Nanostructured SmFeO3 Gas Sensors: Investigation of the Gas Sensing Performance Reproducibility for Colorectal Cancer Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. SmFeO3 Synthesis, Film Deposition and Characterization
2.2. Experimental Setups
3. Results and Discussion
3.1. Sensors Characterization
3.2. Sensor Calibration with CO
3.3. Tests with Feces
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKercher, G.R.; Salmond, J.A.; Vanos, J.K. Characteristics and applications of small, portable gaseous air pollution monitors. Environ. Pollut. 2017, 223, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, B.; Valt, M.; Parretta, C.; Gherardi, S.; Gaiardo, A.; Malagù, C.; Mantovani, F.; Strati, V.; Guidi, V. Correlation of gaseous emissions to water stress in tomato and maize crops: From field to laboratory and back. Sens. Actuators B Chem. 2020, 303, 127227. [Google Scholar] [CrossRef]
- Zonta, G.; Anania, G.; Astolfi, M.; Feo, C.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Palmonari, C.; et al. Chemoresistive sensors for colorectal cancer preventive screening through fecal odor: Double-blind approach. Sens. Actuators B Chem. 2019, 301, 127062. [Google Scholar] [CrossRef]
- Zonta, G.; Anania, G.; de Togni, A.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Palmonari, C.; Ricci, L.; et al. Use of Gas Sensors and FOBT for the Early Detection of Colorectal Cancer. Sens. Actuators B Chem. 2018, 262, 884–891. [Google Scholar] [CrossRef]
- Comini Elisabetta Faglia, G.; Sberveglieri, G. (Eds.) Solid State Gas Sensing; Springer Science & Business Media: New York, NY, USA, 2008; Volume 20. [Google Scholar]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Bagolini, A.; Gaiardo, A.; Crivellari, M.; Demenev, E.; Bartali, R.; Picciotto, A.; Valt, M.; Ficorella, F.; Guidi, V.; Bellutti, P. Development of MEMS MOS gas sensors with CMOS compatible PECVD inter-metal passivation. Sens. Actuators B Chem. 2019, 292, 225–232. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, Selectivity, and Stability of Gas-Sensitive Metal-Oxide Nanostructures. In Metal Oxide Nanostructures and Their Applications; American Scientific Publishers: Valencia, CA, USA, 2010; pp. 31–52. [Google Scholar]
- Fonollosa, J.; Rodríguez-Luján, I.; Huerta, R. Chemical gas sensor array dataset. Data Brief. 2015, 3, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Magna, G.; Di Natale, C.; Martinelli, E. Self-Repairing classification algorithms for chemical sensor array. Sens. Actuators B Chem. 2019, 297, 126721. [Google Scholar] [CrossRef] [Green Version]
- Guidi, V.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Malagù, C.; Zonta, G.; Bellutti, P. Metal sulfides as a new class of sensing materials. Procedia Eng. 2015, 120, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Gaiardo, A.; Fabbri, B.; Giberti, A.; Valt, M.; Gherardi, S.; Guidi, V.; Malagù, C.; Bellutti, P.; Pepponi, G.; Casotti, D.; et al. Tunable formation of nanostructured SiC/SiOC core-shell for selective detection of SO2. Sens. Actuators B Chem. 2020, 305, 127485. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gómez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Valt, M.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Casotti, D.; Cruciani, G.; Pepponi, G.; Vanzetti, L.; Iacob, E.; Malagu, C.; et al. Aza-crown-ether functionalized graphene oxide for gas sensing and cation trapping applications. Mater. Res. Express. 2019, 6, 075603. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Kusior, A.; Rekas, M.; Zukrowski, J.; Gajewska, M.; Michalow-Mauke, K.; Graule, T.; Radecka, M.; Zakrzewska, K. Nanocrystalline TiO2-SnO2 heterostructures for gas sensing. Beilstein J. Nanotechnol. 2017, 8, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Mori, M.; Itagaki, Y.; Iseda, J.; Sadaoka, Y.; Ueda, T.; Mitsuhashi, H.; Nakatani, M. Influence of VOC structures on sensing property of SmFeO3 semiconductive gas sensor. Sens. Actuators B Chem. 2014, 202, 873–877. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.-Q.; Ji, H.-M.; Yu, J.-J. Semiconducting nano-structured SmFeO3-based thin films prepared by novel sol-gel method for acetone gas sensors. Integr. Ferroelectr. 2014, 152, 29–35. [Google Scholar] [CrossRef]
- Aono, H.; Sato, M.; Traversa, E.; Sakamoto, M.; Sadaoka, Y. Design of ceramic materials for chemical sensors: Effect of SmFeO3 processing on surface and electrical properties. J. Am. Ceram. Soc. 2001, 84, 341–347. [Google Scholar] [CrossRef]
- Huang, H.-T.; Zhang, W.-L.; Zhang, X.-D.; Guo, X. NO2 sensing properties of SmFeO3 porous hollow microspheres. Sens. Actuators B Chem. 2018, 265, 443–451. [Google Scholar] [CrossRef]
- Mori, M.; Fujita, J.; Itagaki, Y.; Sadaoka, Y. Ozone detection in air using SmFeO3 gas sensor for air quality classification. J. Ceram. Soc. Jpn. 2011, 119, 926–928. [Google Scholar] [CrossRef] [Green Version]
- Tomoda, M.; Okano, S.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Air quality prediction by using semiconducting gas sensor with newly fabricated SmFeO3 film. Sens. Actuators B Chem. 2004, 97, 190–197. [Google Scholar] [CrossRef]
- Zonta, G.; Astolfi, M.; Casotti, D.; Cruciani, G.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Guidi, V.; Landini, N.; Valt, M.; et al. Reproducibility tests with zinc oxide thick-film sensors. Ceram. Int. 2020, 46, 6847–6855. [Google Scholar] [CrossRef]
- Zonta, G.; Anania, G.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Landini, N.; Malagù, C.; Scagliarini, L.; Guidi, V. Preventive screening of colorectal cancer with a device based on chemoresistive sensors. Sens. Actuators B Chem. 2016, 238, 1098–1101. [Google Scholar] [CrossRef]
- Scholkopf, B.; Smola, A.J. Learning with Kernels: Support Vector Machines, Regularization, Optimization and Beyond; The MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Aharon, M.; Elad, M.; Bruckstein, A. K-SVD: An Algorithm for Designing Overcomplete Dictionaries for Sparse Representation. IEEE Trans. Signal Process. 2006, 54, 4311–4322. [Google Scholar] [CrossRef]
- Bruker, A.X.S. TOPAS 4−1 User Manual; Bruker AXS GmbH: Karlsruhe, Germany, 2009. [Google Scholar]
- Cheary, R.W.; Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental parameters line profile fitting in laboratory diffractometers. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 1. [Google Scholar] [CrossRef]
- Kern, A.; Coelho, A.A.; Cheary, R.W. Convolution based profile fitting. In Diffraction Analysis of the Microstructure of Materials; Springer: Berlin/Heidelberg, Germany, 2004; pp. 17–50. [Google Scholar]
- Scardi, P.; Leoni, M. Whole powder pattern modelling: Theory and applications. In Diffraction Analysis of the Microstructure of Materials; Springer: Berlin/Heidelberg, Germany, 2004; pp. 51–91. [Google Scholar]
- Balzar, D.; Popa, N.C. Crystallite size and residual strain/stress modeling in rietveld refinement. In Diffraction Analysis of the Microstructure of Materials; Springer: Berlin/Heidelberg, Germany, 2004; pp. 125–145. [Google Scholar]
- Snyder, R.; Bunge, H.; Fiala, J. Defect and Microstructure Analysis by Diffraction; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Speranza, G.; Canteri, R. RxpsG a new open project for Photoelectron and Electron Spectroscopy data processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- SCENT A1, Italian Patent Number: RM20144000595, Patent and Intellectual Property of SCENT S.r.l. European Patent Number (UK, Germany Extension) 3210013, 30 August 2017.
- Acceptance Letter from the Ethics Committee of Ferrara, Valutazione Dell’accuratezza della Determinazione di Composti Organici Volatili Quali Biomarker di Neoplasia Colo-Rettale Nelle feci di Soggetti FOBT-Positivi Sottoposti a Colonscopia, 21/01/2016, Protocol Number: 151298. Available online: https://www.quadernodiepidemiologia.it/epi/campion/dimens.htm (accessed on 14 October 2020).
- Sone, B.T.; Manikandan, E.; Gurib-Fakim, A.; Maaza, M. Sm2O3 nanoparticles green synthesis via Callistemon viminalis’ extract. J. Alloys Compd. 2015, 650, 357–362. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention Website. Available online: https://www.cdc.gov/niosh/pel88/630-08.html (accessed on 14 September 2020).
- Qi, Q.; Zhang, T.; Liu, L.; Zheng, X.; Yu, Q.; Zeng, Y.; Yang, H. Selective acetone sensor based on dumbbell-like ZnO with rapid response and recovery. Sens. Actuators B Chem. 2008, 134, 166–170. [Google Scholar] [CrossRef]



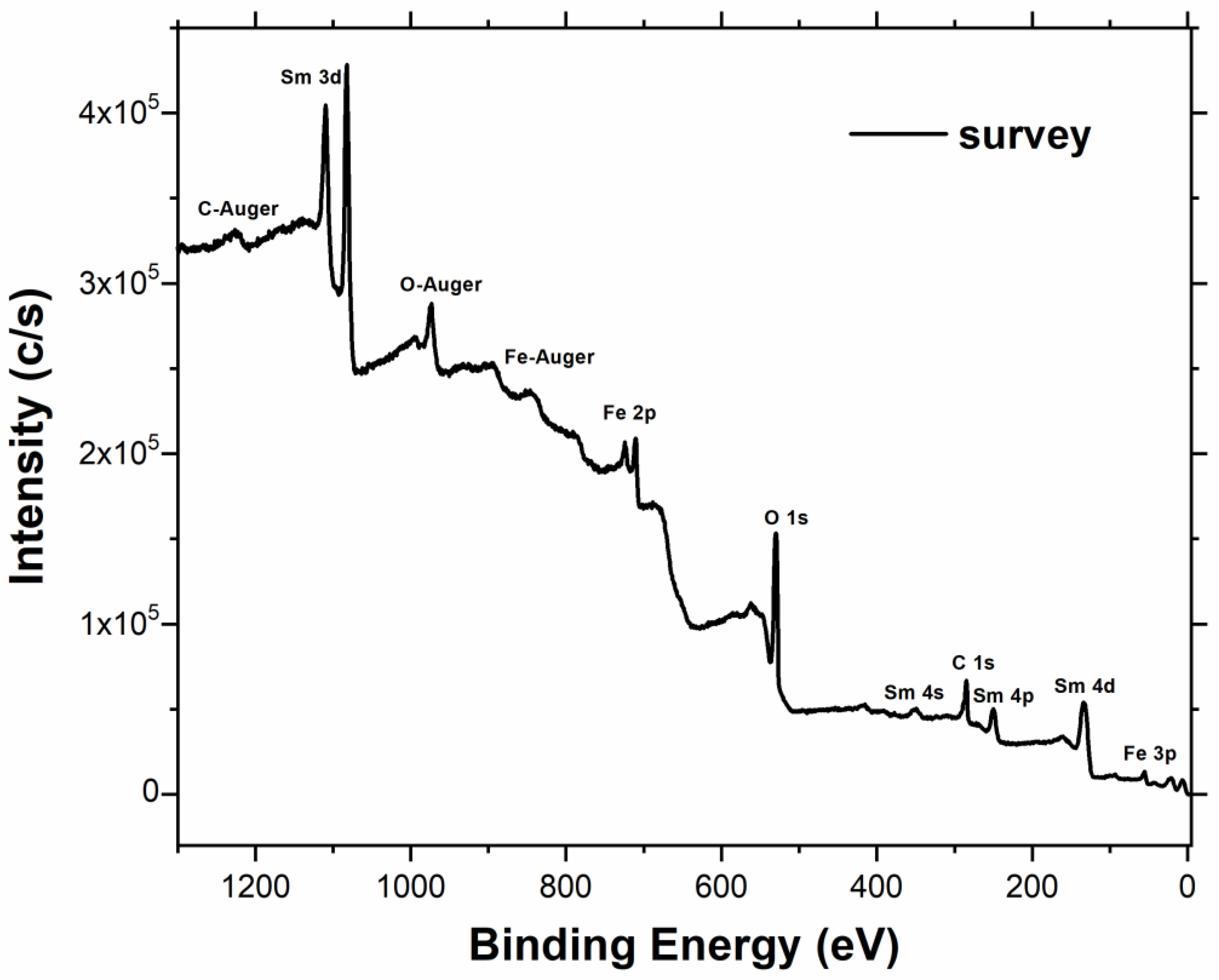



| a (Å) | 5.40077 ± 0.00023 |
| b (Å) | 5.59920 ± 0.00024 |
| c (Å) | 7.71295 ± 0.00034 |
| Cell Volume (Å3) | 233.239 |
| Sm (at%) | Fe (at%) | O (at%) | C (at%) |
|---|---|---|---|
| 16.67 | 17.25 | 56.82 | 9.26 |
| Sm (at%) | Fe (at%) | O (at%) | C (at%) |
|---|---|---|---|
| 15.5 | 7.0 | 33.0 | 44.5 |
| SENSORS | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| R (100 ppm) | 1.39 | 1.36 | 1.40 | 1.38 |
| |R − <R>| | 0.00750 | 0.0225 | 0.0175 | 0.00250 |
| 2% R | 0.0278 | 0.0272 | 0.0280 | 0.0276 |
| R (50 ppm) | 1.23 | 1.24 | 1.24 | 1.25 |
| |R − <R>| | 0.0100 | 0 | 0 | 0.0100 |
| 1% R | 0.0123 | 0.0124 | 0.0124 | 0.0125 |
| R (25 ppm) | 1.16 | 1.16 | 1.17 | 1.16 |
| |R − <R>| | 0.00250 | 0.00250 | 0.00750 | 0.00250 |
| 1% R | 0.0116 | 0.0116 | 0.0117 | 0.0116 |
| SENSORS | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
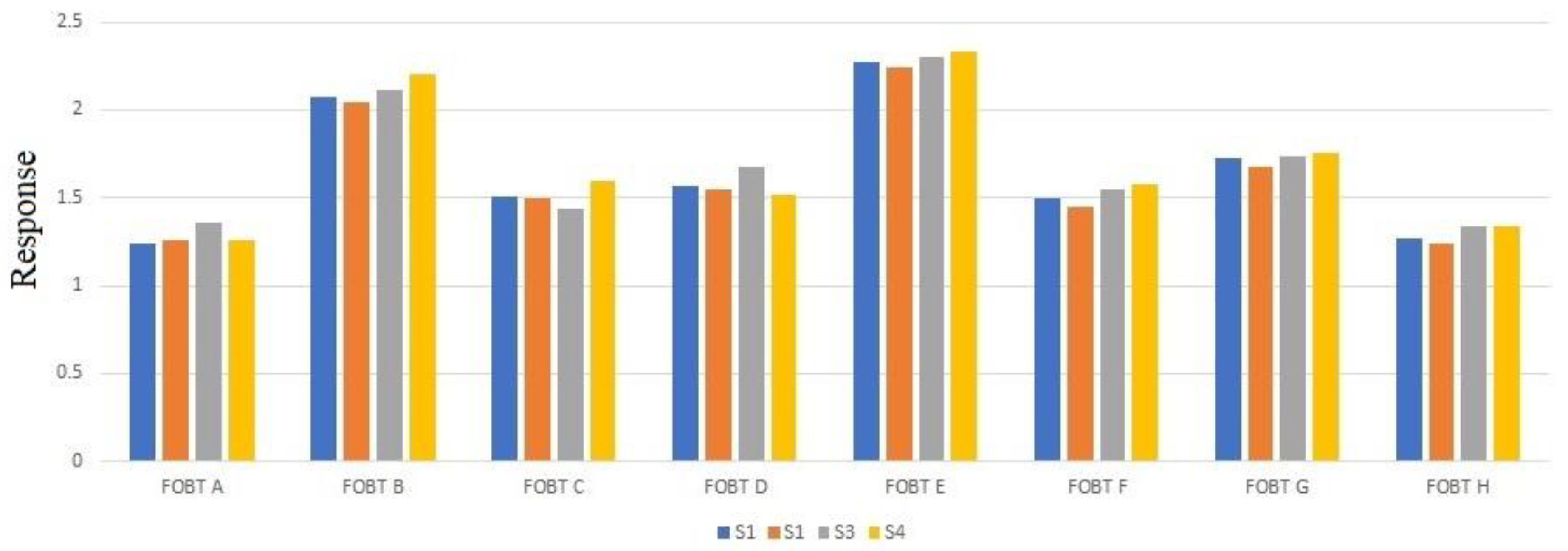
| FOBT A | 1.24 | 1.26 | 1.36 | 1.26 |
| |R − <R>| | 0.0400 | 0.0200 | 0.0800 | 0.0200 |
| 6% R | 0.0744 | 0.0756 | 0.0816 | 0.0756 |
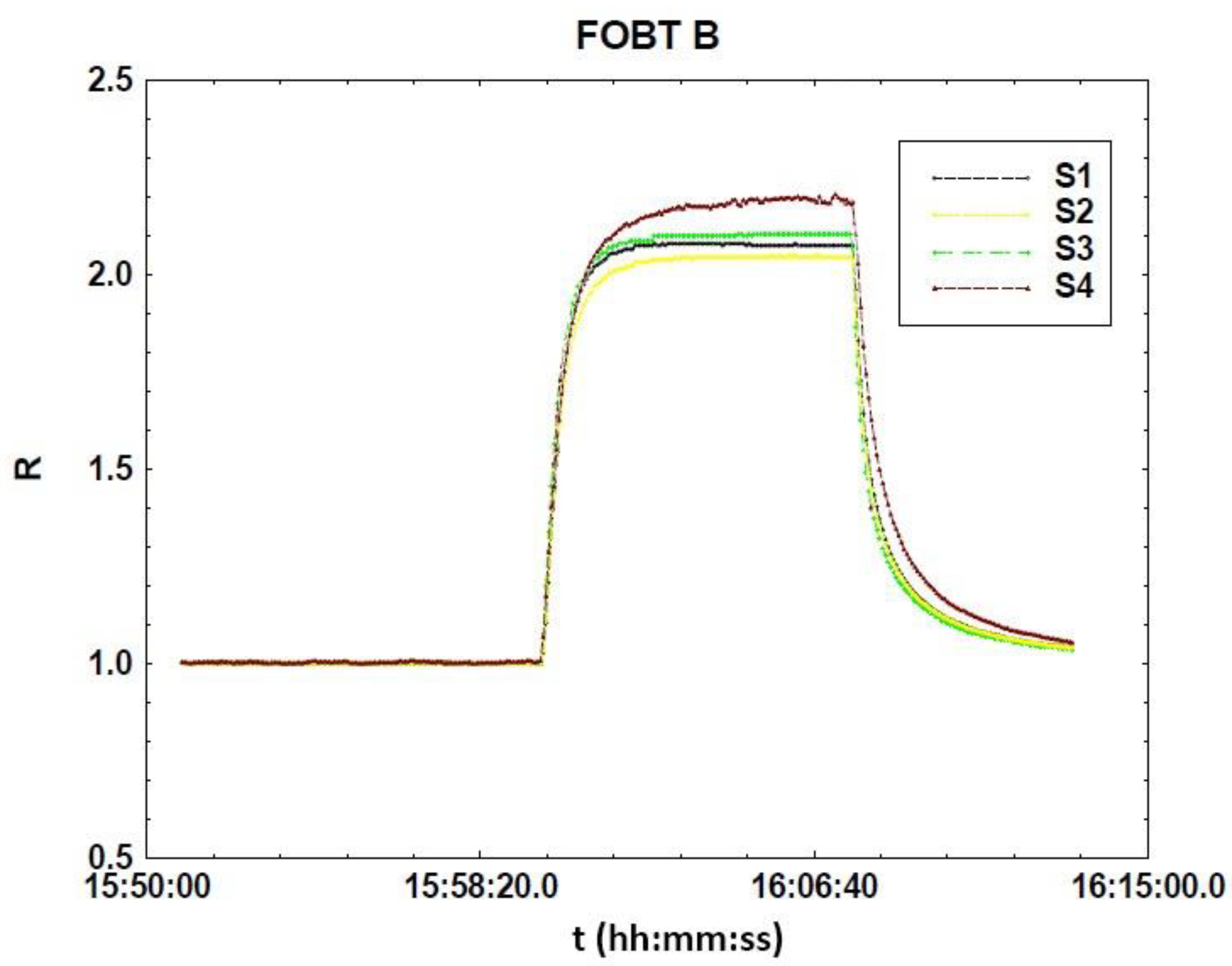
| FOBT B | 2.07 | 2.04 | 2.11 | 2.2 |
| |R − <R>| | 0.0350 | 0.0650 | 0.00500 | 0.0950 |
| 4% R | 0.104 | 0.102 | 0.106 | 0.110 |
| FOBT C | 1.51 | 1.50 | 1.44 | 1.60 |
| |R − <R>| | 0.00250 | 0.0125 | 0.0725 | 0.0950 |
| 6% R | 0.0906 | 0.0900 | 0.0864 | 0.0960 |
| FOBT D | 1.57 | 1.55 | 1.68 | 1.52 |
| |R − <R>| | 0.0100 | 0.0300 | 0.100 | 0.0600 |
| 6% R | 0.0942 | 0.0930 | 0.101 | 0.0912 |
| FOBT E | 2.27 | 2.24 | 2.30 | 2.33 |
| |R − <R>| | 0.0150 | 0.0450 | 0.0150 | 0.0450 |
| 3% R | 0.0681 | 0.0672 | 0.0690 | 0.0699 |
| FOBT F | 1.50 | 1.45 | 1.55 | 1.58 |
| |R − <R>| | 0.0200 | 0.0700 | 0.0300 | 0.0600 |
| 5% R | 0.0750 | 0.0725 | 0.0775 | 0.0790 |
| FOBT G | 1.73 | 1.68 | 1.74 | 1.76 |
| |R − <R>| | 0.00250 | 0.0475 | 0.0125 | 0.0325 |
| 3% R | 0.0519 | 0.0504 | 0.0522 | 0.0528 |
| FOBT H | 1.27 | 1.24 | 1.34 | 1.34 |
| |R − <R>| | 0.0275 | 0.0575 | 0.0425 | 0.0425 |
| 5% R | 0.0635 | 0.0620 | 0.0670 | 0.0670 |
| Response Time (s) | ||||
|---|---|---|---|---|
| SENSORS | S1 | S2 | S3 | S4 |
| FOBT A | 72 | 75 | 78 | 70 |
| |ResT − <ResT>| | 1.75 | 1.25 | 4.25 | 3.75 |
| 6% ResT | 4.32 | 4.50 | 4.68 | 4.20 |
| FOBT B | 65 | 69 | 67 | 74 |
| |ResT − <ResT>| | 3.75 | 0.25 | 1.75 | 5.25 |
| 8% ResT | 5.20 | 5.52 | 5.36 | 5.92 |
| FOBT C | 103 | 100 | 96 | 92 |
| |ResT − <ResT>| | 5.25 | 2.25 | 1.75 | 5.75 |
| 7% ResT | 7.21 | 7.00 | 6.72 | 6.44 |
| FOBT D | 131 | 135 | 118 | 119 |
| |ResT − <ResT>| | 5.25 | 9.25 | 7.75 | 6.75 |
| 7% ResT | 9.17 | 9.45 | 8.26 | 8.33 |
| FOBT E | 73 | 71 | 77 | 81 |
| |ResT − <ResT>| | 2.50 | 4.50 | 1.50 | 5.50 |
| 7% ResT | 5.11 | 4.97 | 5.39 | 5.67 |
| FOBT F | 93 | 95 | 89 | 86 |
| |ResT − <ResT>| | 2.25 | 4.25 | 1.75 | 4.75 |
| 6% ResT | 5.58 | 5.70 | 5.34 | 5.16 |
| FOBT G | 101 | 96 | 93 | 99 |
| |ResT − <ResT>| | 3.75 | 1.25 | 4.25 | 1.75 |
| 5% ResT | 5.05 | 4.80 | 4.65 | 4.95 |
| FOBT H | 83 | 86 | 81 | 79 |
| |ResT − <ResT>| | 0.75 | 3.75 | 1.25 | 3.25 |
| 5% ResT | 4.15 | 4.30 | 4.05 | 3.95 |
| Recovery Time (s) | ||||
|---|---|---|---|---|
| SENSORS | S1 | S2 | S3 | S4 |
| FOBT A | 227 | 224 | 231 | 211 |
| |RecT − <RecT>| | 3.75 | 0.75 | 7.75 | 12.25 |
| 6% RecT | 13.62 | 13.44 | 13.86 | 12.66 |
| FOBT B | 157 | 153 | 147 | 168 |
| |RecT − <RecT>| | 0.75 | 3.25 | 9.25 | 11.75 |
| 7% RecT | 10.99 | 10.71 | 10.29 | 11.76 |
| FOBT C | 132 | 131 | 121 | 124 |
| |RecT − <RecT>| | 5 | 4 | 6 | 3 |
| 5% RecT | 6.6 | 6.55 | 6.05 | 6.2 |
| FOBT D | 231 | 220 | 239 | 219 |
| |RecT − <RecT>| | 3.75 | 7.25 | 11.75 | 8.25 |
| 5% RecT | 11.55 | 11 | 11.95 | 10.95 |
| FOBT E | 182 | 173 | 175 | 167 |
| |RecT − <RecT>| | 7.75 | 1.25 | 0.75 | 7.25 |
| 5% RecT | 9.1 | 8.65 | 8.75 | 8.35 |
| FOBT F | 204 | 209 | 193 | 211 |
| |RecT − <RecT>| | 0.25 | 4.75 | 11.25 | 6.75 |
| 6% RecT | 12.24 | 12.54 | 11.58 | 12.66 |
| FOBT G | 171 | 173 | 166 | 181 |
| |RecT − <RecT>| | 1.75 | 0.25 | 6.75 | 8.25 |
| 5% RecT | 8.55 | 8.65 | 8.3 | 9.05 |
| FOBT H | 214 | 221 | 218 | 208 |
| |RecT − <RecT>| | 1.25 | 5.75 | 2.75 | 7.25 |
| 4% RecT | 8.56 | 8.84 | 8.72 | 8.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaiardo, A.; Zonta, G.; Gherardi, S.; Malagù, C.; Fabbri, B.; Valt, M.; Vanzetti, L.; Landini, N.; Casotti, D.; Cruciani, G.; et al. Nanostructured SmFeO3 Gas Sensors: Investigation of the Gas Sensing Performance Reproducibility for Colorectal Cancer Screening. Sensors 2020, 20, 5910. https://doi.org/10.3390/s20205910
Gaiardo A, Zonta G, Gherardi S, Malagù C, Fabbri B, Valt M, Vanzetti L, Landini N, Casotti D, Cruciani G, et al. Nanostructured SmFeO3 Gas Sensors: Investigation of the Gas Sensing Performance Reproducibility for Colorectal Cancer Screening. Sensors. 2020; 20(20):5910. https://doi.org/10.3390/s20205910
Chicago/Turabian StyleGaiardo, Andrea, Giulia Zonta, Sandro Gherardi, Cesare Malagù, Barbara Fabbri, Matteo Valt, Lia Vanzetti, Nicolò Landini, Davide Casotti, Giuseppe Cruciani, and et al. 2020. "Nanostructured SmFeO3 Gas Sensors: Investigation of the Gas Sensing Performance Reproducibility for Colorectal Cancer Screening" Sensors 20, no. 20: 5910. https://doi.org/10.3390/s20205910








