Dual-Task Gait Stability after Concussion and Subsequent Injury: An Exploratory Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical Evaluation
2.3. Instrumented Dual-Task Gait Evaluation
2.4. CRQA Data Processing and Analysis
2.5. Statistical Analysis
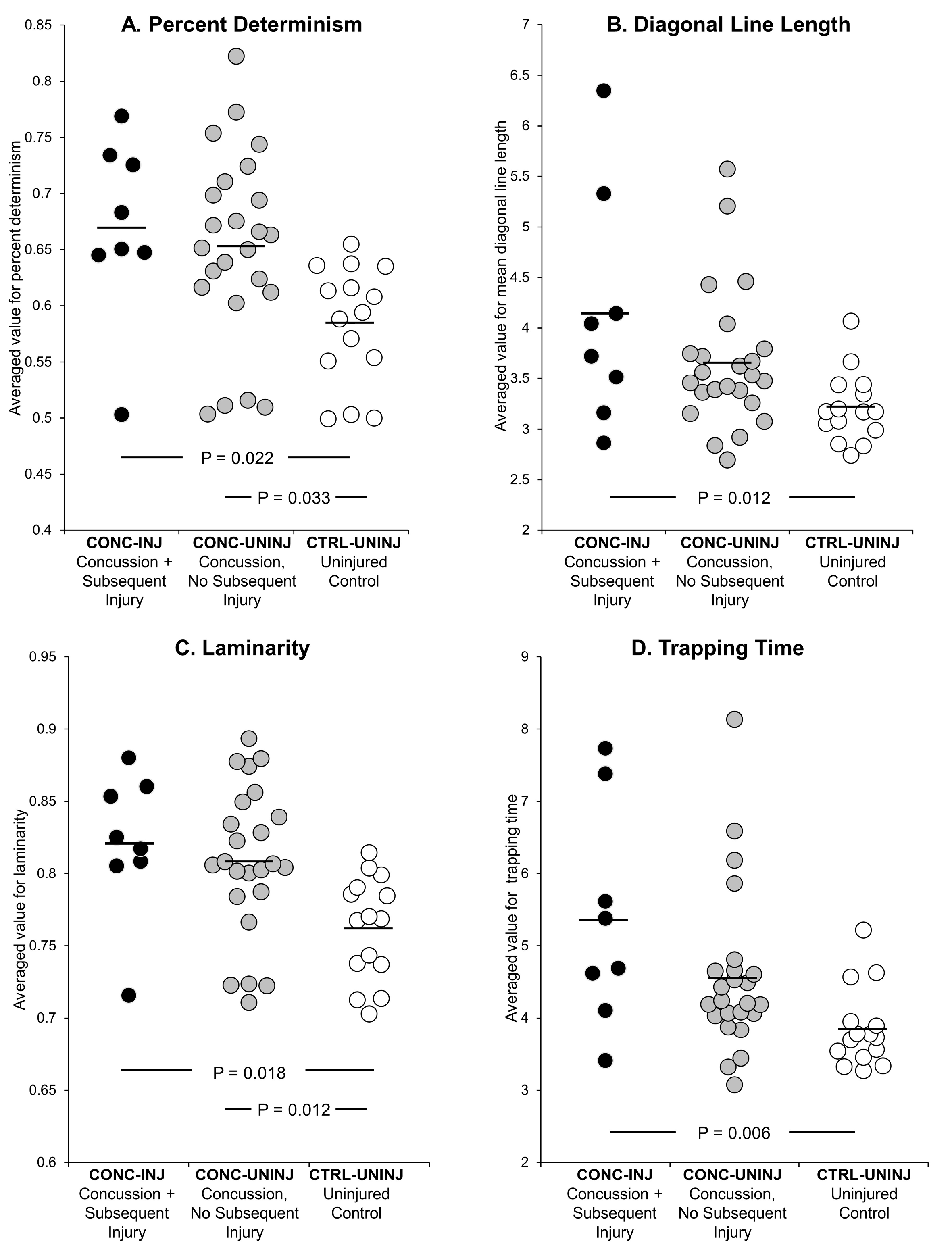
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCrea, M.; Broglio, S.; McAllister, T.; Zhou, W.; Zhao, S.; Katz, B.; Kudela, M.; Harezlak, J.; Nelson, L.; Meier, T.; et al. Return to play and risk of repeat concussion in collegiate football players: Comparative analysis from the NCAA Concussion Study (1999–2001) and CARE Consortium (2014–2017). Br. J. Sports Med. 2019, 54, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.P.; Katz, B.P.; Zhao, S.; McCrea, M.; McAllister, T. CARE Consortium Investigators. Test-Retest Reliability and Interpretation of Common Concussion Assessment Tools: Findings from the NCAA-DoD CARE Consortium. Sports Med. 2017, 48, 1255–1268. [Google Scholar] [CrossRef]
- Kamins, J.; Bigler, E.; Covassin, T.; Henry, L.; Kemp, S.; Leddy, J.J.; Mayer, A.; McCrea, M.; Prins, M.; Schneider, K.J.; et al. What is the physiological time to recovery after concussion? A systematic review. Br. J. Sports Med. 2017, 51, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Büttner, F.; Howell, D.R.; Ardern, C.L.; Doherty, C.; Blake, C.; Ryan, J.; Catena, R.; Chou, L.-S.; Fino, P.; Rochefort, C.; et al. Concussed athletes walk slower than non-concussed athletes during cognitive-motor dual-task assessments but not during single-task assessments 2 months after sports concussion: A systematic review and meta-analysis using individual participant data. Br. J. Sports Med. 2019, 54, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Osternig, L.R.; Chou, L.S. Detection of acute and long-term effects of concussion: Dual-task gait balance control vs. computerized neurocognitive test. Arch. Phys. Med. Rehabil. 2018, 99, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Myer, G.D.; Brilliant, A.; Barber Foss, K.; Meehan, W.P.I. Quantitative Multimodal Assessment of Concussion Recovery in Youth Athletes. Clin. J. Sport Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Lynall, R.C.; Buckley, T.A.; Herman, D.C. Neuromuscular control deficits and the risk of subsequent injury after a concussion: A scoping review. Sports Med. 2018, 48, 1097–1115. [Google Scholar] [CrossRef]
- McPherson, A.L.; Nagai, T.; Webster, K.E.; Hewett, T.E. Musculoskeletal Injury Risk After Sport-Related Concussion: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 47, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Reneker, J.C.; Babl, R.; Flowers, M.M. History of concussion and risk of subsequent injury in athletes and service members: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 173–185. [Google Scholar] [CrossRef]
- Buckley, T.A.; Howard, C.M.; Oldham, J.R.; Lynall, R.C.; Swanik, C.B.; Getchell, N. No Clinical Predictors of Postconcussion Musculoskeletal Injury in College Athletes. Med. Sci. Sports Exerc. 2020, 52, 1256–1262. [Google Scholar] [CrossRef]
- Howell, D.R.; Buckley, T.A.; Lynall, R.C.; Meehan, W.P., III. Worsening dual-task gait costs after concussion and their association with subsequent sport-related injury. J. Neurotrauma 2018, 35, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.R.; Howell, D.R.; Knight, C.A.; Crenshaw, J.R.; Buckley, T.A. Gait Performance is Associated with Subsequent Lower Extremity Injury following Concussion. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef]
- Guy, J.A.; Knight, L.M.; Wang, Y.; Jerrell, J.M. Factors associated with musculoskeletal injuries in children and adolescents with attention-deficit/hyperactivity disorder. Prim. Care Companion CNS Disord 2016, 18, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Read, P.J.; Oliver, J.L.; De Ste Croix, M.B.A.; Myer, G.D.; Lloyd, R.S. Neuromuscular risk factors for knee and ankle ligament injuries in male youth soccer players. Sports Med. 2016, 46, 1059–1066. [Google Scholar] [CrossRef]
- Howell, D.R.; Bonnette, S.; Diekfuss, J.A.; Grooms, D.R.; Myer, G.D.; Meehan, W.P. Youth With Concussion Have Less Adaptable Gait Patterns Than Their Uninjured Peers: Implications for Concussion Management. J. Orthop. Sports Phys. Ther. 2020, 50, 438–446. [Google Scholar] [CrossRef]
- Rhea, C.K.; Kiefer, A.W. Patterned variability in gait behavior: How can it be measured and what does it mean. In Gait Biometrics: Basic Patterns, Role of Neurological Disorders and Effects of Physical Activity; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 17–44. [Google Scholar]
- Bonnette, S.; Diekfuss, J.A.; Grooms, D.; Myer, G.D.; Meehan, W.P.; Howell, D.R. Integrated linear and nonlinear trunk dynamics identify residual concussion deficits. Neurosci. Lett. 2020, 729, 134975. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Cross, M.; Kemp, S.; Smith, A.; Trewartha, G.; Stokes, K. Professional Rugby Union players have a 60% greater risk of time loss injury after concussion: A 2-season prospective study of clinical outcomes. Br. J. Sports Med. 2016, 50, 926–931. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Finch, C.F.; Bennell, K.L.; Wajswelner, H. How valid is a self reported 12 month sports injury history? Br. J. Sports Med. 2003, 37, 545–547. [Google Scholar] [CrossRef]
- Kontos, A.P.; Elbin, R.J.; Schatz, P.; Covassin, T.; Henry, L.; Pardini, J.; Collins, M.W. A revised factor structure for the post-concussion symptom scale: Baseline and postconcussion factors. Am. J. Sports Med. 2012, 40, 2375–2384. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M.; et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5). Br. J. Sports Med. 2017, 51, 848–850. [Google Scholar] [CrossRef]
- Howell, D.R.; Berkstresser, B.; Wang, F.; Buckley, T.A.; Mannix, R.; Stillman, A.; Meehan, W.P., III. Self-reported sleep duration affects tandem gait, but not steady-state gait outcomes among healthy collegiate athletes. Gait Posture 2018, 62, 291–296. [Google Scholar] [CrossRef]
- Howell, D.R.; Brilliant, A.N.; Meehan, W.P. Tandem Gait Test-Retest Reliability among Healthy Child and Adolescent Athletes. J. Athl. Train. 2019, 54, 1254–1259. [Google Scholar] [CrossRef]
- Howell, D.R.; Stillman, A.; Buckley, T.A.; Berkstresser, B.; Wang, F.; Meehan, W.P. The utility of instrumented dual-task gait and tablet-based neurocognitive measurements after concussion. J. Sci. Med. Sport 2018, 21, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Brilliant, A.; Berkstresser, B.; Wang, F.; Fraser, J.; Meehan, W. The association between dual-task gait after concussion and prolonged symptom duration. J. Neurotrauma 2017, 34, 3288–3294. [Google Scholar] [CrossRef]
- Zbilut, J.P.; Giuliani, A.; Webber, C.L. Detecting deterministic signals in exceptionally noisy environments using cross-recurrence quantification. Phys. Lett. A 1998, 246, 122–128. [Google Scholar] [CrossRef]
- Shockley, K.; Butwill, M.; Zbilut, J.P.; Webber, C.L. Cross recurrence quantification of coupled oscillators. Phys. Lett. A 2002, 305, 59–69. [Google Scholar] [CrossRef]
- Webber, C.L.; Zbilut, J.P. Dynamical assessment of physiological systems and states using recurrence plot strategies. J. Appl. Physiol. 1994, 76, 965–973. [Google Scholar] [CrossRef]
- Bisi, M.C.; Stagni, R. Development of gait motor control: What happens after a sudden increase in height during adolescence? Biomed. Eng. Online 2016, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait variability and stability measures: Minimum number of strides and within-session reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Labini, F.S.; Meli, A.; Ivanenko, Y.P.; Tufarelli, D. Recurrence quantification analysis of gait in normal and hypovestibular subjects. Gait Posture 2012, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bonnette, S.; Diekfuss, J.A.; Kiefer, A.W.; Riley, M.A.; Barber Foss, K.D.; Thomas, S.; DiCesare, C.A.; Yuan, W.; Dudley, J.; Reches, A.; et al. A jugular vein compression collar prevents alterations of endogenous electrocortical dynamics following blast exposure during special weapons and tactical (SWAT) breacher training. Exp. Brain Res. 2018, 236, 2691–2701. [Google Scholar] [CrossRef]
- Rizzi, M.; Weissberg, I.; Milikovsky, D.Z.; Friedman, A. Following a potential epileptogenic insult, prolonged high rates of nonlinear dynamical regimes of intermittency type is the hallmark of epileptogenesis. Sci. Rep. 2016, 6, 31129. [Google Scholar] [CrossRef] [PubMed]
- Roulston, M.S. Estimating the errors on measured entropy and mutual information. Physica D Nonlinear Phenom. 1999, 125, 285–294. [Google Scholar] [CrossRef]
- Kennel, M.B.; Abarbanel, H.D.I. False neighbors and false strands: A reliable minimum embedding dimension algorithm. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002, 66, 026209. [Google Scholar] [CrossRef]
- Marwan, N.; Kurths, J. Nonlinear analysis of bivariate data with cross recurrence plots. Phys. Lett. A 2002, 302, 299–307. [Google Scholar] [CrossRef]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002, 66, 026702. [Google Scholar] [CrossRef]
- Marwan, N.; Carmen Romano, M.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Fino, P.C.; Parrington, L.; Pitt, W.; Martini, D.N.; Chesnutt, J.C.; Chou, L.-S.; King, L.A. Detecting gait abnormalities after concussion or mild traumatic brain injury: A systematic review of single-task, dual-task, and complex gait. Gait Posture 2018, 62, 157–166. [Google Scholar] [CrossRef]
- Howell, D.R.; Osternig, L.R.; Chou, L.-S. Return to activity after concussion affects dual-task gait balance control recovery. Med. Sci. Sports Exerc. 2015, 47, 673–680. [Google Scholar] [CrossRef]
- Stergiou, N.; Harbourne, R.; Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.W.; Myer, G.D. Training the Antifragile Athlete: A Preliminary Analysis of Neuromuscular Training Effects on Muscle Activation Dynamics. Nonlinear Dyn. Psychol Life Sci. 2015, 19, 489–510. [Google Scholar]
- Wilkerson, G.B.; Grooms, D.R.; Acocello, S.N. Neuromechanical Considerations for Postconcussion Musculoskeletal Injury Risk Management. Curr. Sports Med. Rep. 2017, 16, 419–427. [Google Scholar] [CrossRef]
- Eagle, S.R.; Kontos, A.P.; Pepping, G.-J.; Johnson, C.D.; Sinnott, A.; LaGoy, A.; Connaboy, C. Increased Risk of Musculoskeletal Injury Following Sport-Related Concussion: A Perception-Action Coupling Approach. Sports Med. 2020, 50, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Soon, K.; Lee, M.; Tsai, W.; Lin, C. A new trunk sway assessment protocol using biofeedback inertial-based sensing modality for stroke patients. In Proceedings of the 2011 International Conference on System Science and Engineering, Macao, China, 8–10 June 2011; pp. 675–678. [Google Scholar]
- Valdés, B.A.; Schneider, A.N.; Van der Loos, H.F.M. Reducing Trunk Compensation in Stroke Survivors: A Randomized Crossover Trial Comparing Visual and Force Feedback Modalities. Arch. Phys. Med. Rehabil. 2017, 98, 1932–1940. [Google Scholar] [CrossRef]
- Dozza, M.; Chiari, L.; Chan, B.; Rocchi, L.; Horak, F.B.; Cappello, A. Influence of a portable audio-biofeedback device on structural properties of postural sway. J. Neuroeng. Rehabil. 2005, 2, 13. [Google Scholar] [CrossRef]
- Dozza, M.; Chiari, L.; Horak, F.B. Audio-biofeedback improves balance in patients with bilateral vestibular loss. Arch. Phys. Med. Rehabil. 2005, 86, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Herman, T.; Nicolai, S.; Zijlstra, A.; Zijlstra, W.; Becker, C.; Chiari, L.; Hausdorff, J.M. Audio-biofeedback training for posture and balance in patients with Parkinson’s disease. J. Neuroeng. Rehabil. 2011, 8, 35. [Google Scholar] [CrossRef]
- Caudron, S.; Guerraz, M.; Eusebio, A.; Gros, J.-P.; Azulay, J.-P.; Vaugoyeau, M. Evaluation of a visual biofeedback on the postural control in Parkinson’s disease. Neurophysiol. Clin. 2014, 44, 77–86. [Google Scholar] [CrossRef]
- Pataky, Z.; De León Rodriguez, D.; Golay, A.; Assal, M.; Assal, J.-P.; Hauert, C.-A. Biofeedback training for partial weight bearing in patients after total hip arthroplasty. Arch. Phys. Med. Rehabil. 2009, 90, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Lifeso, R.M. Altering asymmetric limb loading after hip arthroplasty using real-time dynamic feedback when walking. Arch. Phys. Med. Rehabil. 2005, 86, 1958–1963. [Google Scholar] [CrossRef]
| Variable | CONC-INJ Concussion + Subsequent Injury (n = 8) | CONC-UNINJ Concussion, No Subsequent Injury (n = 24) | CTRL-UNINJ Control, No Subsequent Injury (n = 15) | p Value |
|---|---|---|---|---|
| Age (years) | 15.4 (3.5) | 14.0 (2.6) | 14.2 (1.9) | 0.40 |
| Sex (female) | 5 (63%) | 13 (46%) | 7 (53%) | 0.86 |
| Assessment Time (days post-injury †) | 50.8 (57.5) | 45.7 (21.4) | 28.6 (21.6) | 0.18 |
| Assessment Time (days after return-to-play clearance) | 9.1 (9.0) | 12.9 (9.8) | - | 0.31 |
| Symptom Resolution Time (days post-injury) | 41.7 (56.8) | 32.7 (19.9) | - | 0.50 |
| Height (cm) | 163.1 (13.3) | 160.8 (13.5) | 158.2 (11.7) | 0.66 |
| Mass (kg) | 63.3 (16.5) | 54.3 (15.6) | 48.3 (12.0) | 0.12 |
| LOC at Time of Injury | 1 (13%) | 2 (8%) | - | >0.99 |
| History of Concussion | 5 (63%) | 14 (58%) | 2 (13%) | 0.02 * |
| Initial Symptom Severity (PCSS score) | 19.6 (12.7) | 31.2 (18.1) | 2.4 (3.6) | <0.001 * |
| Variable | CONC-INJ Concussion + Subsequent Injury | CONC-UNINJ Concussion, No Subsequent Injury | CTRL-UNINJ Control, No Subsequent Injury | p Value |
|---|---|---|---|---|
| Hours of Week in Organized Sport Participation During the Year After Assessment | 11.8 (5.8) | 8.6 (4.4) | 10.9 (4.3) | 0.37 |
| Number of Sport Seasons Completed During the Year after Assessment | 2.7 (1.5) | 3.0 (0.9) | 3.7 (0.5) | 0.09 |
| Follow-up Time (days from assessment—questionnaire completion) | 369 (21) | 374 (13) | 377 (12) | 0.41 |
| Type of Subsequent Injury | Lower extremity injury: 5 Ankle sprain: 2 Ankle fracture: 1 Hamstring strain: 1 Knee sprain: 1 Concussion: 3 | - | - | - |
| Days Missed Due to Subsequent Injury | 45 (69) | - | - | - |
| Time from Concussion to Subsequent Injury | 158 (91) | - | - | - |
| Variable | CONC-INJ Concussion + Subsequent Injury | CONC-UNINJ Concussion, No Subsequent Injury | CTRL-UNINJ Control, No Subsequent Injury | p Value |
|---|---|---|---|---|
| Dual-task self-selected Average Walking Speed (m/s) * | 0.76 (0.14) | 0.84 (0.15) | 0.95 (0.14) | 0.009 |
| Dual-task Cognitive Accuracy (% correct) | 88.6 (11.2)% | 89.2 (15.4)% | 95.5 (4.5)% | 0.24 |
| Dual-task Tandem Gait Time (s) | 19.2 (5.4) | 17.6 (5.7) | 16.1 (5.1) | 0.78 |
| Symptom Severity (PCSS score) | 2.0 (4.9) | 3.1 (6.3) | 1.0 (1.9) | 0.44 |
| Predictor Variable | Hazard Ratio | Standard Error | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Percent Determinism | 1.92 | 0.86 | 0.80, 4.63 | 0.15 |
| Diagonal Line Length * | 1.95 | 0.61 | 1.05, 3.60 | 0.03 |
| Laminarity | 3.24 | 2.44 | 0.74, 14.22 | 0.12 |
| Trapping Time * | 1.66 | 0.36 | 1.09, 2.52 | 0.02 |
| Average Walking Speed * | 0.01 | 0.02 | 0.00, 0.51 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, D.R.; Bonnette, S.; Diekfuss, J.A.; Grooms, D.R.; Myer, G.D.; Wilson, J.C.; Meehan, W.P., III. Dual-Task Gait Stability after Concussion and Subsequent Injury: An Exploratory Investigation. Sensors 2020, 20, 6297. https://doi.org/10.3390/s20216297
Howell DR, Bonnette S, Diekfuss JA, Grooms DR, Myer GD, Wilson JC, Meehan WP III. Dual-Task Gait Stability after Concussion and Subsequent Injury: An Exploratory Investigation. Sensors. 2020; 20(21):6297. https://doi.org/10.3390/s20216297
Chicago/Turabian StyleHowell, David R., Scott Bonnette, Jed A. Diekfuss, Dustin R. Grooms, Gregory D. Myer, Julie C. Wilson, and William P. Meehan, III. 2020. "Dual-Task Gait Stability after Concussion and Subsequent Injury: An Exploratory Investigation" Sensors 20, no. 21: 6297. https://doi.org/10.3390/s20216297
APA StyleHowell, D. R., Bonnette, S., Diekfuss, J. A., Grooms, D. R., Myer, G. D., Wilson, J. C., & Meehan, W. P., III. (2020). Dual-Task Gait Stability after Concussion and Subsequent Injury: An Exploratory Investigation. Sensors, 20(21), 6297. https://doi.org/10.3390/s20216297





