Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor
Abstract
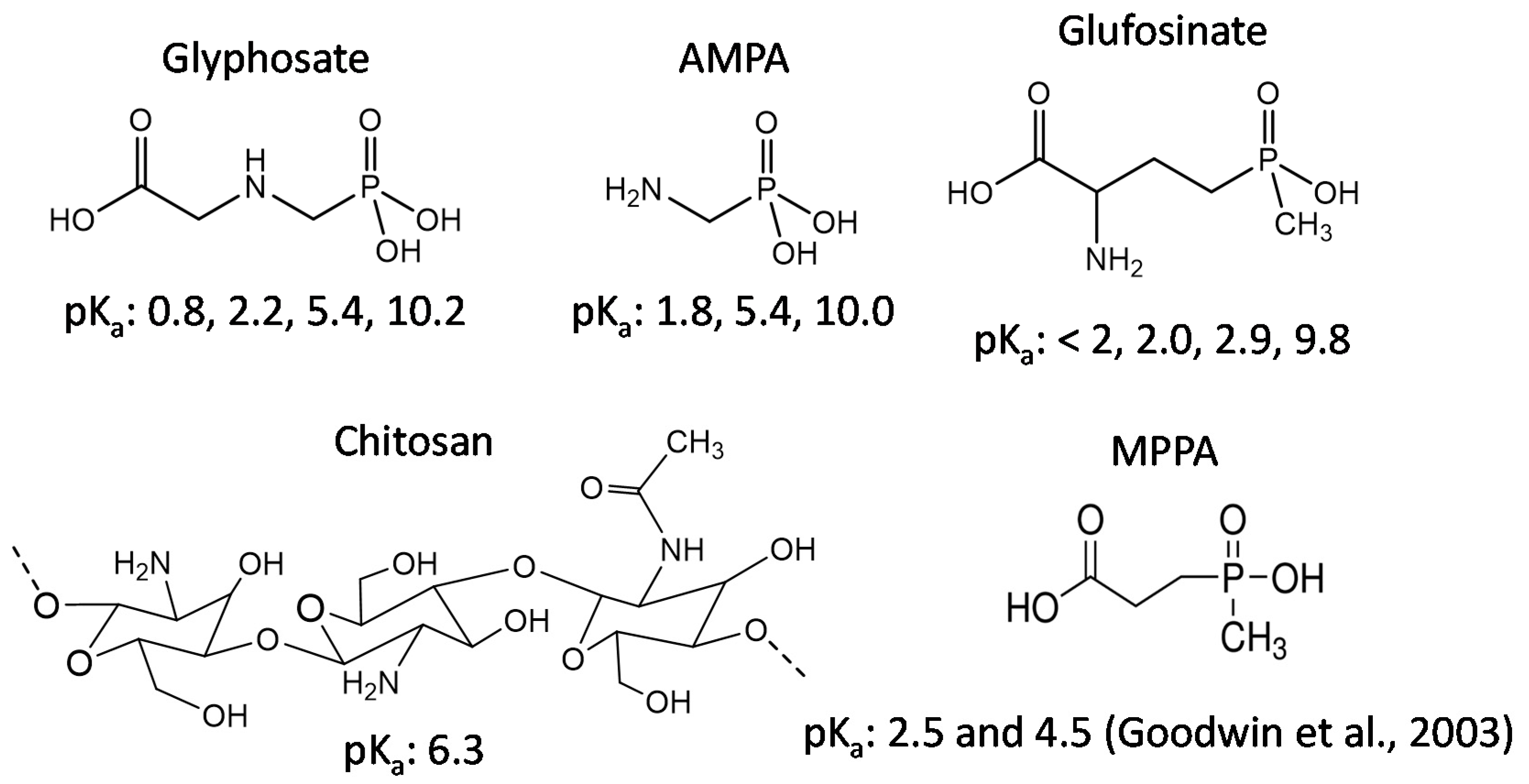
:1. Introduction
2. Kinetic and Equilibrium Interaction Models
3. Material and Methods
3.1. Chemicals and Instrumentation
3.2. Preparation of Thin Film
3.3. Procedure of the Analysis
3.4. Evaluation of CS/ZnO SPR Sensor Response
3.5. Statistical Analysis
4. Results and Discussion
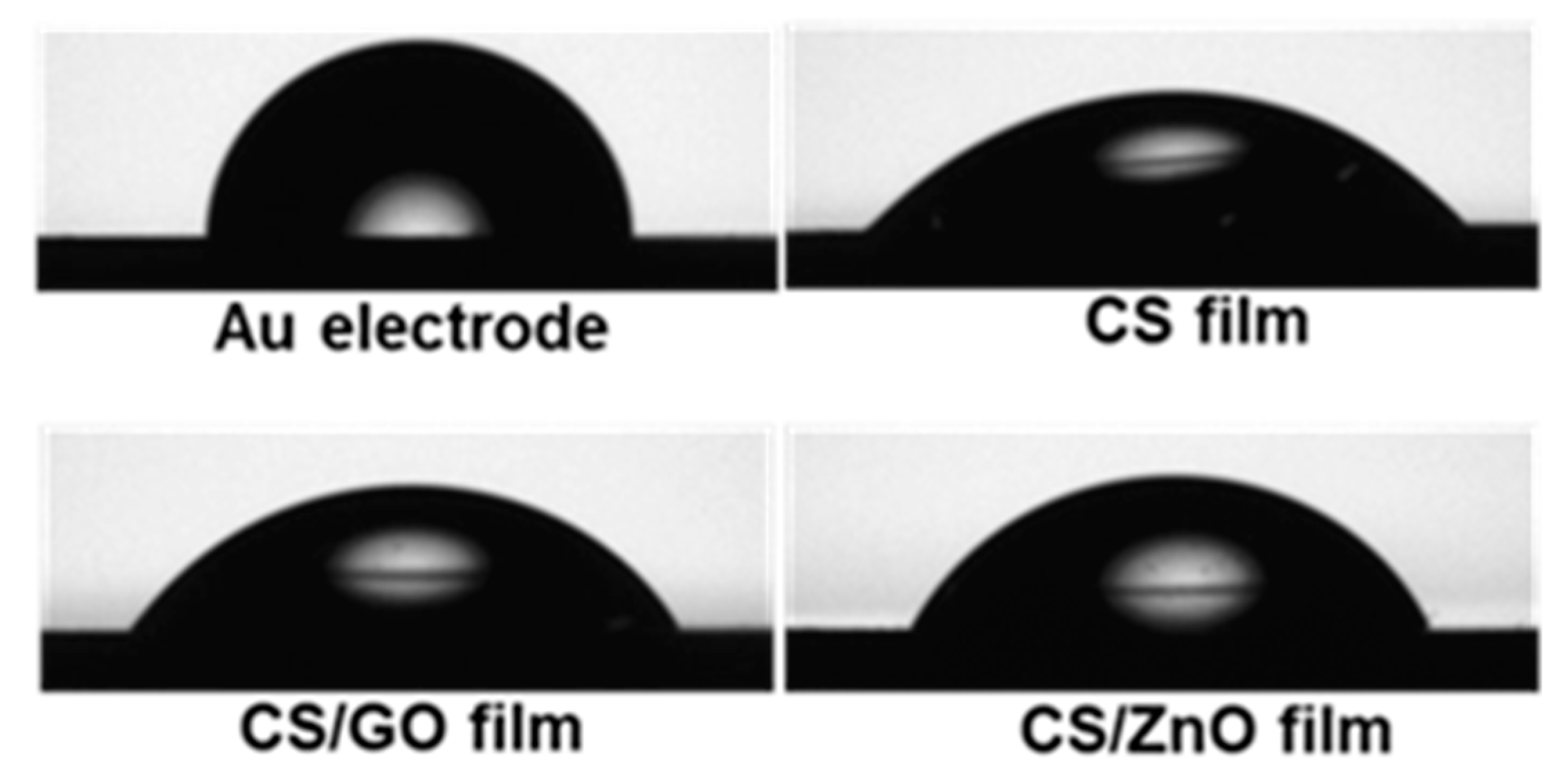
4.1. Characterization of CS-Based Nanocomposite Films
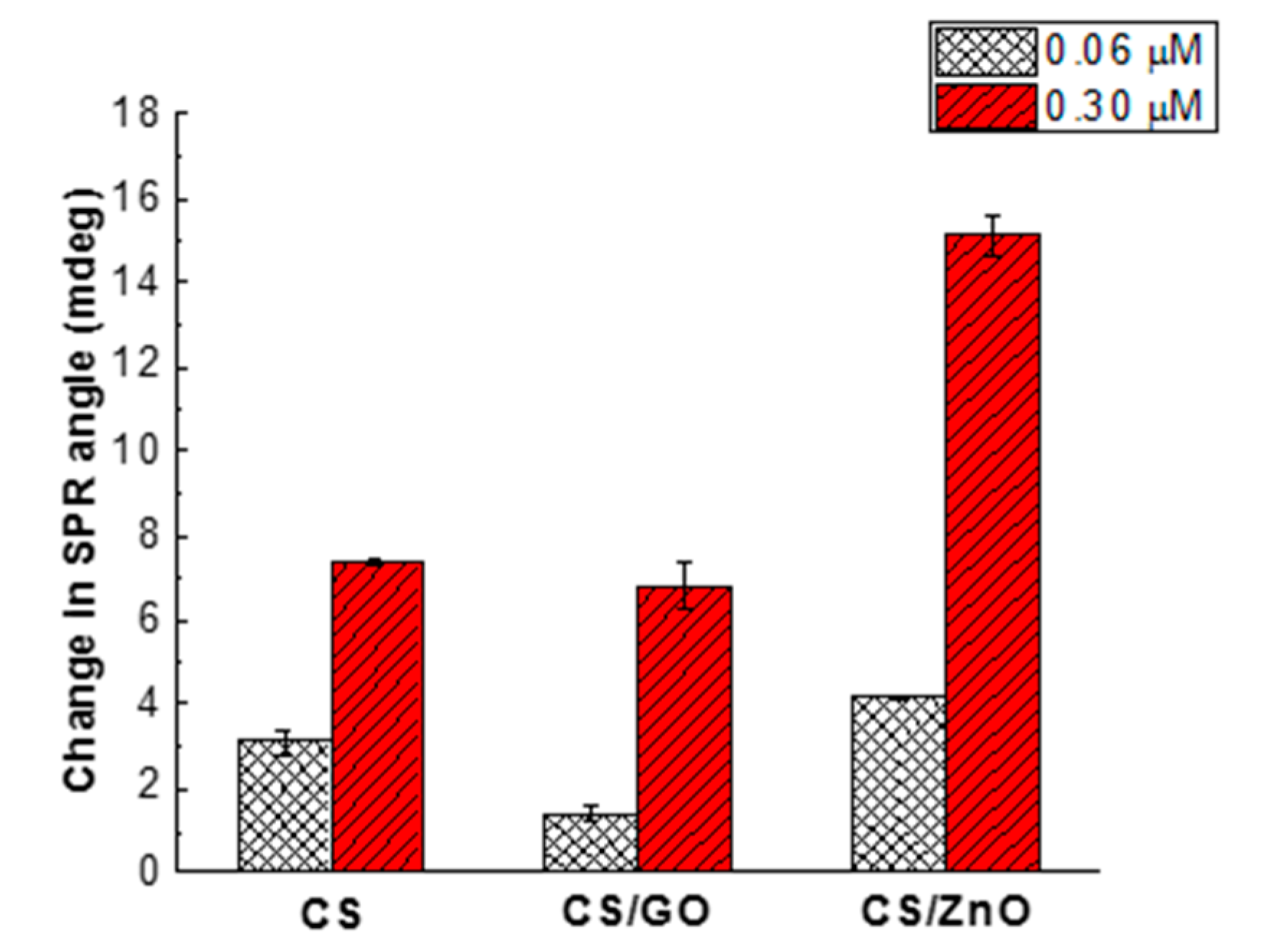
4.2. Sensitivity of CS-Based Nanocomposite Films for Glyphosate Detection
4.3. Effect of Cross-Linking Conditions on the Sensitivity of CS/ZnO Composite Films
4.4. Effect of pH on the Sorption Capacity of CS/ZnO Composite Films
4.5. CS/ZnO SPR Sensor Response for Glyphosate at pH 5.5 and Ionic Strength (I) Effect
4.6. Sorption Mechanisms
4.7. Selectivity of CS/ZnO Composite Films
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolpin, D.W.; Thurman, E.M.; Lee, E.A.; Meyer, M.T.; Furlong, E.T.; Glassmeyer, S.T. Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci. Total Environ. 2006, 354, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2010, 38, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Martins-Júnior, H.A.; Lebre, D.T.; Wang, A.Y.; Pires, M.A.F.; Bustillos, O.V. An alternative and fast method for determination of glyphosate and aminomethylphosphonic acid (AMPA) residues in soybean using liquid chromatography coupled with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.K. Effects of 2,4-D, glyphosate and paraquat on growth, photosynthesis and chlorophyll–a synthesis of Scenedesmus quadricauda Berb 614. Chemosphere 2000, 41, 177–182. [Google Scholar] [CrossRef]
- Grunewald, K.; Schmidt, W.; Unger, C.; Hanschmann, G. Behavior of glyphosate and aminomethylphosphonic acid (AMPA) in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J. Plant Nutr. Soil Sci. 2001, 164, 65–70. [Google Scholar] [CrossRef]
- Shea, P.J.; Tupy, D.R. Reversal of Cation-Induced Reduction in Glyphosate Activity by EDTA. Weed Sci. 1984, 32, 802–806. [Google Scholar] [CrossRef]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; dos Santos Afonso, M.; de Molina, M.D.C.R.; Juárez, Á.B. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Dżygiel, P.; Wieczorek, P. Extraction of glyphosate by a supported liquid membrane technique. J. Chromatogr. A 2000, 889, 93–98. [Google Scholar] [CrossRef]
- Mallat, E.; Barceló, D. Analysis and degradation study of glyphosate and of aminomethylphosphonic acid in natural waters by means of polymeric and ion-exchange solid-phase extraction columns followed by ion chromatography–post-column derivatization with fluorescence detection. J. Chromatogr. A 1998, 823, 129–136. [Google Scholar] [CrossRef]
- Xu, J.; Smith, S.; Smith, G.; Wang, W.; Li, Y. Glyphosate contamination in grains and foods: An overview. Food Control 2019, 106, 106710. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Belpoggi, F. The need for independent research on the health effects of glyphosate-based herbicides. Environ. Health 2018, 17, 51. [Google Scholar] [CrossRef]
- Xu, Y.; Li, A.J.; Li, K.; Qin, J.; Li, H. Effects of glyphosate-based herbicides on survival, development and growth of invasive snail (Pomacea canaliculata). Aquat. Toxicol. 2017, 193, 136–143. [Google Scholar] [CrossRef]
- González-Martínez, M.Á.; Brun, E.M.; Puchades, R.; Maquieira, Á.; Ramsey, K.; Rubio, F. Glyphosate Immunosensor. Application for Water and Soil Analysis. Anal. Chem. 2005, 77, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Preston, C.; Bryan, I.B.; Jutsum, A.R. Herbicide Resistance: Impact and Management. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 1997; Volume 58, pp. 57–93. [Google Scholar]
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic Acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lund-HØie, K.; Friestad, H.O. Photodegradation of the herbicide glyphosate in water. Bull. Environ. Contam. Toxicol. 1986, 36, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils – A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- Saunders, E.L.; Pezeshki, R. Glyphosate in Runoff Waters and in the Root-Zone: A Review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendon-von Osten, J.; Dzul-Caamal, R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Perry, M.J.; Mandrioli, D.; Belpoggi, F.; Manservisi, F.; Panzacchi, S.; Irwin, C. Historical evidence of glyphosate exposure from a US agricultural cohort. Environ. Health 2019, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Williams, G.M.; Aardema, M.; Acquavella, J.; Berry, S.C.; Brusick, D.; Burns, M.M.; de Camargo, J.L.V.; Garabrant, D.; Greim, H.A.; Kier, L.D.; et al. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit. Rev. Toxicol. 2016, 46, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, T.; Howsam, P.; Parsons, D.J.; Whelan, M.J. Is the EU drinking water directive standard for pesticides in drinking water consistent with the precautionary principle? Environ. Sci. Technol. 2013, 47, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ryu, S.; Sakiyama, N.; Makita, M. Simple and rapid determination of the herbicides glyphosate and glufosinate in river water, soil and carrot samples by gas chromatography with flame photometric detection. J. Chromatogr. A 1996, 726, 253–258. [Google Scholar] [CrossRef]
- Royer, A.; Beguin, S.; Tabet, J.C.; Hulot, S.; Reding, M.A.; Communal, P.Y. Determination of Glyphosate and Aminomethylphosphonic Acid Residues in Water by Gas Chromatography with Tandem Mass Spectrometry after Exchange Ion Resin Purification and Derivatization. Application on Vegetable Matrixes. Anal. Chem. 2000, 72, 3826–3832. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate detection: Methods, needs and challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Stalikas, C.D.; Pilidis, G.A.; Karayannis, M.I. An integrated gas chromatographic method towards the simultaneous determination of phosphoric and amino acid group containing pesticides. Chromatographia 2000, 51, 741–746. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, L.; Startin, J.R.; Keely, B.J.; Goodall, D.M. Analysis of glyphosate and glufosinate by capillary electrophoresis–mass spectrometry utilising a sheathless microelectrospray interface. J. Chromatogr. A 2003, 1004, 107–119. [Google Scholar] [CrossRef]
- Noori, J.S.; Dimaki, M.; Mortensen, J.; Svendsen, W.E. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmohseni, A.; Farhadi, K.; Jahangiri, S. Application of polydimethylsiloxane/acrylic resins coated quartz crystal nano balance sensor for detection of glyphosate pesticide. Int. J. Environ. Anal. Chem. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Rettke, D.; Döring, J.; Martin, S.; Venus, T.; Estrela-Lopis, I.; Schmidt, S.; Ostermann, K.; Pompe, T. Picomolar glyphosate sensitivity of an optical particle-based sensor utilizing biomimetic interaction principles. Biosens. Bioelectron. 2020, 165, 11226. [Google Scholar] [CrossRef]
- Skeff, W.; Recknagel, C.; Düwel, Y.; Schulz-Bull, D.E. Adsorption behaviors of glyphosate, glufosinate, aminomethylphosphonic acid, and 2-aminoethylphosphonic acid on three typical Baltic Sea sediments. Mar. Chem. 2018, 198, 1–9. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Daniyal, W.M.E.M.M. Optical and structural characterization of immobilized 4-(2-pyridylazo)resorcinol in chitosan-graphene oxide composite thin film and its potential for Co2+ sensing using surface plasmon resonance technique. Results Phys. 2018, 11, 118–122. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Hinman, S.S.; McKeating, K.S.; Cheng, Q. Surface Plasmon Resonance: Material and Interface Design for Universal Accessibility. Anal. Chem. 2018, 90, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, Q.; Wang, Y.; Liu, X.; Wei, T. Surface plasmon resonance sensor for theophylline using a water-compatible molecularly imprinted film. Anal. Methods 2016, 8, 2349–2356. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.-M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, C.; Ge, L.; Hayashi, K. Localized surface plasmon resonance gas sensor of Au nano-islands coated with molecularly imprinted polymer: Influence of polymer thickness on sensitivity and selectivity. Sens. Actuators B Chem. 2016, 231, 787–792. [Google Scholar] [CrossRef]
- Dhara, P.; Singh, V.K.; Olivero, M.; Perrone, G. Reflectance-based low-cost disposable optical fiber surface plasmon resonance probe with enhanced biochemical sensitivity. Opt. Eng. 2016, 55, 046114. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Qi, K.; Hu, C. Novel SPR sensing platform based on superstructure MoS2 nanosheets for ultrasensitive detection of mercury ion. Sens. Actuators B Chem. 2019, 284, 589–594. [Google Scholar] [CrossRef]
- Aguirre, M.N.; Pérez, M.L.; Colín, A.J.; Buenrostro-Gonzalez, E. Development of a Surface Plasmon Resonance n-dodecane Vapor Sensor. Sensors 2007, 7, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. Chembioeng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; El-Eswed, B.I.; Abu-Sbeih, K.A.; Arafat, T.A.; Al Omari, M.M.H.; Darras, F.H.; Badwan, A.A. Preparation of Chito-Oligomers by Hydrolysis of Chitosan in the Presence of Zeolite as Adsorbent. Mar. Drugs 2016, 14, 43. [Google Scholar]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar]
- Birlik, E.; Büyüktiryaki, S.; Ersöz, A.; Denizli, A.; Say, R. Selective Separation of Thorium Using Ion Imprinted Chitosan-Phthalate Particles via Solid Phase Extraction. Sep. Sci. Technol. 2006, 41, 3109–3121. [Google Scholar]
- Monteiro, O.A.C.; Airoldi, C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [PubMed]
- Gonçalves, V.; Fávere, V.; Rozangela, P.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polímeros 2005, 15, 6–12. [Google Scholar]
- Luk, C.; Yip, J.; Yuen, C.M.; Kan, C.; Lam, K. A Comprehensive Study on Adsorption Behaviour of Direct, Reactive and Acid Dyes on Crosslinked and Non-crosslinked Chitosan Beads. J. Fiber Bioeng. Inform. 2014, 7, 35–52. [Google Scholar]
- McIlwee, H.A.; Schauer, C.L.; Praig, V.G.; Boukherroub, R.; Szunerits, S. Thin chitosan films as a platform for SPR sensing of ferric ions. Analyst 2008, 133, 673–677. [Google Scholar]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar]
- Tabassum, R.; Gupta, B.D. Fiber optic manganese ions sensor using SPR and nanocomposite of ZnO–polypyrrole. Sens. Actuators B Chem. 2015, 220, 903–909. [Google Scholar]
- Baccarin, M.; Santos, F.A.; Vicentini, F.C.; Zucolotto, V.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical sensor based on reduced graphene oxide/carbon black/chitosan composite for the simultaneous determination of dopamine and paracetamol concentrations in urine samples. J. Electroanal. Chem. 2017, 799, 436–443. [Google Scholar]
- Magesh, G.; Bhoopathi, G.; Nithya, N.; Arun, A.P.; Ranjith Kumar, E. Tuning effect of polysaccharide Chitosan on structural, morphological, optical and photoluminescence properties of ZnO nanoparticles. Superlattices Microstruct. 2018, 117, 36–45. [Google Scholar] [CrossRef]
- Sari, E.; Üzek, R.; Duman, M.; Denizli, A. Fabrication of surface plasmon resonance nanosensor for the selective determination of erythromycin via molecular imprinted nanoparticles. Talanta 2016, 150, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Sigg, L.; Behra, P.; Stumm, W. Chimie des Milieux Aquatiques; Dunod: Paris, France, 2014. [Google Scholar]
- Charrière, D.; Cortázar, M.D.A.H.; Behra, P. Effect of the presence of pyrite traces on silver behavior in natural porous media. J. Colloid Interface Sci. 2015, 446, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, E.; Özgür, E.; Bereli, N.; Türkmen, D.; Denizli, A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater. Sci. Eng. C 2017, 73, 603–610. [Google Scholar] [CrossRef]
- Yola, M.L.; Eren, T.; Atar, N. Molecular imprinted nanosensor based on surface plasmon resonance: Application to the sensitive determination of amoxicillin. Sens. Actuators B Chem. 2014, 195, 28–35. [Google Scholar] [CrossRef]
- Dabrowski, A.; Jaroniec, M. Effects of surface heterogeneity in adsorption from binary liquid mixtures: III. Analysis of experimental data by using Langmuir—Freundlich type equations. J. Colloid Interface Sci. 1980, 73, 475–482. [Google Scholar]
- Burghoff, H.G.; Pusch, W. A model of physical adsorption of gases. J. Appl. Polym. Sci. 1979, 24, 1479–1495. [Google Scholar] [CrossRef]
- Gustavo González, A.; Ángeles Herrador, M. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef]
- Pires, N.R.; Cunha, P.L.R.; Maciel, J.S.; Angelim, A.L.; Melo, V.M.M.; de Paula, R.C.M.; Feitosa, J.P.A. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr. Polym. 2013, 91, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yang, C.Y.; Chen, A.H. Biosorption of Cu(II), Zn(II), Ni(II) and Pb(II) ions by cross-linked metal-imprinted chitosans with epichlorohydrin. J. Environ. Manag. 2011, 92, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Gök, M.K.; Demir, K.; Cevher, E.; Özgümüş, S.; Pabuccuoğlu, S. Effect of the linear aliphatic amine functionalization on in vitro transfection efficiency of chitosan nanoparticles. Carbohydr. Polym. 2019, 207, 580–587. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Wang, W.-X.; Chu, L.M. Influence of glyphosate and its formulation (Roundup®) on the toxicity and bioavailability of metals to Ceriodaphnia dubia. Environ. Pollut. 2005, 138, 59–68. [Google Scholar] [CrossRef]
- Anandhavelu, S.; Thambidurai, S. Single step synthesis of chitin/chitosan-based graphene oxide–ZnO hybrid composites for better electrical conductivity and optical properties. Electrochim. Acta 2013, 90, 194–202. [Google Scholar] [CrossRef]
- Torres, M.A.; Beppu, M.M.; Santana, C.C. Characterization of chemically modified chitosan microspheres as adsorbents using standard Proteins. Braz. J. Chem. Eng. 2007, 24, 325–336. [Google Scholar]
- Webster, A.; Halling, M.D.; Grant, D.M. Metal complexation of chitosan and its glutaraldehyde cross-linked derivative. Carbohydr. Res. 2007, 342, 1189–1201. [Google Scholar] [CrossRef]
- Yong, S.K.; Shrivastava, M.; Srivastava, P.; Kunhikrishnan, A.; Bolan, N. Environmental Applications of Chitosan and Its Derivatives. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 233, pp. 1–43. [Google Scholar]
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Mash, H.E.; Chin, Y.P.; Sigg, L.; Hari, R.; Xue, H.H. Complexation of copper by zwitterionic aminosulfonic (good) buffers. Anal. Chem. 2003, 75, 671–677. [Google Scholar] [CrossRef]
- Wernert, V. Rôle de la Matière Organique Dans le Transport et la Spéciation du Mercure. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2004. (In French). [Google Scholar]
- Kamaruddin, N.H.; Bakar, A.A.A.; Mobarak, N.N.; Zan, M.S.D.; Arsad, N. Binding affinity of a highly sensitive au/ag/au/chitosan-graphene oxide sensor based on direct detection of Pb2+ and Hg2+ ions. Sensors 2017, 17, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaruddin, N.H.; Bakar, A.A.A.; Yaacob, M.H.; Mahdi, M.A.; Zan, M.S.D.; Shaari, S. Enhancement of chitosan-graphene oxide SPR sensor with a multi-metallic layers of Au–Ag–Au nanostructure for lead(II) ion detection. Appl. Surf. Sci. 2016, 361, 177–184. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Özkütük, E.B.; Diltemiz, S.E.; Özalp, E.; Gedikbey, T.; Ersöz, A. Paraoxon imprinted biopolymer based QCM sensor. Mater. Chem. Phys. 2013, 139, 107–112. [Google Scholar] [CrossRef]
- Góes, R.E.D.; Possetti, G.R.C.; Muller, M.; Fabris, J.L. Optical Detection of Glyphosate in Water. In Proceedings of the 25th Optical Fiber Sensors Conference (OFS), Jeju, Korea, 24–28 April 2017; pp. 1–4. [Google Scholar]
- De Góes, R.E.; Muller, M.; Fabris, J.L. Spectroscopic Detection of Glyphosate in Water Assisted by Laser-Ablated Silver Nanoparticles. Sensors 2017, 17, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, L.K.S.; Chigome, S.; Torto, N.; Frost, C.L.; Pletschke, B.I. A novel colorimetric sensor strip for the detection of glyphosate in water. Sens. Actuators B Chem. 2015, 206, 357–363. [Google Scholar] [CrossRef]
- Wang, D.; Lin, B.; Cao, Y.; Guo, M.; Yu, Y. A Highly Selective and Sensitive Fluorescence Detection Method of Glyphosate Based on an Immune Reaction Strategy of Carbon Dot Labeled Antibody and Antigen Magnetic Beads. J. Agric. Food Chem. 2016, 64, 6042–6050. [Google Scholar] [CrossRef]
- Rawat, K.A.; Majithiya, R.P.; Rohit, J.V.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Mg2+ ion as a tuner for colorimetric sensing of glyphosate with improved sensitivity via the aggregation of 2-mercapto-5-nitrobenzimidazole capped silver nanoparticles. RSC Adv. 2016, 6, 47741–47752. [Google Scholar] [CrossRef]
- Döring, J.; Rettke, D.; Rödel, G.; Pompe, T.; Ostermann, K. Surface Functionalization by Hydrophobin-EPSPS Fusion Protein Allows for the Fast and Simple Detection of Glyphosate. Biosensors 2019, 29, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Sensor | Linear Range | LOD | Ref. |
|---|---|---|---|
| Colloidal Ag nanoparticles/SERS a | 237–350 µM | 10 µM | [91] |
| AgNPs b/SERS | - | 5.3 µM | [92] |
| Cu doped poly(vinyl) alcohol)) nanofiber/colorimetric | 0.59–2958 µM | 0.59 µM | [93] |
| Carbon dot labelled antibodies/magnetic NPs/fluorescence | 0.059–473 µM | 0.047 µM | [94] |
| 2-mercapto-5-nitrobenzimidazole capped AgNPs (MNBZ-AgNPs)/Mg2+/colorimetric | 399–517 nM | 17.1 nM | [95] |
| Hydrophobin-EPSPS c Fusion Protein/Spectrophotometer. | - | 0.005 μM | [96] |
| CS/ZnO/SPR | 0–0.59 μM | 0.008 μM | This work |
| Pseudo-First-Order Kinetics | |||||||||
| ka (M−1 s−1) | kd (s−1) | KA (M−1) | R2 | ||||||
| 1.9 × 104 | 5.8 × 10−4 | 3.3 × 107 | 0.998 | ||||||
| Equilibrium Isotherm Models | |||||||||
| Langmuir-type | Freundlich-type | Langmuir–Freundlich-type | |||||||
| ΔRmax (mdeg) | KL (M−1) | R2 | n | KF (mdeg.M−n) | R2 | ΔRmax (mdeg) | n | KLF,n (M−n) | R2 |
| 399 | 2.5 × 104 | 0.994 | 0.44 | 1.6 × 104 | 0.991 | 550 | 0.71 | 2.7 × 105 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, M.H.; Dubreuil, B.; Peydecastaing, J.; Vaca-Medina, G.; Nhu-Trang, T.-T.; Jaffrezic-Renault, N.; Behra, P. Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor. Sensors 2020, 20, 5942. https://doi.org/10.3390/s20205942
Do MH, Dubreuil B, Peydecastaing J, Vaca-Medina G, Nhu-Trang T-T, Jaffrezic-Renault N, Behra P. Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor. Sensors. 2020; 20(20):5942. https://doi.org/10.3390/s20205942
Chicago/Turabian StyleDo, Minh Huy, Brigitte Dubreuil, Jérôme Peydecastaing, Guadalupe Vaca-Medina, Tran-Thi Nhu-Trang, Nicole Jaffrezic-Renault, and Philippe Behra. 2020. "Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor" Sensors 20, no. 20: 5942. https://doi.org/10.3390/s20205942
APA StyleDo, M. H., Dubreuil, B., Peydecastaing, J., Vaca-Medina, G., Nhu-Trang, T.-T., Jaffrezic-Renault, N., & Behra, P. (2020). Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor. Sensors, 20(20), 5942. https://doi.org/10.3390/s20205942







