Surface Acoustic Wave Sensor for C-Reactive Protein Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Fabrication
2.2. Chemicals and Materials
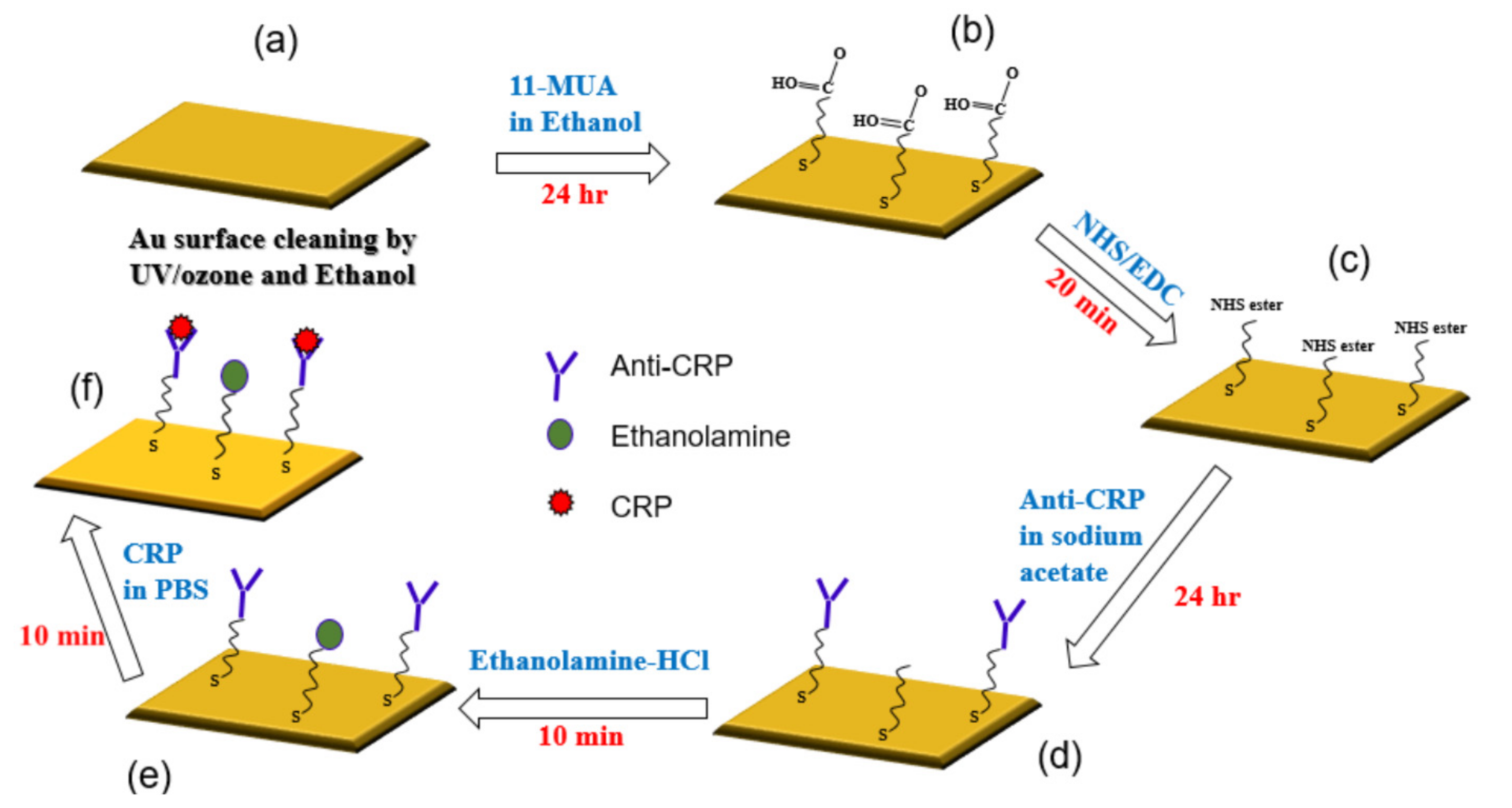
2.3. Method of Immobilization
- (a)
- The gold surface was cleaned by UV/ozone and ethanol and blown dry with nitrogen gas before use.
- (b)
- For the surface modification binding, 11-mercaptoundencanoic acid (11-MUA) in absolute ethanol (4 mM) was injected into the gold sensing area and incubated for 24 h, then washed by absolute ethanol and deionized (DI) water, and dried by nitrogen (N2) gas. This was the way the self-assembled monolayer (SAM) was attached to the gold surface.
- (c)
- This reactive SAM layer was activated by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC)/N-hydroxy succinimide (NHS) mixture (1:1) for 20 min and washed by sodium acetate buffer (10 mM, pH 5.0).
- (d)
- Immobilization of anti-CRP was done in sodium acetate buffer (10 mM, pH 5.0) for 24 h.
- (e)
- Blocking was performed by incubating in ethanolamine-HCl (pH 8.5) solutions for 10 min and then washed and incubated in phosphate buffered saline (PBS) buffer. This process was done for blocking the remaining non-specific binding of antibody active sites or removing the interference.
- (f)
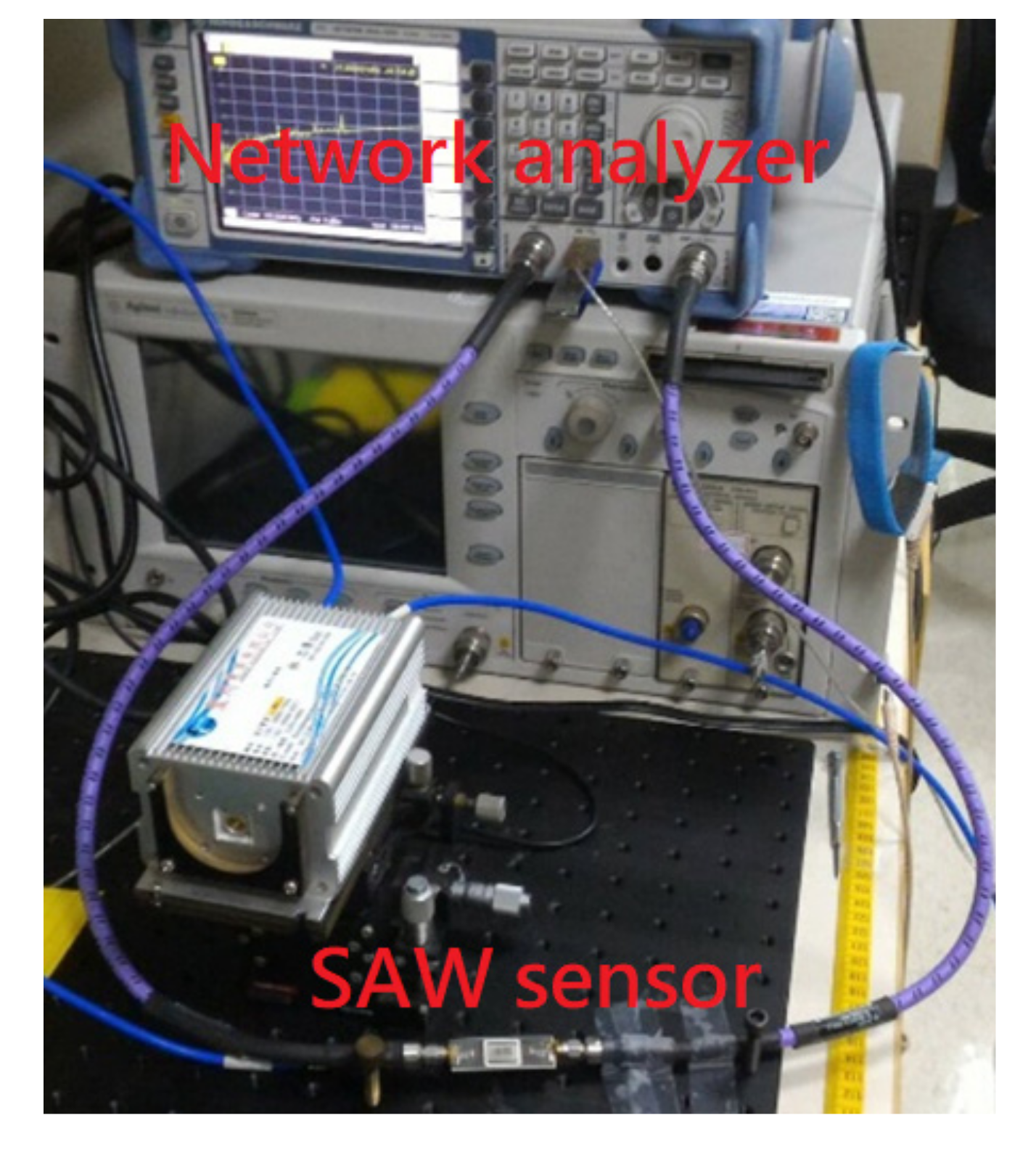
- For evaluating the interaction between CRP and anti-CRP, the prepared CRP with PBS buffer solution was injected over the sensing area and allowed to react with anti-CRP for 10 min. We prepared the CRP solution of 10 mL individually for each CRP concentration (0.1, 1, 10, 100, and 1000 ug/mL), which was diluted by PBS buffer. It means that we had five bottles of CRP solution with different concentrations. The proposed SAW sensor was exposed to CRP solutions of increasing concentrations (0.1, 1, 10, 100, and 1000 μg/mL). We used a pipette to suck 50 uL from the prepared CRP solutions and cover its sensing surface to interact with the CRP antibodies for each measurement. After an interaction time of 10 min, the CRP solution was carefully removed by a pipette and the surface was rinsed with PBS (500 μL) and DI water (1000 μL), separately, for the removal of non-specifically bound molecules. Next, the residue water at the surface of the SAW sensor was also carefully removed by a low nitrogen gas flow for 30 sec. After the nitrogen drying process, the frequency response of SAW sensor for detecting the CRP was recorded by a vector network analyzer (VNA). We repeated three times the abovementioned four steps (CRP interaction, removal of CRP solution, surface drying, and record spectrum response) for each CRP concentration. It should be noted that surface liquid residues should be completely removed to avoid the radiation of the acoustic energy into the liquid. For Rayleigh SAW sensors, this will cause a severe attenuation of the acoustic wave propagating at the solid/liquid interface [34]. No resonance peak was observed when the surface liquid residues had not been removed.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pai, J.; Pischon, T.; Ma, J.; Manson, J.; Hankinson, S.; Joshipura, K. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gaw, A.; Scherbakova, O.; Ford, I.; O’Reilly, D.S.J.; Haffner, S.M.; Isles, C.; Macfarlane, P.W.; Packard, C.J.; Cobbe, S.M. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003, 108, 414–419. [Google Scholar] [CrossRef]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Harris, R.; Stone, P.; Hudson, A.; Stuart, J. C reactive protein rapid assay techniques for monitoring resolution of infection in immunosuppressed patients. J. Clin. Pathol. 1984, 37, 821–825. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of 185 immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Jiang, H.; Hou, Y.; Rao, Q.; Guo, P.; Ding, S. A novel quantum dot-based fluoroimmunoassay method for detection of Enrofloxacin residue in chicken muscle tissue. Food Chem. 2009, 113, 1197–1201. [Google Scholar] [CrossRef]
- Beloglazova, N.; Speranskaya, E.; De Saeger, S.; Hens, Z.; Abé, S.; Goryacheva, I.Y. Quantum dot based rapid tests for zearalenone detection. Anal. Bioanal. Chem. 2012, 403, 3013–3024. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Gizeli, E. Detection of multiple cardiac markers with an integrated acoustic platform for cardiovascular risk assessment. Anal. Chim. Acta 2011, 699, 1–5. [Google Scholar] [CrossRef]
- Bing, X.; Wang, G. Label free C-reactive protein detection based on an electrochemical sensor for clinical application. Int. J. Electrochem. Sci. 2017, 12, 6304–6314. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Santschi, C.; Martin, O.J.F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Vance, S.A.; Sandros, M.G. Zeptomole detection of C-reactive protein in serum by a nanoparticle amplified surface plasmon resonance imaging aptasensor. Sci. Rep. 2014, 4, 5129. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Schneider, E.; Luong, J.H. Surface plasmon resonance-based immunoassay for human C-reactive protein. Analyst 2015, 140, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.K.; Lai, Y.C.; Hong, W.T.; Pheanpanitporn, Y.; Chen, C.S.; Huang, L.S. Electrical detection of C-reactive protein using a single free-standing, thermally controlled piezoresistive microcantilever for highly reproducible and accurate measurements. Sensors 2013, 13, 9653–9668. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, D.K.; Cho, Y.J. Development of indirect-competitive quartz crystal microbalance immunosensor for C-reactive protein. Sens. Actuators B Chem. 2009, 143, 444–448. [Google Scholar] [CrossRef]
- Gao, K.; Cui, S.; Liu, S. Development of an electrochemical quartz crystal microbalance-based immunosensor for C-reactive protein determination. Int. J. Electrochem. Sci. 2018, 13, 812–821. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and biosensors for c-reactive protein, temperature and ph, and their applications for monitoring wound healing: A review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef]
- Khan, M.S.; Yamashita, K.; Sharma, V.; Ranjan, R.; Selzman, C.H.; Dosdall, D.J. Perioperative biomarkers predicting postoperative atrial fibrillation risk after coronary artery bypass grafting: A narrative review. J. Cardiothor. Vasc. Anesth. 2020, 34, 1933–1941. [Google Scholar] [CrossRef]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef]
- Hashimoto, K.Y. Surface Acoustic Wave Devices in Telecommunications; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Wang, T.; Ni, Q.; Crane, N.; Guldiken, R. Surface acoustic wave based pumping in a microchannel. Microsyst. Technol. 2017, 23, 1335–1342. [Google Scholar] [CrossRef]
- Gell, J.; Ward, M.; Young, R.; Stevenson, R.; Atkinson, P.; Anderson, D.; Jones, G.; Ritchie, D.; Shields, A. Modulation of single quantum dot energy levels by a surface-acoustic-wave. Appl. Phys. Lett. 2008, 93, 081115. [Google Scholar] [CrossRef]
- Aigner, R. SAW and BAW technologies for RF filter applications: A review of the relative strengths and weaknesses. IEEE Int. Ultrason. Symp. 2008, 582–589. [Google Scholar]
- Onen, O.; Sisman, A.; Gallant, N.D.; Kruk, P.; Guldiken, R. A urinary Bcl-2 surface acoustic wave biosensor for early ovarian cancer detection. Sensors 2012, 12, 7423–7437. [Google Scholar] [CrossRef] [PubMed]
- Onen, O.; Ahmad, A.A.; Guldiken, R.; Gallant, N.D. Surface modification on acoustic wave biosensors for enhanced specificity. Sensors 2012, 12, 12317–12328. [Google Scholar] [CrossRef]
- Drobe, H.; Leidl, A.; Rost, M.; Ruge, I. Acoustic sensors based on surface-localized HPSWs for measurements in liquids. Sensor Actuators A Phys. 1993, 37, 141–148. [Google Scholar] [CrossRef]
- Rocha-Gaso, M.I.; García, J.V.; García, P.; March-Iborra, C.; Jiménez, Y.; Francis, L.A.; Montoya, Á.; Arnau, A. Love wave immunosensor for the detection of carbaryl pesticide. Sensors 2014, 14, 16434–16453. [Google Scholar] [CrossRef]
- Petroni, S.; Tripoli, G.; Combi, C.; Vigna, B.; De Vittorio, M.; Todaro, M.; Epifani, G.; Cingolani, R.; Passaseo, A. Noise reduction in GaN-based radio frequencysurface acoustic wave filters. Appl. Phys. Let. 2004, 85, 1039–1041. [Google Scholar] [CrossRef]
- Go, D.B.; Atashbar, M.Z.; Ramshani, Z.; Chang, H.C. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal. Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef]
- Smith, W.R.; Gerard, H.M.; Collins, J.H.; Reeder, T.M.; Shaw, H.J. Design of surface wave delay lines with interdigital transducers. IEEE Trans. Microw. Theory Tech. 1969, 17, 865–873. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamanouchi, K. Field analysis of SAW single-phase unidirectional transducers using internal floating electrodes. IEEE Ultrason. Symp. 1988, 57–61. [Google Scholar] [CrossRef]
- Shu, L.; Peng, B.; Li, C.; Gong, D.; Yang, Z.; Liu, X.; Zhang, W. The characterization of surface acoustic wave devices based on AlN-metal structures. Sensors 2016, 16, 526. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Greco, G.; Cecchini, M. A Rayleigh surface acoustic wave (R-SAW) resonator biosensor based on positive and negative reflectors with sub-nanomolar limit of detection. Sens. Actuators B Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Gizeli, E.; Stevenson, A.C.; Goddard, N.J.; Lowe, C.R. A novel Love-plate acoustic sensor utilizing polymer overlayers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 657–659. [Google Scholar] [CrossRef]
- Herrmann, F.; Weihnacht, M.; Buttgenbach, S. Properties of sensors based on shear-horizontal surface acoustic waves in LiTaO3/SiO2 and quartz SiO2 structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 268–273. [Google Scholar] [CrossRef]
- Powell, D.A.; Kalantar-zadeh, K.; Ippolito, S.; Wlodarski, W. A layered SAW device based on ZnO/LiTaO3 for liquid media sensing applications. IEEE Int. Ultrason. Symp. 2002, 1, 493–496. [Google Scholar]
- Herrmann, F.; Hahn, D.; Büttgenbach, S. Separate determination of liquid density and viscosity with sagittally corrugated Love-mode sensors. Sens. Actuators A Phys. 1999, 78, 99–107. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef]
- Martin, S.J.; Frye, G.C.; Senturla, S.D. Dynamics and response of polymer-coated surface acoustic wave devices: Effect of viscoelastic properties and film resonance. Anal. Chem. 1994, 66, 2201–2219. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Wang, S.; Wang, T.; Zhao, Y.; Li, J.; Hu, X.; Liang, H.; Zhu, J.; Sun, X.; et al. Detection of Clenbuterol Hydrochloride Residuals in Pork Liver Using a Customized Surface Plasmon Resonance Bioanalyzer. PLoS ONE 2015, 10, e0122005. [Google Scholar] [CrossRef]
- Dunér, G.; Anderson, H.; Pei, Z.; Ingemarsson, B.; Aastrup, T.; Ramström, O. Signal enhancement in ligand–receptor interactions using dynamic polymers at quartz crystal microbalance sensors. Analyst 2016, 141, 3993–3996. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.; Stakenborg, T.; Reekmans, G.; Laureyn, W.; Lagae, L.; Aerschot, A.V.; Ranst, M.V. Impact of spacers on the hybridization efficiency of mixed self-assembled DNA/alkanethiol films. Biosens. Bioelectron. 2008, 24, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Jans, K.; Bryce, G.; Ebrahim, S.; Lagae, L.; Witvrouw, A. Improving the selectivity by using different blocking agents in DNA hybridization assays for SiGe bio-molecular sensors. Microelectron. Eng. 2013, 111, 421–424. [Google Scholar] [CrossRef]




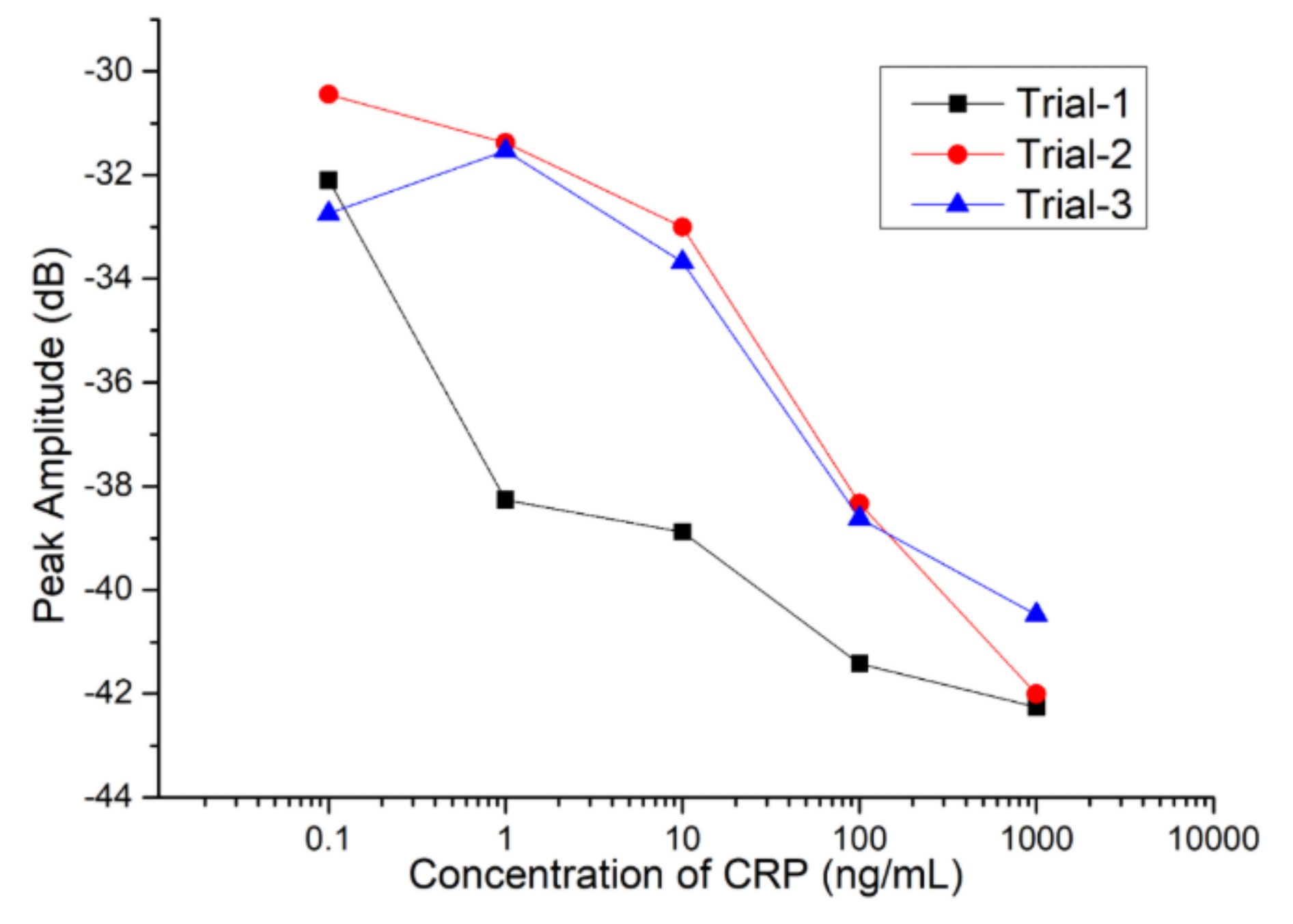

| CRP Concentration (μg/mL) | Average Peak Amplitude (dB) | Standard Deviation (dB) |
|---|---|---|
| 0.1 | −31.763 | 1.187 |
| 1 | −33.726 | 3.93 |
| 10 | −35.184 | 3.213 |
| 100 | −39.5 | 1.706 |
| 1000 | −41.413 | 0.884 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeng, M.-J.; Sharma, M.; Li, Y.-C.; Lu, Y.-C.; Yu, C.-Y.; Tsai, C.-L.; Huang, S.-F.; Chang, L.-B.; Lai, C.-S. Surface Acoustic Wave Sensor for C-Reactive Protein Detection. Sensors 2020, 20, 6640. https://doi.org/10.3390/s20226640
Jeng M-J, Sharma M, Li Y-C, Lu Y-C, Yu C-Y, Tsai C-L, Huang S-F, Chang L-B, Lai C-S. Surface Acoustic Wave Sensor for C-Reactive Protein Detection. Sensors. 2020; 20(22):6640. https://doi.org/10.3390/s20226640
Chicago/Turabian StyleJeng, Ming-Jer, Mukta Sharma, Ying-Chang Li, Yi-Chen Lu, Chia-Yu Yu, Chia-Lung Tsai, Shiang-Fu Huang, Liann-Be Chang, and Chao-Sung Lai. 2020. "Surface Acoustic Wave Sensor for C-Reactive Protein Detection" Sensors 20, no. 22: 6640. https://doi.org/10.3390/s20226640
APA StyleJeng, M.-J., Sharma, M., Li, Y.-C., Lu, Y.-C., Yu, C.-Y., Tsai, C.-L., Huang, S.-F., Chang, L.-B., & Lai, C.-S. (2020). Surface Acoustic Wave Sensor for C-Reactive Protein Detection. Sensors, 20(22), 6640. https://doi.org/10.3390/s20226640






