A Study on an Organic Semiconductor-Based Indirect X-ray Detector with Cd-Free QDs for Sensitivity Improvement
Abstract
1. Introduction
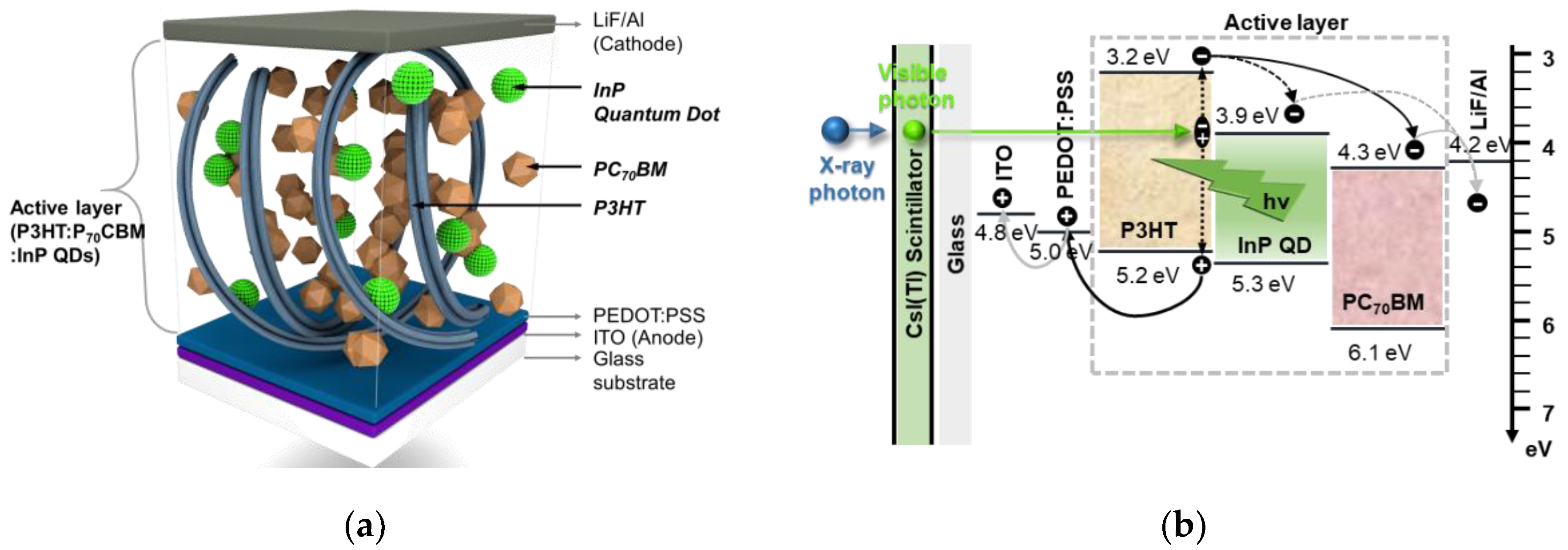
2. Experimental Detail
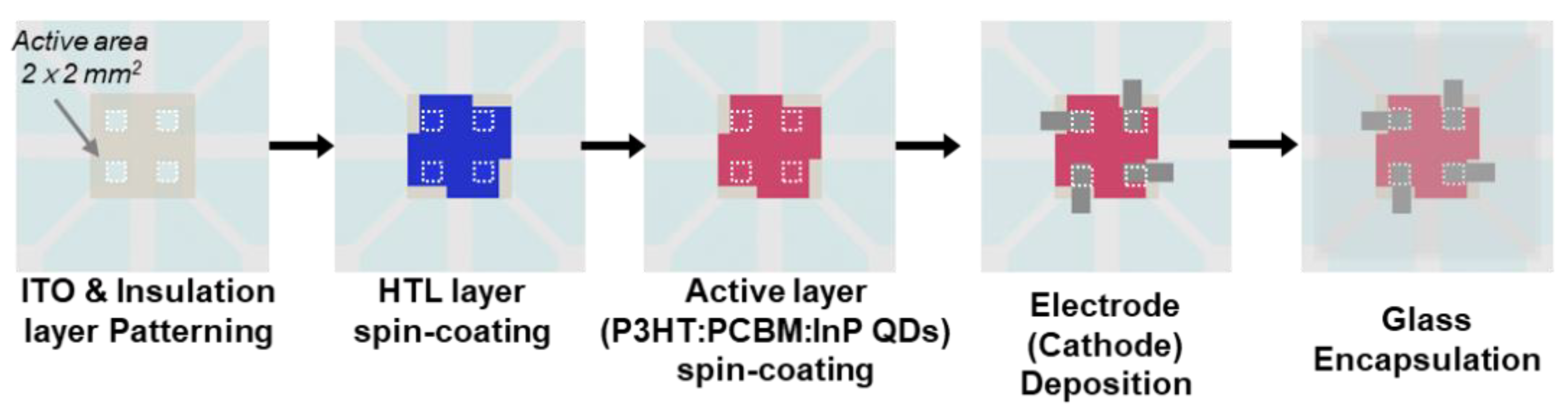
2.1. Detector Fabrication
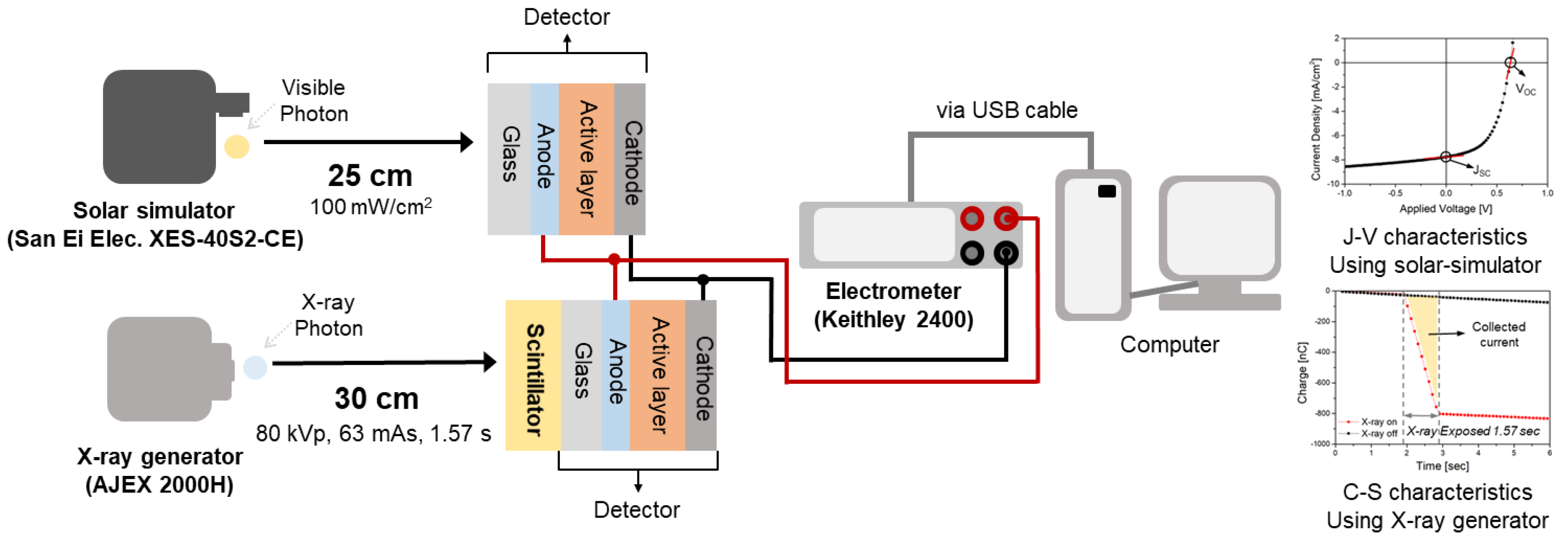
2.2. Experimental Setup
3. Result and Discussion
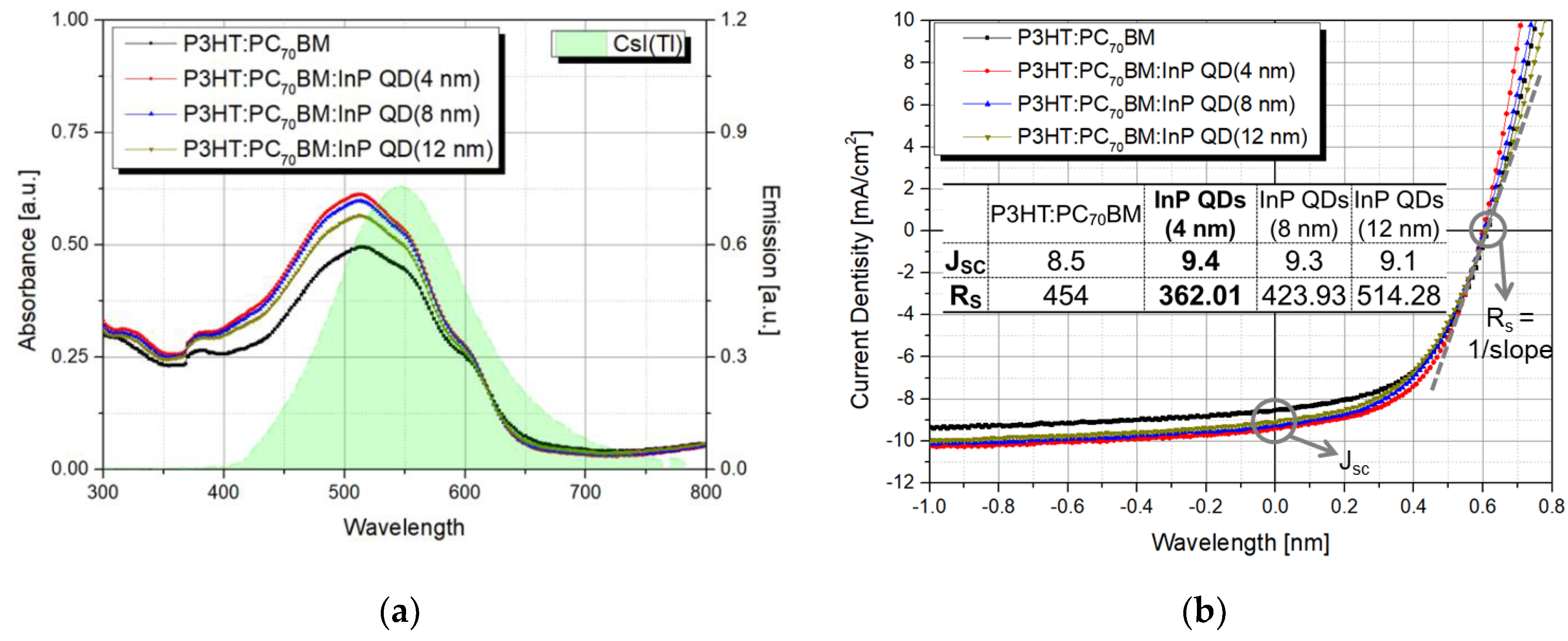
3.1. Experiment on QD Size Change
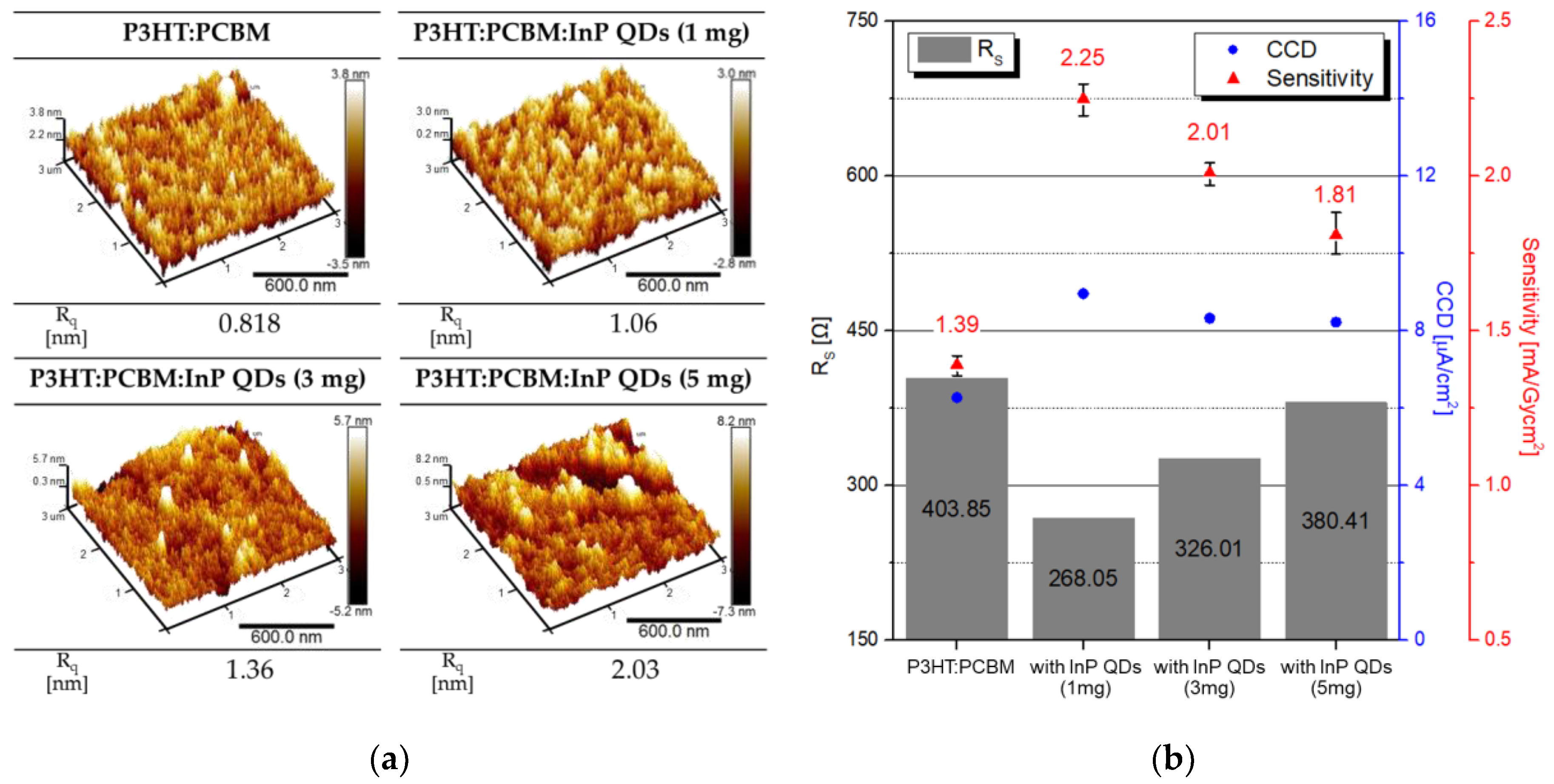
3.2. Experiment on QD Amount Change
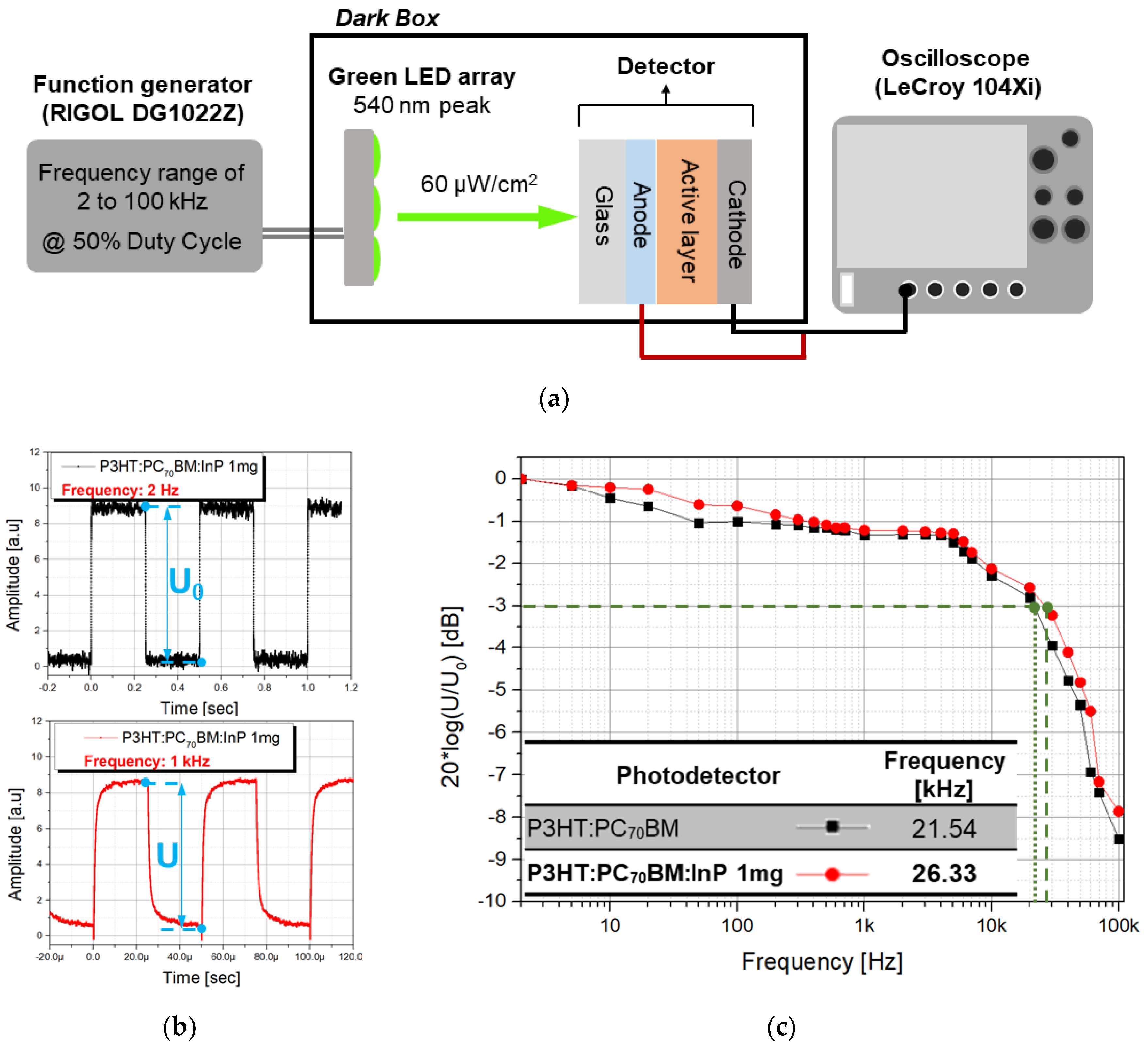
3.3. Changes in Frequency Response According to QD Addition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Losch, P.; Huang, W.; Goodman, E.D.; Wrasman, C.J.; Holm, A.; Riscoe, A.R.; Schwalbe, J.A.; Cagnello, M. Colloidal nanocrystals for heterogeneous catalysis. Nano Today 2019, 24, 15–47. [Google Scholar] [CrossRef]
- Nasilowski, M.; Mahler, B.; Lhuillier, E.; Ithurria, S.; Dubertret, B. Two-Dimensional Colloidal Nanocrystals. Chem. Rev. 2016, 116, 10934–10982. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bian, K.; Zhou, X.; Lu, P.; Liu, S.; Brener, I.; Sinclair, M.; Luk, T.; Schunk, H.; Alarid, L.; et al. Pressure compression of CdSe nanoparticles into luminescent nanowires. Sci. Adv. 2017, 3, e1602916. [Google Scholar] [CrossRef] [PubMed]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Sci. Chem. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Roh, J.; Park, Y.S.; Lim, J.; Klimov, V.I. Optically pumped colloidal-quantum-dot lasing in LED-like devices with an integrated optical cavity. Nat. Commun. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, Z.; Liu, H.; Wang, L.; Deng, Y.; Li, P.; Li, H.; Zhang, Z.H.; Fan, C.; Bi, W. One Stone, Two Birds High-Efficiency Blue-Emitting Perovskite Nanocrystals for LED and Security Ink Applications. Chem. Mater. 2019, 31, 5116–5123. [Google Scholar] [CrossRef]
- Qiao, F.; Xie, Y. Strategies for enhancing conductivity of colloidal nanocrystals and their photoelectronic applications. J. Energy Chem. 2020, 48, 29–42. [Google Scholar] [CrossRef]
- Pan, A.; Ma, X.; Huang, S.; Wu, Y.; Jia, M.; Shi, Y.; Liu, Y.; Wangyang, P.; He, L.; Liu, Y. CsPbBr3 Perovskite Nanocrystal Grown on MXene Nanosheets for Enhanced Photoelectric Detection and Photocatalytic CO2 Reduction. J. Phys. Chem. Lett. 2019, 10, 6590–6597. [Google Scholar] [CrossRef]
- Yan, X.; Remond, M.; Zheng, Z.; Hoibian, E.; Soulage, C.; Chambert, S.; Andraud, C.; Van der Sanden, B.; Ganachaud, F.; Bretonniere, Y.; et al. General and Scalable Approach to Bright, Stable, and Functional AIE Fluorogen Colloidal Nanocrystals for in Vivo Imaging. ACS Appl. Mater. Interfaces 2018, 10, 25154–25165. [Google Scholar] [CrossRef]
- Zamberlan, F.; Turyanska, L.; Patane, A.; Liu, Z.; Williams, H.E.L.; Fay, M.W.; Clarke, P.A.; Imamaura, Y.; Jin, T.; Bradshaw, T.D.; et al. Stable DHLA–PEG capped PbS quantum dots: From synthesis to near-infrared biomedical imaging. J. Mater. Chem. B 2018, 6, 550. [Google Scholar] [CrossRef]
- Turtos, R.M.; Gundacker, S.; Polovisyn, A.; Christodoulou, S.; Salomoni, M.; Auffray, E.; Moreels, I.; Lecoq, P.; Grim, J.Q. Ultrafast emission from colloidal nanocrystals under pulsed X-ray excitation. J. Intrum. 2016, 11, P10015. [Google Scholar] [CrossRef]
- Sytnyk, M.; Deumel, S.; Tedde, S.F.; Matt, G.J.; Heiss, W. A perspective on the bright future of metal halide perovskites for X-ray detection. Appl. Phys. Lett. 2019, 115, 190501. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kang, J. Sensitivity Improvement of Quantum Dot-Blended Hybrid Detector for X-ray Imaging. Coatings 2020, 10, 222. [Google Scholar] [CrossRef]
- Thirimanne, H.M.; Jayawardena, K.D.G.I.; Parnell, A.J.; Bandara, R.M.; Karalasingam, A.; Pani, S.; Huerdler, J.E.; Lidzey, D.G.; Tedde, S.F.; Nisbet, A.; et al. High sensitivity organic inorganic hybrid X-ray detectors with direct transduction and broadband response. Nat. Commun. 2018, 9, 2926. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.; Kang, J. Improving the sensitivity of indirect-type organic X-ray detector by blending with CdSe quantum dots. J. Intrum. 2016, 12, C01009. [Google Scholar] [CrossRef]
- Kumari, M.; Rana, P.; Chauhan, R.P. Modifications in structural and electrical properties of gamma irradiated CdSe nanowire. Nucl. Instrum. Methods Phys. Res. A 2014, 753, 116–120. [Google Scholar] [CrossRef]
- Turtos, R.M.; Gundacker, S.; Omelkov, S.; Mahler, B.; Khan, A.H.; Saaring, J.; Meng, Z.; Vasilev, A.; Dujardin, C.; Krim, M.; et al. On the use of CdSe scintillating nanoplatelets as time taggers for high-energy gamma detection. NPJ 2D Mater. Appl. 2019, 3, 37. [Google Scholar] [CrossRef]
- Abbaspour, S.; Mahmoudian, B.; Islamian, J.P. Cadmium Telluride Semiconductor Detector for Improved Spatial and Energy Resolution Radioisotopic Imaging. World J. Nucl. Med. 2017, 16, 101–107. [Google Scholar]
- Kang, Z.; Zhang, Y.; Menkara, H.; Wagner, B.K.; Semmers, C.J.; Lawrence, W.; Nagarkar, V. CdTe quantum dots and polymer nanocomposites for X-ray scintillation and imaging. Appl. Phys. Lett. 2011, 98, 181914. [Google Scholar] [CrossRef]
- Ankah, G.N.; Buchele, P.; Poulsen, K.; Rauch, T.; Tedde, S.F.; Gimmler, C.; Schmidt, O.; Kraus, T. PbS quantum dot based hybrid-organic photodetectors for X-ray sensing. Org. Electron. 2016, 33, 201–206. [Google Scholar] [CrossRef]
- Crisp, R.W.; Hashemi, F.S.M.; Alkemade, J.; Kirkwod, N.; Grimaldi, G.; Kinge, S.; Siebbeles, L.D.A.; Ommen, R.; Houtepen, A.J. Atomic Layer Deposition of ZnO on InP Quantum Dot Films for Charge Separation, Stabilization, and Solar Cell Formation. Adv. Mater. Interfaces 2020, 7, 1901600. [Google Scholar] [CrossRef]
- Tyazhev, A.V.; Budnitsky, D.L.; Koretskay, O.B.; Novikov, V.A.; Okaevich, L.S.; Potapov, A.I.; Tolbanov, O.P.; Vorobiev, A.P. CaAs radiation imaging detectors with an active layer thickness up to 1 mm. Nucl. Instrum. Methods Phys. Res. A 2003, 509, 34–39. [Google Scholar] [CrossRef]
- Ciavatti, A.; Cramer, T.; Carroli, M.; Basirico, L.; Fuher, R.; Leeuw, D.M.D.; Fraboni, B. Dynamics of direct X-ray detection processes in High-Z Bi2O3 nanoparticles-loaded PFO polymer-based diodes. Appl. Phys. Lett. 2017, 111, 183301. [Google Scholar] [CrossRef]
- Jayawardena, K.D.G.I.; Thirimanne, H.M.; Tedde, S.F.; Huerdler, J.E.; Parnell, A.J.; Bandara, R.M.I.; Mills, C.A.; Silva, S.R. Millimeter-Scale Unipolar Transport in High Sensitivity Organic-Inorganic Semiconductor X-ray Detectors. ACS Nano. 2019, 13, 6973–6981. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tong, X.; Wang, W.; Sun, J.; Yu, P.; Ji, H.; Niu, X.; Wang, Z.M. Eco-Friendly Colloidal Quantum Dot-Based Luminescent Solar Cenecntrators. Adv. Sci. 2019, 6, 1801967. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, L.; Xu, G.; Wang, X.; Wang, J.; Chen, Y.; Jiang, W.; Yang, Z.; Lin, G. Cytoxicity of InP/ZnS Quantum Dots with Different Surface Functional Groups Toward Two Lung-Derived Cell Lines. Front. Pharmacol. 2018, 9, 736. [Google Scholar] [CrossRef]
- Pan, Z.; Mora-Sero, I.; Shen, Q.; Zhang, H.; Li, Y.; Zhao, K.; Wang, J.; Zhong, X.; Bisquert, J. High-Efficiency “Green” Quantum Dots Solar Cell. J. Am. Chem. Soc. 2014, 136, 9203–9210. [Google Scholar] [CrossRef]
- Crips, R.W.; Kirkwood, N.; Frimaldi, G.; Kinge, S.; Siebbeles, L.D.A.; Houtepen, A.J. Highly Photoconductive InP Quantum Dots Films and Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6569–6576. [Google Scholar]
- Mahmoud, W.E.; Chang, Y.C.; Al-Ghamdi, A.A.; Al-Marzouki, F.; Bronstein, L.M. Development of nanoimprinted InP QDs decorated polyaniline solar cell with conversion efficiency 3%. Org. Electron. 2013, 14, 2762–2769. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Hu, R.; Roy, I.; Yong, K.T. Cadmiun-Free Quantum Dots for Biophotonic Imaging and Sensing. In Handbook of Photonics for Biomedical Engineering, 1st ed.; Ho, A.H.P., Kim, D., Somekh, M.G., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 841–870. [Google Scholar]
- Lee, J.; Kang, J. Characteristics of a Flexible Radiation Detector Fabricated with Non-Fullerene Acceptor for an Indirect-type X-ray Imaging. J. Instrum. 2019, 14, C03008. [Google Scholar] [CrossRef]
- Starkenburg, D.J.; Johns, P.M.; Baciak, J.E.; Nino, J.C.; Xue, J. Thin film organic photodetectors for indirect X-ray detection demonstrating low dose rate sensitivity at low voltage. J. Appl. Phys. 2017, 122, 225502. [Google Scholar] [CrossRef]
- Basirico, L.; Ciavatti, A.; Cramer, T.; Cosseddu, P.; Bonfiglio, A.; Fraboni, B. Direct X-ray photoconversion in flexible organic thin film devices operated below 1 V. Nat. Commun. 2016, 7, 13063. [Google Scholar] [CrossRef] [PubMed]
- Cherstha, S.; Fischer, R.; Matt, G.J.; Feldner, P.; Michel, T.; Osvet, A.; Levchuk, I.; Merle, B.; Golkar, S.; Chen, H.; et al. High-performance direct conversion X-ray detectors based on sintered hybrid lead triiodide perovskite wafers. Nat. Photonics 2017, 11, 436–441. [Google Scholar]
- Han, Y.W.; Lee, E.J.; Joo, J.; Park, J.; Sung, T.H.; Moon, D.K. Photon energy transfer by quantum dots in organic-inorganic hybrid solar cells through FRET. J. Mater. Chem. A 2016, 4, 10444. [Google Scholar] [CrossRef]
- Han, Y.H.; Jeon, S.J.; Choi, J.Y.; Kim, J.H.; Moon, D.K. Highly Efficient Ternary Solar Cells of 10.2% with Core/Shell Quantum Dots via FRET Effect. Sol. RRL 2018, 2, 1800077. [Google Scholar] [CrossRef]
- Yin, J.; Kumar, M.; Lei, Q.; Ma, L.; Raavi, S.S.K.; Gurzadyan, G.G.; Soci, C. Small-Size Effects on Electron Transfer in P3HT/InP Quantum Dots. J. Phys. Chem. C 2015, 119, 26783–26792. [Google Scholar] [CrossRef]
- Lee, J.; Seon, H.; Kang, J. Comparative studies between photovoltaic and radiation parameters in indirect-type organic X-ray detector with a P3HT: PCBM active layer. Nanosci. Nanotechnol. Lett. 2017, 9, 1159–1164. [Google Scholar] [CrossRef]
- Masi, S.; Echeverria-Arrondo, C.; Salim, K.M.M.; Ngo, T.T.; Mendez, P.F.; Lopez-Fraguas, E.; Macias-Pinilla, D.F.; Planelles, J.; Climente, J.I.; Mora-Sero, I. Chemi-Structural Stabilization of Formamidinium Lead Iodide Perovskite by Using Embedded Quantum Dots. ACS Energy Lett. 2020, 5, 418–427. [Google Scholar] [CrossRef]
- Treat, N.D.; Malik, J.A.N.; Reid, O.; Yu, L.; Shettle, C.G.; Rumbles, G.; Hawker, C.J.; Chabinyc, M.L.; Smith, P.; Stingelin, N. Microstructure formation in molecular and polymer semiconductors assisted by nucleation agents. Nat. Mater. 2013, 12, 628–633. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W.; Herwig, P.; et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Zheng, K. Insights into charge carrier dynamics in organo-metal halide perovskites: From neat films to solar cells. Chem. Soc. Rev. 2017, 46, 5714–5729. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S. Modeling of Space-Charge-Limited Current Injection Incorporating an Advanced Model of the Poole-Frenkel Effect. Master’s Thesis, Clemson University, Clemson, SC, USA, December 2008. [Google Scholar]
- Malliaras, G.G.; Scott, J.C. Numeric simulations of the electronical characteristics and the efficiencies of single-layer organic light emitting diodes. J. Appl. Phys. 1999, 85, 7426–7432. [Google Scholar] [CrossRef]
- Abkowitz, M.; Facci, J.S.; Stolka, M. Influence of effective-medium dielectric constant on electronic transport in an arylamine-containing glassy polymer. Appl. Phys. Lett. 1949, 65, 1127. [Google Scholar] [CrossRef]
- Tsang, S.W.; So, S.K. Application of admittance spectroscopy to evaluate carrier mobility in organic charge transport materials. J. Appl. Phys. 2006, 99, 013706. [Google Scholar] [CrossRef]
- Kwan, C.P.; Street, M.; Mahmood, A.; Echtenkamp, W.; Randle, M.; He, K.; Nathawat, J.; Arabchigavkani, N.; Barut, B.; Yin, S.; et al. Space-charge limited conduction in epitaxial chromia films grown on elemental and oxide-based metallic substrates. AIP Adv. 2019, 9, 055018. [Google Scholar] [CrossRef]
- Jhamba, L.; Wamwangi, D.; Chiguvare, Z. Dependence of mobility and charge injection on active layer thickness of bulk heterojunction organic solar cells: PCBM:P3HT. Opt. Quantum Electron. 2020, 52, 245. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Sareni, B.; Krrahenbuhl, L.; Brosseau, C.; Beroual, A. Effective dielectric constant of random composite materials. J. Appl. Phys. 1997, 81, 2375. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Liu, H.; Kang, J. A Study on an Organic Semiconductor-Based Indirect X-ray Detector with Cd-Free QDs for Sensitivity Improvement. Sensors 2020, 20, 6562. https://doi.org/10.3390/s20226562
Lee J, Liu H, Kang J. A Study on an Organic Semiconductor-Based Indirect X-ray Detector with Cd-Free QDs for Sensitivity Improvement. Sensors. 2020; 20(22):6562. https://doi.org/10.3390/s20226562
Chicago/Turabian StyleLee, Jehoon, Hailiang Liu, and Jungwon Kang. 2020. "A Study on an Organic Semiconductor-Based Indirect X-ray Detector with Cd-Free QDs for Sensitivity Improvement" Sensors 20, no. 22: 6562. https://doi.org/10.3390/s20226562
APA StyleLee, J., Liu, H., & Kang, J. (2020). A Study on an Organic Semiconductor-Based Indirect X-ray Detector with Cd-Free QDs for Sensitivity Improvement. Sensors, 20(22), 6562. https://doi.org/10.3390/s20226562




