 282.5 | 0.9988 | 0.0123 | 460 | 0.3920 | 0.0037 | 637.5 | 0.2724 | 0.0068 |
| 285 | 0.9983 | 0.0139 | 462.5 | 0.4002 | 0.0043 | 640 | 0.2909 | 0.0066 |
| 287.5 | 0.9984 | 0.0126 | 465 | 0.3283 | 0.0047 | 642.5 | 0.3857 | 0.0085 |
| 290 | 0.9982 | 0.0124 | 467.5 | 0.4024 | 0.0050 | 645 | 0.4697 | 0.0079 |
| 292.5 | 0.9966 | 0.0157 | 470 | 0.2940 | 0.0049 | 647.5 | 0.4383 | 0.0077 |
| 295 | 0.9930 | 0.0210 | 472.5 | 0.2715 | 0.0054 | 650 | 0.3744 | 0.0094 |
| 297.5 | 0.9896 | 0.0240 | 475 | 0.1537 | 0.0058 | 652.5 | 0.2757 | 0.0100 |
| 300 | 0.9883 | 0.0238 | 477.5 | 0.1678 | 0.0062 | 655 | 0.4766 | 0.0079 |
| 302.5 | 0.9883 | 0.0225 | 480 | 0.1382 | 0.0052 | 657.5 | 0.4586 | 0.0085 |
| 305 | 0.9883 | 0.0211 | 482.5 | 0.4923 | 0.0052 | 660 | 0.3474 | 0.0079 |
| 307.5 | 0.9873 | 0.0206 | 485 | 0.7846 | 0.0065 | 662.5 | 0.3433 | 0.0088 |
| 310 | 0.9862 | 0.0202 | 487.5 | 0.7375 | 0.0065 | 665 | 0.4337 | 0.0089 |
| 312.5 | 0.9858 | 0.0192 | 490 | 0.5191 | 0.0057 | 667.5 | 0.2991 | 0.0097 |
| 315 | 0.9836 | 0.0197 | 492.5 | 0.6808 | 0.0050 | 670 | 0.2686 | 0.0117 |
| 317.5 | 0.9788 | 0.0216 | 495 | 0.5347 | 0.0048 | 672.5 | 0.3862 | 0.0086 |
| 320 | 0.9735 | 0.0231 | 497.5 | 0.6630 | 0.0047 | 675 | 0.5706 | 0.0111 |
| 322.5 | 0.9686 | 0.0240 | 500 | 0.6226 | 0.0058 | 677.5 | 0.5329 | 0.0099 |
| 325 | 0.9663 | 0.0237 | 502.5 | 0.4703 | 0.0057 | 680 | 0.4096 | 0.0102 |
| 327.5 | 0.9629 | 0.0238 | 505 | 0.1710 | 0.0048 | 682.5 | 0.4444 | 0.0106 |
| 330 | 0.9591 | 0.0240 | 507.5 | 0.4694 | 0.0044 | 685 | 0.4717 | 0.0113 |
| 332.5 | 0.9573 | 0.0233 | 510 | 0.3895 | 0.0050 | 687.5 | 0.4326 | 0.0114 |
| 335 | 0.9540 | 0.0232 | 512.5 | 0.4054 | 0.0049 | 690 | 0.4302 | 0.0113 |
| 337.5 | 0.9508 | 0.0229 | 515 | 0.3332 | 0.0060 | 692.5 | 0.3367 | 0.0107 |
| 340 | 0.9484 | 0.0223 | 517.5 | 0.2726 | 0.0051 | 695 | 0.4827 | 0.0100 |
| 342.5 | 0.9475 | 0.0216 | 520 | 0.2026 | 0.0045 | 697.5 | 0.5418 | 0.0121 |
| 345 | 0.9425 | 0.0217 | 522.5 | 0.3525 | 0.0040 | 700 | 0.5341 | 0.0108 |
| 347.5 | 0.9301 | 0.0232 | 525 | 0.5074 | 0.0047 | 702.5 | 0.4690 | 0.0099 |
| 350 | 0.9232 | 0.0234 | 527.5 | 0.4303 | 0.0049 | 705 | 0.4626 | 0.0120 |
| 352.5 | 0.9174 | 0.0236 | 530 | 0.6237 | 0.0053 | 707.5 | 0.4440 | 0.0133 |
| 355 | 0.9025 | 0.0253 | 532.5 | 0.5686 | 0.0061 | 710 | 0.3627 | 0.0114 |
| 357.5 | 0.8788 | 0.0281 | 535 | 0.5214 | 0.0073 | 712.5 | 0.3789 | 0.0126 |
| 360 | 0.8600 | 0.0301 | 537.5 | 0.6233 | 0.0051 | 715 | 0.3239 | 0.0143 |
| 362.5 | 0.8398 | 0.0315 | 540 | 0.3470 | 0.0053 | 717.5 | 0.3650 | 0.0122 |
| 365 | 0.8335 | 0.0304 | 542.5 | 0.3967 | 0.0046 | 720 | 0.4993 | 0.0127 |
| 367.5 | 0.8570 | 0.0266 | 545 | 0.3976 | 0.0058 | 722.5 | 0.5222 | 0.0124 |
| 370 | 0.8721 | 0.0234 | 547.5 | 0.4221 | 0.0063 | 725 | 0.4412 | 0.0124 |
| 372.5 | 0.8718 | 0.0214 | 550 | 0.5608 | 0.0056 | 727.5 | 0.4156 | 0.0129 |
| 375 | 0.8614 | 0.0209 | 552.5 | 0.3943 | 0.0058 | 730 | 0.4511 | 0.0135 |
| 377.5 | 0.8361 | 0.0221 | 555 | 0.1816 | 0.0048 | 732.5 | 0.4651 | 0.0140 |
| 380 | 0.8178 | 0.0226 | 557.5 | 0.1285 | 0.0042 | 735 | 0.4389 | 0.0143 |
| 382.5 | 0.8214 | 0.0216 | 560 | 0.1987 | 0.0045 | 737.5 | 0.3717 | 0.0140 |
| 385 | 0.8174 | 0.0204 | 562.5 | 0.1783 | 0.0044 | 740 | 0.3918 | 0.0147 |
| 387.5 | 0.7958 | 0.0200 | 565 | 0.3906 | 0.0042 | 742.5 | 0.4228 | 0.0175 |
| 390 | 0.7743 | 0.0203 | 567.5 | 0.4372 | 0.0055 | 745 | 0.4344 | 0.0169 |
| 392.5 | 0.7586 | 0.0208 | 570 | 0.2402 | 0.0047 | 747.5 | 0.3577 | 0.0193 |
| 395 | 0.7567 | 0.0202 | 572.5 | 0.0906 | 0.0045 | - |
| Uncompensated or Raw Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.206 | 0.655 |
| Polynomial kernel | 0.219 | 0.633 |
| RBF kernel | 0.028 | 0.994 |
| Sigmoid kernel | 0.243 | 0.541 |
| Particle Compensated Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.175 | 0.746 |
| Polynomial kernel | 0.010 | 0.999 |
| RBF kernel | 0.074 | 0.957 |
| Sigmoid kernel | 0.176 | 0.743 |
| Particle, DOC and NO3-N Compensated Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.170 | 0.760 |
| Polynomial kernel | 0.010 | 0.999 |
| RBF kernel | 0.064 | 0.967 |
| Sigmoid kernel | 0.170 | 0.758 |
| Uncompensated or Raw Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.211 | 0.633 |
| Polynomial kernel | 0.259 | 0.472 |
| RBF kernel | 0.199 | 0.680 |
| Sigmoid kernel | 0.245 | 0.532 |
| Particle Compensated Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.203 | 0.659 |
| Polynomial kernel | 0.210 | 0.658 |
| RBF kernel | 0.180 | 0.732 |
| Sigmoid kernel | 0.204 | 0.656 |
| Particle, DOC and NO3-N Compensated Spectra | ||
| Kernel Function Type | RMSE | R-square |
| Linear kernel | 0.200 | 0.670 |
| Polynomial kernel | 0.184 | 0.720 |
| RBF kernel | 0.176 | 0.760 |
| Sigmoid kernel | 0.200 | 0.670 |
References
- Gray, N.F. Chapter Thirty-One-Free and Combined Chlorine. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 571–590. [Google Scholar]
- Kirmeyer, G.J.; Martel, K.; Thompson, G.; Radder, L.; Klement, W.; LeChevallier, M.; Baribeau, H.; Flores, A. Optimizing Chloramine Treatment; American Water Works Association: Denver, CO, USA, 2004. [Google Scholar]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, K.M. (Eds.) CHAPTER 11-Disinfection of Water. In Water Supply, 6th ed.; Butterworth-Heinemann: Boston, MA, USA, 2009; pp. 425–461. [Google Scholar]
- Wolfe, R.L.; Ward, N.R.; Olson, B.H. Inorganic Chloramines as Drinking Water Disinfectants: A Review. J. Am. Water Works Assoc. 1984, 76, 74–88. [Google Scholar] [CrossRef]
- NHMRC; NRMMC. Australian Drinking Water Guidelines 6: National Water Quality Management Strategy; National Health and Medical Research Council; National Resource Management Ministerial Council: Canberra, Australia, 2011.
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017; p. 1. [Google Scholar]
- Malcov, V.B.; Zachman, B.; Scribner, T. Comparison of On-Line Chlorine Analysis Methods and Instrumentation on Amperometric and Colorimetric Technologies; American Water Works Association: Denver, CO, USA, 2009. [Google Scholar]
- Dibo, H.; Liu, S.; Zhang, J.; Chen, F.; Huang, P.; Zhang, G. Online Monitoring of Water-Quality Anomaly in Water Distribution Systems Based on Probabilistic Principal Component Analysis by UV-Vis Absorption Spectroscopy. J. Spectrosc. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Altmann, J.; Massa, L.; Sperlich, A.; Gnirss, R.; Jekel, M. UV254 absorbance as real-time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res. 2016, 94, 240–245. [Google Scholar] [CrossRef]
- Du, S.; Xiaoli, W.; Tiejun, W. Support vector machine for ultraviolet spectroscopic water quality analyzers. Chin. J. Anal. Chem. 2004, 32, 1227–1230. [Google Scholar]
- Rieger, L.; Langergraber, G.; Thomann, M.; Fleischmann, N.; Siegrist, H. Spectral in-situ analysis of NO2, NO3, COD, DOC and TSS in the effluent of a WWTP. Water Sci. Technol. 2004, 50, 143–152. [Google Scholar] [CrossRef]
- Roccaro, P.; Yan, M.; Korshin, G.V. Use of log-transformed absorbance spectra for online monitoring of the reactivity of natural organic matter. Water Res. 2015, 84, 136–143. [Google Scholar] [CrossRef]
- Wang, D.; Sowlat, M.H.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Development and evaluation of a novel monitor for online measurement of iron, manganese, and chromium in ambient particulate matter (PM). Sci. Total Environ. 2016, 565, 123–131. [Google Scholar] [CrossRef]
- Carreres-Prieto, D.; García, J.T.; Cerdán-Cartagena, F.; Suardiaz-Muro, J. Wastewater Quality Estimation Through Spectrophotometry-Based Statistical Models. Sensors 2020, 20, 5631. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Revealing the mechanism of indirect ammonia electrooxidation. Electrochim. Acta 2012, 63, 209–219. [Google Scholar] [CrossRef]
- Li, J.; Blatchley Iii, E.R. UV Photodegradation of Inorganic Chloramines. Environ. Sci. Technol. 2009, 43, 60–65. [Google Scholar] [CrossRef]
- Poskrebyshev, G.A.; Huie, R.E.; Neta, P. Radiolytic Reactions of Monochloramine in Aqueous Solutions. J. Phys. Chem. A 2003, 107, 7423–7428. [Google Scholar] [CrossRef]
- Ferriol, M.; Gazet, J.; Rizk-Ouaini, R. Ultraviolet absorption spectra of some alkylchloramines. Anal. Chim. Acta 1990, 231, 161–163. [Google Scholar] [CrossRef]
- Thomas, O.; Causse, V. Chapter 2 From spectra to qualitative and quantitative results. In UV-Visible Spectrophotometry of Water and Wastewater, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 27, pp. 21–45. [Google Scholar]
- Alves, E.M.; Rodrigues, R.J.; dos Santos Corrêa, C.; Fidemann, T.; Rocha, J.C.; Buzzo, J.L.L.; de Oliva Neto, P.; Núñez, E.G.F. Use of ultraviolet–visible spectrophotometry associated with artificial neural networks as an alternative for determining the water quality index. Environ. Monit. Assess. 2018, 190, 319. [Google Scholar] [CrossRef] [PubMed]
- Carré, E.; Pérot, J.; Jauzein, V.; Lin, L.; Lopez-Ferber, M. Estimation of water quality by UV/Vis spectrometry in the framework of treated wastewater reuse. Water Sci. Technol. 2017, 76, 633–641. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Ai, W.; Lin, B.; Feng, Q.; Cai, K. Kernel functions embedded in support vector machine learning models for rapid water pollution assessment via near-infrared spectroscopy. Sci. Total Environ. 2020, 714, 136765. [Google Scholar] [CrossRef]
- Wolf, C.; Gaida, D.; Stuhlsatz, A.; Ludwig, T.; McLoone, S.; Bongards, M. Predicting organic acid concentration from UV/vis spectrometry measurements–a comparison of machine learning techniques. Trans. Inst. Meas. Control 2011, 35, 5–15. [Google Scholar] [CrossRef]
- Kim, C.; Eom, J.B.; Jung, S.; Ji, T. Detection of Organic Compounds in Water by an Optical Absorbance Method. Sensors 2016, 16, 61. [Google Scholar] [CrossRef]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Edwards, A.C.; Hooda, P.S.; Cook, Y. Determination of Nitrate in Water Containing Dissolved Organic Carbon by Ultraviolet Spectroscopy. Int. J. Environ. Anal. Chem. 2001, 80, 49–59. [Google Scholar] [CrossRef]
- Huber, E.; Frost, M. Light scattering by small particles. Aqua 1998, 47, 87–94. [Google Scholar] [CrossRef]
- Tang, B.; Wei, B.; Wu, D.-C.; Mi, D.; Zhao, J.-X.; Feng, P.; Jiang, S.-H.; Mao, B.-J. Experimental research of turbidity influence on water quality monitoring of COD in UV-visible spectroscopy. Spectrosc. Spectr. Anal. 2014, 34, 3020–3024. [Google Scholar]
- Wu, X.; Tong, R.; Wang, Y.; Mei, C.; Li, Q. Study on an online detection method for ground water quality and instrument design. Sensors 2019, 19, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wen, Y.; Wang, X. Novel method of turbidity compensation for chemical oxygen demand measurements by using UV–vis spectrometry. Sens. Actuators B Chem. 2016, 227, 393–398. [Google Scholar] [CrossRef]
- Boser, B.E.; Guyon, I.M.; Vapnik, V.N. A training algorithm for optimal margin classifiers. In Proceedings of the Fifth Annual Workshop on Computational Learning Theory, Pittsburgh, PA, USA, 27–29 July 1992; Association for Computing Machinery: New York, NY, USA; pp. 144–152. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Vapnik, V.; Golowich, S.E.; Smola, A. Support Vector Method for Function Approximation, Regression Estimation, and Signal Processing. Adv. Neural Inf. Process. Syst. 1996, 9, 281–287. [Google Scholar]
- Basak, D.; Pal, S.; Patranabis, D.C. Support Vector Regression. Neural Inf. Process. Lett. Rev. 2007, 11, 203–224. [Google Scholar]
- Liao, Y.; Xu, J.; Wang, W. A Method of Water Quality Assessment Based on Biomonitoring and Multiclass Support Vector Machine. Procedia Environ. Sci. 2011, 10, 451–457. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Deka, P.C. Support vector machine applications in the field of hydrology: A review. Appl. Soft Comput. 2014, 19, 372–386. [Google Scholar] [CrossRef]
- Nanda, M.A.; Seminar, K.B.; Nandika, D.; Maddu, A. A Comparison Study of Kernel Functions in the Support Vector Machine and Its Application for Termite Detection. Information 2018, 9, 5. [Google Scholar] [CrossRef]
- Granata, F.; Gargano, R.; De Marinis, G. Support Vector Regression for Rainfall-Runoff Modeling in Urban Drainage: A Comparison with the EPA’s Storm Water Management Model. Water 2016, 8, 69. [Google Scholar] [CrossRef]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef]
- Karush, W. Minima of Functions of Several Variables with Inequalitiesas Side Constraints. Master’s Thesis, Dept. of Mathematics, University of Chicago, Chicago, IL, USA, 1939. [Google Scholar]
- Yang, J.; Liu, L.; Zhang, L.; Li, G.; Sun, Z.; Song, H. Prediction of Marine Pycnocline Based on Kernel Support Vector Machine and Convex Optimization Technology. Sensors 2019, 19, 1562. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Ji, U. Outlier Detection and Smoothing Process for Water Level Data Measured by Ultrasonic Sensor in Stream Flows. Water 2019, 11, 951. [Google Scholar] [CrossRef]
- Henrie, M.; Carpenter, P.; Nicholas, R.E. Chapter 5-Statistical Processing and Leak Detection. In Pipeline Leak Detection Handbook; Henrie, M., Carpenter, P., Nicholas, R.E., Eds.; Gulf Professional Publishing: Boston, MA, USA, 2016; pp. 91–114. [Google Scholar]
- Iglewicz, B.; Hoaglin, D.C. How to Detect and Handle Outliers; ASQC Quality Press: Milwaukee, WI, USA, 1993. [Google Scholar]
- Yoo, C.; Cho, E. Effect of Multicollinearity on the Bivariate Frequency Analysis of Annual Maximum Rainfall Events. Water 2019, 11, 905. [Google Scholar] [CrossRef]
- Mattera, D.; Haykin, S. Support Vector Machines for Dynamic Reconstruction of a Chaotic System; Schölkopf, B., Burges, C.J.C., Smola, A.J., Eds.; MIT Press: Cambridge, MA, USA, 1999; pp. 211–242. [Google Scholar]
- Hossain, S.; Hewa, G.; Wella-Hewage, S. A Comparison of Continuous and Event-Based Rainfall–Runoff (RR) Modelling Using EPA-SWMM. Water 2019, 11, 611. [Google Scholar] [CrossRef]
- Roccaro, P.; Chang, H.-S.; Vagliasindi, F.G.A.; Korshin, G.V. Differential absorbance study of effects of temperature on chlorine consumption and formation of disinfection by-products in chlorinated water. Water Res. 2008, 42, 1879–1888. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, S.; Chow, C.W.K.; Hewa, G.A.; Cook, D.; Harris, M. Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach. Sensors 2020, 20, 6671. https://doi.org/10.3390/s20226671
Hossain S, Chow CWK, Hewa GA, Cook D, Harris M. Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach. Sensors. 2020; 20(22):6671. https://doi.org/10.3390/s20226671
Chicago/Turabian StyleHossain, Sharif, Christopher W.K. Chow, Guna A. Hewa, David Cook, and Martin Harris. 2020. "Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach" Sensors 20, no. 22: 6671. https://doi.org/10.3390/s20226671
APA StyleHossain, S., Chow, C. W. K., Hewa, G. A., Cook, D., & Harris, M. (2020). Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach. Sensors, 20(22), 6671. https://doi.org/10.3390/s20226671






