Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field
Abstract
1. Introduction
2. The Current Situation in Clinical Care
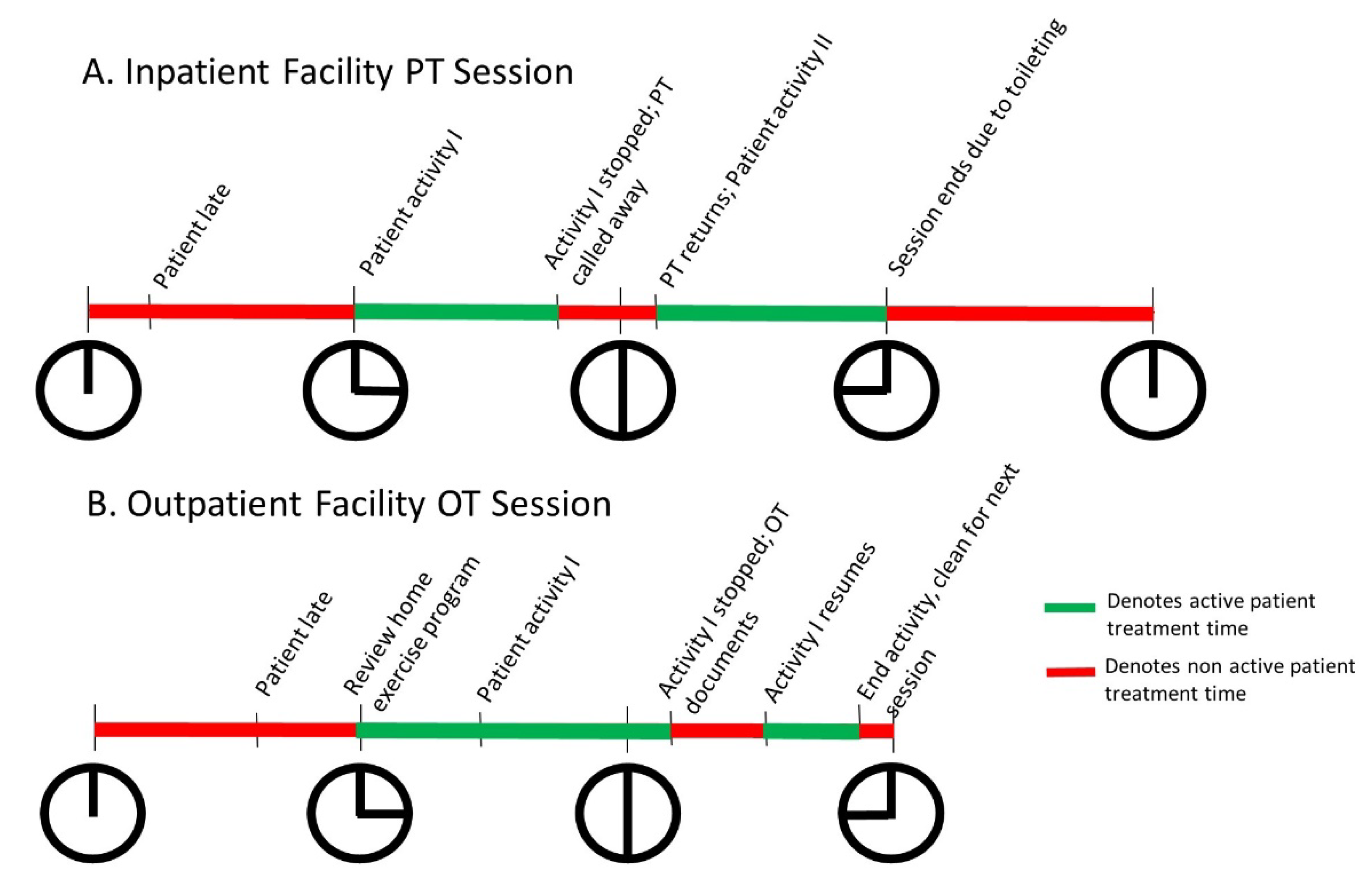
2.1. Busy Clinical Practice Affords Little Time for Anything Else
2.2. Clinicians Are Still Building towards Understanding the Added Value of Wearable Sensor Data for Clinical Rehabilitation Practice
3. The Current Situation with Wearable Device Systems
3.1. Commerically-Available, Consumer-Grade Device Algorithms Have Limited Accuracy in Disabled Patient Populations
3.2. Research-Grade Device Systems Are Expensive and Not yet Clinician- and Patient-Friendly
3.3. Standardization of Output Variables in Research Is Limited to Date, with Much Work to Do
3.4. Different Clinical Populations Will Need Different Metrics for Clinical Decision-Making
3.5. Special Considerations for Complexity in Some Populations
3.5.1. Children
3.5.2. Individuals with Cognitive Deficits
4. Benchmarks for Future Development
4.1. Proposed Benchmarks
4.2. Example Application of Benchmarks to A Currently-Available System
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Towards A Common Language for Functioning, Disability, and Health: ICF; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Dobkin, B.H.; Martinez, C. Wearable Sensors to Monitor, Enable Feedback, and Measure Outcomes of Activity and Practice. Curr. Neurol. Neurosci. Rep. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.R.; Bird, M.L.; Menon, C.; Eng, J.J. Perspectives on the prospective development of stroke-specific lower extremity wearable monitoring technology: A qualitative focus group study with physical therapists and individuals with stroke. J. Neuroeng. Rehabil. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C. Measuring by Value Not Volume. Available online: https://www.apta.org/apta-magazine/2015/07/01/measuring-by-value-not-volume (accessed on 15 July 2020).
- Host, H.H.; Lang, C.E.; Hildebrand, M.W.; Zou, D.; Binder, E.F.; Baum, C.M.; Freedland, K.E.; Morrow-Howell, N.; Lenze, E.J. Patient Active Time During Therapy Sessions in Postacute Rehabilitation: Development and Validation of a New Measure. Phys. Occup. Ther. Geriatr. 2014, 32, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Talkowski, J.B.; Lenze, E.J.; Munin, M.C.; Harrison, C.; Brach, J.S. Patient participation and physical activity during rehabilitation and future functional outcomes in patients after hip fracture. Arch. Phys. Med. Rehabil. 2009, 90, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Bayley, M.T.; Hurdowar, A.; Richards, C.L.; Korner-Bitensky, N.; Wood-Dauphinee, S.; Eng, J.J.; McKay-Lyons, M.; Harrison, E.; Teasell, R.; Harrison, M.; et al. Barriers to implementation of stroke rehabilitation evidence: Findings from a multi-site pilot project. Disabil. Rehabil. 2012, 34, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Noonan, V.K.; Moore, J.L. Knowledge Translation: The Catalyst for Innovation of Neurologic Physical Therapy. J. Neurol. Phys. Ther. 2016, 40, 67–70. [Google Scholar] [CrossRef]
- Wright, S.P.; Hall Brown, T.S.; Collier, S.R.; Sandberg, K. How consumer physical activity monitors could transform human physiology research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R358–R367. [Google Scholar] [CrossRef]
- Standards of Practice for Physical Therapy. Available online: https://www.apta.org/apta-and-you/leadership-and-governance/policies/standards-of-practice-pt (accessed on 15 July 2020).
- Blau, R.; Bolus, S.; Carolan, T.; Kramer, D.; Mahoney, E.; Jette, D.U.; Beal, J.A. The experience of providing physical therapy in a changing health care environment. Phys. Ther. 2002, 82, 648–657. [Google Scholar] [CrossRef]
- Lang, C.E.; Waddell, K.J.; Klaesner, J.W.; Bland, M.D. A Method for Quantifying Upper Limb Performance in Daily Life Using Accelerometers. J. Vis. Exp. 2017, 122, 55673. [Google Scholar] [CrossRef]
- Physical Therapy Outcomes Registry. Available online: http://www.ptoutcomes.com/home.aspx (accessed on 4 September 2020).
- Moore, J.L.; Potter, K.; Blankshain, K.; Kaplan, S.L.; O’Dwyer, L.C.; Sullivan, J.E. A Core Set of Outcome Measures for Adults with Neurologic Conditions Undergoing Rehabilitation: A CLINICAL PRACTICE GUIDELINE. J. Neurol. Phys. Ther. 2018, 42, 174–220. [Google Scholar] [CrossRef]
- Waddell, K.J.; Birkenmeier, R.L.; Bland, M.D.; Lang, C.E. An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disabil. Rehabil. 2016, 38, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Danks, K.A.; Pohlig, R.T.; Roos, M.; Wright, T.R.; Reisman, D.S. Relationship Between Walking Capacity, Biopsychosocial Factors, Self-efficacy, and Walking Activity in Persons Poststroke. J. Neurol. Phys. Ther. 2016, 40, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated with Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Holleran, C.L.; Bland, M.D.; Reisman, D.S.; Ellis, T.D.; Earhart, G.M.; Lang, C.E. Day-to-Day Variability of Walking Performance Measures in Individuals Poststroke and Individuals with Parkinson Disease. J. Neurol. Phys. Ther. 2020, 44, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Waddell, K.J.; Lang, C.E. Comparison of Self-Report Versus Sensor-Based Methods for Measuring the Amount of Upper Limb Activity Outside the Clinic. Arch. Phys. Med. Rehabil. 2018, 99, 1913–1916. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil. Neural. Repair. 2012, 26, 76–84. [Google Scholar] [CrossRef]
- Doman, C.A.; Waddell, K.J.; Bailey, R.R.; Moore, J.L.; Lang, C.E. Changes in Upper-Extremity Functional Capacity and Daily Performance During Outpatient Occupational Therapy for People with Stroke. Am. J. Occup. Ther. 2016, 70, 7003290040p1–7003290040p11. [Google Scholar] [CrossRef]
- Waddell, K.J.; Strube, M.J.; Bailey, R.R.; Klaesner, J.W.; Birkenmeier, R.L.; Dromerick, A.W.; Lang, C.E. Does Task-Specific Training Improve Upper Limb Performance in Daily Life Poststroke? Neurorehabil. Neural. Repair. 2017, 31, 290–300. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Henderson, C.E.; Hornby, T.G. Improved walking function in laboratory does not guarantee increased community walking in stroke survivors: Potential role of gait biomechanics. J. Biomech. 2019, 91, 151–159. [Google Scholar] [CrossRef]
- Benson, L.C.; Clermont, C.A.; Bosnjak, E.; Ferber, R. The use of wearable devices for walking and running gait analysis outside of the lab: A systematic review. Gait Posture 2018, 63, 124–138. [Google Scholar] [CrossRef]
- Braito, I.; Maselli, M.; Sgandurra, G.; Inguaggiato, E.; Beani, E.; Cecchi, F.; Cioni, G.; Boyd, R. Assessment of upper limb use in children with typical development and neurodevelopmental disorders by inertial sensors: A systematic review. J. Neuroeng. Rehabil. 2018, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, K.J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef] [PubMed]
- Danks, K.A.; Pohlig, R.; Reisman, D.S. Combining Fast-Walking Training and a Step Activity Monitoring Program to Improve Daily Walking Activity After Stroke: A Preliminary Study. Arch. Phys. Med. Rehabil. 2016, 97 (Suppl. 9), S185–S193. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Dorsch, A. The promise of mHealth: Daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil. Neural. Repair. 2011, 25, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Danks, K.A.; Roos, M.A.; McCoy, D.; Reisman, D.S. A step activity monitoring program improves real world walking activity post stroke. Disabil. Rehabil. 2014, 36, 2233–2236. [Google Scholar] [CrossRef]
- Moore, J.L.; Roth, E.J.; Killian, C.; Hornby, T.G. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke 2010, 41, 129–135. [Google Scholar] [CrossRef]
- Urbin, M.A.; Waddell, K.J.; Lang, C.E. Acceleration Metrics Are Responsive to Change in Upper Extremity Function of Stroke Survivors. Arch. Phys. Med. Rehabil. 2015, 96, 854–861. [Google Scholar] [CrossRef]
- Moore, S.A.; Hallsworth, K.; Plotz, T.; Ford, G.A.; Rochester, L.; Trenell, M.I. Physical activity, sedentary behaviour and metabolic control following stroke: A cross-sectional and longitudinal study. PLoS ONE 2013, 8, e55263. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Ellis, T.D.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Capturing ambulatory activity decline in Parkinson’s disease. J. Neurol. Phys. Ther. 2012, 36, 51–57. [Google Scholar] [CrossRef]
- Goldsack, J.C.; Coravos, A.; Bakker, J.P.; Bent, B.; Dowling, A.V.; Fitzer-Attas, C.; Godfrey, A.; Godino, J.G.; Gujar, N.; Izmailova, E.; et al. Verification, analytical validation, and clinical validation (V3): The foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit. Med. 2020, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, T.; Kooiman, T.J.; Krijnen, W.P.; Van der Schans, C.P.; De Groot, M. Reliability and Validity of Ten Consumer Activity Trackers Depend on Walking Speed. Med. Sci. Sports Exerc. 2017, 49, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Tophoj, K.H.; Petersen, M.G.; Saebye, C.; Baad-Hansen, T.; Wagner, S. Validity and Reliability Evaluation of Four Commercial Activity Trackers’ Step Counting Performance. Telemed. J. E Health 2018, 24, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Duclos, N.C.; Aguiar, L.T.; Aissaoui, R.; Faria, C.; Nadeau, S.; Duclos, C. Activity Monitor Placed at the Nonparetic Ankle Is Accurate in Measuring Step Counts During Community Walking in Poststroke Individuals: A Validation Study. PM R 2019, 11, 963–971. [Google Scholar] [CrossRef]
- Macko, R.F.; Haeuber, E.; Shaughnessy, M.; Coleman, K.L.; Boone, D.A.; Smith, G.V.; Silver, K.H. Microprocessor-based ambulatory activity monitoring in stroke patients. Med. Sci. Sports Exerc. 2002, 34, 394–399. [Google Scholar] [CrossRef]
- Fulk, G.D.; Combs, S.A.; Danks, K.A.; Nirider, C.D.; Raja, B.; Reisman, D.S. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys. Ther. 2014, 94, 222–229. [Google Scholar] [CrossRef]
- Tedesco, S.; Sica, M.; Ancillao, A.; Timmons, S.; Barton, J.; O’Flynn, B. Accuracy of consumer-level and research-grade activity trackers in ambulatory settings in older adults. PLoS ONE 2019, 14, e0216891. [Google Scholar] [CrossRef]
- Feehan, L.M.; Geldman, J.; Sayre, E.C.; Park, C.; Ezzat, A.M.; Yoo, J.Y.; Hamilton, C.B.; Li, L.C. Accuracy of Fitbit Devices: Systematic Review and Narrative Syntheses of Quantitative Data. JMIR Mhealth Uhealth 2018, 6, e10527. [Google Scholar] [CrossRef]
- Larsen, R.T.; Korfitsen, C.B.; Juhl, C.B.; Andersen, H.B.; Langberg, H.; Christensen, J. Criterion validity for step counting in four consumer-grade physical activity monitors among older adults with and without rollators. Eur. Rev. Aging Phys. Act. 2020, 17, 1. [Google Scholar] [CrossRef]
- Rozanski, G.M.; Aqui, A.; Sivakumaran, S.; Mansfield, A. Consumer Wearable Devices for Activity Monitoring Among Individuals After a Stroke: A Prospective Comparison. JMIR Cardio 2018, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.L.; Taylor, J.; Crighton, L.J.; Goodbrand, J.A.; McMurdo, M.E.T.; Witham, M.D. Validation of the AX3 triaxial accelerometer in older functionally impaired people. Aging Clin. Exp. Res. 2017, 29, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Wendel, N.; Macpherson, C.E.; Webber, K.; Hendron, K.; DeAngelis, T.; Colon-Semenza, C.; Ellis, T. Accuracy of Activity Trackers in Parkinson Disease: Should We Prescribe Them? Phys. Ther. 2018, 98, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Maganja, S.A.; Clarke, D.C.; Lear, S.A.; Mackey, D.C. Formative Evaluation of Consumer-Grade Activity Monitors Worn by Older Adults: Test-Retest Reliability and Criterion Validity of Step Counts. JMIR Form. Res. 2020, 4, e16537. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Motl, R.W.; Pilutti, L.A.; Learmonth, Y.C.; Ensari, I.; Dlugonski, D.; Klaren, R.E.; Balantrapu, S.; Riskin, B.J. Accuracy of StepWatch and ActiGraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS ONE 2014, 9, e93511. [Google Scholar] [CrossRef] [PubMed]
- Wendland, D.M.; Sprigle, S.H. Activity monitor accuracy in persons using canes. J. Rehabil. Res. Dev. 2012, 49, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.; Stott, N.S. Test--retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin. Rehabil. 2008, 22, 871–877. [Google Scholar] [CrossRef]
- Bowden, M.G.; Behrman, A.L. Step Activity Monitor: Accuracy and test-retest reliability in persons with incomplete spinal cord injury. J. Rehabil. Res. Dev. 2007, 44, 355–362. [Google Scholar] [CrossRef]
- Haeuber, E.; Shaughnessy, M.; Forrester, L.W.; Coleman, K.L.; Macko, R.F. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1997–2001. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Pennypacker, M.L.; Thrush, A.H.; Leiper, C.I.; Craik, R.L. Validity of the StepWatch Step Activity Monitor: Preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J. Geriatr. Phys. Ther. 2011, 34, 41–45. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J.; Tang, P.F.; Jeng, J.S.; Hung, C. How active are people with stroke? Use of accelerometers to assess physical activity. Stroke 2009, 40, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Ellis, T.D.; Dibble, L.E.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Cavanaugh, J.T. Obtaining Reliable Estimates of Ambulatory Physical Activity in People with Parkinson’s Disease. J. Parkinsons. Dis. 2016, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, A.K.; Thomas, S.; Xu, X.; Kaiser, W.; Dobkin, B.H.; Investigators, S. SIRRACT: An International Randomized Clinical Trial of Activity Feedback During Inpatient Stroke Rehabilitation Enabled by Wireless Sensing. Neurorehabil. Neural. Repair. 2015, 29, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Yang, A.H.; Christophe, B.R.; Bruce, E.M.; Slomian, J.; Bruyere, O.; Bruce, S.S.; Zacharia, B.E.; Reginster, J.Y.; Connolly, E.S., Jr. The promise of wearable activity sensors to define patient recovery. J. Clin. Neurosci. 2014, 21, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Jones, M.; Thompson, N.; Wallace, T.; DeRuyter, F. Clinician Perspectives on mRehab Interventions and Technologies for People with Disabilities in the United States: A National Survey. Int. J. Environ. Res. Public Health 2019, 16, 4220. [Google Scholar] [CrossRef] [PubMed]
- Jerosch-Herold, C. An evidenced-based approach to choosing outcome measures: A checklist for the critical appraisal of validity, reliability, and responsiveness studies. Brit. J. Occup. Ther. 2005, 68, 347–353. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J. Clin. Epidemiol. 2010, 63, 737–745. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Clinical Practice, 1st ed.; Appleton & Lange: Norwalk, CT, USA, 1993; p. 722. [Google Scholar]
- Guidance for Industry: Patient-Reported Outcome Measures Use in Medical Product Development to Support Labeling Claims; Center for Biologics Evaluation and Research: Washington DC, USA, 2009.
- Cavanaugh, J.T.; Ellis, T.D.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Toward Understanding Ambulatory Activity Decline in Parkinson Disease. Phys. Ther. 2015, 95, 1142–1150. [Google Scholar] [CrossRef]
- Roos, M.A.; Rudolph, K.S.; Reisman, D.S. The structure of walking activity in people after stroke compared with older adults without disability: A cross-sectional study. Phys. Ther. 2012, 92, 1141–1147. [Google Scholar] [CrossRef]
- Orendurff, M.S.; Schoen, J.A.; Bernatz, G.C.; Segal, A.D.; Klute, G.K. How humans walk: Bout duration, steps per bout, and rest duration. J. Rehabil. Res. Dev. 2008, 45, 1077–1089. [Google Scholar] [CrossRef]
- Fulk, G.D.; Reynolds, C.; Mondal, S.; Deutsch, J.E. Predicting home and community walking activity in people with stroke. Arch. Phys. Med. Rehabil. 2010, 91, 1582–1586. [Google Scholar] [CrossRef]
- Mudge, S.; Stott, N.S.; Walt, S.E. Criterion validity of the StepWatch Activity Monitor as a measure of walking activity in patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1710–1715. [Google Scholar] [CrossRef]
- Jarchi, D.; Pope, J.; Lee, T.K.M.; Tamjidi, L.; Mirzaei, A.; Sanei, S. A Review on Accelerometry-Based Gait Analysis and Emerging Clinical Applications. IEEE Rev. Biomed. Eng. 2018, 11, 177–194. [Google Scholar] [CrossRef]
- Bregou Bourgeois, A.; Mariani, B.; Aminian, K.; Zambelli, P.Y.; Newman, C.J. Spatio-temporal gait analysis in children with cerebral palsy using, foot-worn inertial sensors. Gait Posture 2014, 39, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Chadwell, A.; Kenney, L.; Granat, M.; Thies, S.; Head, J.S.; Galpin, A. Visualisation of upper limb activity using spirals: A new approach to the assessment of daily prosthesis usage. Prosthet. Orthot. Int. 2018, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chadwell, A.; Kenney, L.; Granat, M.H.; Thies, S.; Head, J.; Galpin, A.; Baker, R.; Kulkarni, J. Upper limb activity in myoelectric prosthesis users is biased towards the intact limb and appears unrelated to goal-directed task performance. Sci. Rep. 2018, 8, 11084. [Google Scholar] [CrossRef]
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of physical activity after stroke: A systematic review of accelerometry-based measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef]
- Trujillo-Priego, I.A.; Lane, C.J.; Vanderbilt, D.L.; Deng, W.; Loeb, G.E.; Shida, J.; Smith, B.A. Development of a Wearable Sensor Algorithm to Detect the Quantity and Kinematic Characteristics of Infant Arm Movement Bouts Produced across a Full Day in the Natural Environment. Technologies 2017, 5, 39. [Google Scholar] [CrossRef]
- de Lucena, D.S.; Stoller, O.; Rowe, J.B.; Chan, V.; Reinkensmeyer, D.J. Wearable sensing for rehabilitation after stroke: Bimanual jerk asymmetry encodes unique information about the variability of upper extremity recovery. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 1603–1608. [Google Scholar]
- Urbin, M.A.; Bailey, R.R.; Lang, C.E. Validity of body-worn sensor acceleration metrics to index upper extremity function in hemiparetic stroke. J. Neurol. Phys. Ther. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Urbin, M.A.; Hong, X.; Lang, C.E.; Carter, A.R. Resting-State Functional Connectivity and Its Association with Multiple Domains of Upper-Extremity Function in Chronic Stroke. Neurorehabil. Neural. Repair. 2014, 28, 761–769. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, S.C.; Verbunt, J.A.; Breukelaar, D.E.; van Woerden, R.; Seelen, H.A. Assessment of arm activity using triaxial accelerometry in patients with a stroke. Arch. Phys. Med. Rehabil. 2011, 92, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the extremity constraint-induced therapy evaluation trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Miltner, W.H.; Foo, B.; Varma, M.; Moran, S.; Taub, E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke 2000, 31, 662–667. [Google Scholar] [CrossRef]
- Hoyt, C.R.; Brown, S.K.; Sherman, S.K.; Wood-Smith, M.; Van, A.N.; Ortega, M.; Nguyen, A.L.; Lang, C.E.; Schlaggar, B.L.; Dosenbach, N.U.F. Using accelerometry for measurement of motor behavior in children: Relationship of real-world movement to standardized evaluation. Res. Dev. Disabil. 2020, 96, 103546. [Google Scholar] [CrossRef]
- Hoyt, C.R.; Van, A.N.; Ortega, M.; Koller, J.M.; Everett, E.A.; Nguyen, A.L.; Lang, C.E.; Schlaggar, B.L.; Dosenbach, N.U.F. Detection of Pediatric Upper Extremity Motor Activity and Deficits with Accelerometry. JAMA Netw. Open 2019, 2, e192970. [Google Scholar] [CrossRef]
- Bailey, R.R. Assessment of Real-World Upper Limb Activity in Adults with Chronic Stroke; Washington University: St. Louis, MO, USA, 2015. [Google Scholar]
- Bailey, R.R.; Birkenmeier, R.L.; Lang, C.E. Real-world affected upper limb activity in chronic stroke: An examination of potential modifying factors. Top. Stroke Rehabil. 2015, 22, 26–33. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLoS ONE 2014, 9, e103135. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults with Chronic Stroke. Neurorehabilit. Neural Repair 2015, 29, 969–978. [Google Scholar] [CrossRef]
- Bailey, R.R.; Lang, C.E. Upper-limb activity in adults: Referent values using accelerometry. J. Rehabil. Res. Dev. 2013, 50, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, R.J.; Timmermans, A.A.; Janssen-Potten, Y.J.; Pulles, S.A.; Geers, R.P.; Bakx, W.G.; Smeets, R.J.; Seelen, H.A. Accelerometry measuring the outcome of robot-supported upper limb training in chronic stroke: A randomized controlled trial. PLoS ONE 2014, 9, e96414. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Wagner, J.M.; Edwards, D.F.; Dromerick, A.W. Upper Extremity Use in People with Hemiparesis in the First Few Weeks After Stroke. J. Neurol. Phys. Ther. 2007, 31, 56–63. [Google Scholar] [CrossRef]
- Lang, C.E.; Edwards, D.F.; Birkenmeier, R.L.; Dromerick, A.W. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Sokal, B.; Uswatte, G.; Vogtle, L.; Byrom, E.; Barman, J. Everyday movement and use of the arms: Relationship in children with hemiparesis differs from adults. J. Pediatr. Rehabil. Med. 2015, 8, 197–206. [Google Scholar] [CrossRef]
- Seitz, R.J.; Hildebold, T.; Simeria, K. Spontaneous arm movement activity assessed by accelerometry is a marker for early recovery after stroke. J. Neurol. 2011, 258, 457–463. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Melendez-Calderon, A.; Burdet, E. A robust and sensitive metric for quantifying movement smoothness. IEEE Trans. Biomed. Eng. 2012, 59, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Melendez-Calderon, A.; Roby-Brami, A.; Burdet, E. On the analysis of movement smoothness. J. Neuroeng. Rehabil. 2015, 12, 112. [Google Scholar] [CrossRef]
- Thrane, G.; Emaus, N.; Askim, T.; Anke, A. Arm use in patients with subacute stroke monitored by accelerometry: Association with motor impairment and influence on self-dependence. J. Rehabil. Med. 2011, 43, 299–304. [Google Scholar] [CrossRef]
- Lang, C.E.; Cade, W.T. A step toward the future of seamless measurement with wearable sensors in pediatric populations with neuromuscular diseases. Muscle Nerve 2020, 61, 265–267. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, A.; Essers, J.M.N.; Bergsma, A.; Jansen, M.; de Groot, I.J.M. Monitoring daily physical activity of upper extremity in young and adolescent boys with Duchenne muscular dystrophy: A pilot study. Muscle Nerve 2020, 61, 293–300. [Google Scholar] [CrossRef]
- Smith, B.A.; Lang, C.E. Sensor Measures of Symmetry Quantify Upper Limb Movement in the Natural Environment Across the Lifespan. Arch. Phys. Med. Rehabil. 2019, 100, 1176–1183. [Google Scholar] [CrossRef]
- Lang, C.E.; Bland, M.D.; Bailey, R.R.; Schaefer, S.Y.; Birkenmeier, R.L. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. J. Hand. Ther. 2013, 26, 104–115. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Cardinal, B.J. Measuring children’s physical activity and sedentary behaviors. J. Exerc. Sci. Fit. 2011, 9, 15–23. [Google Scholar] [CrossRef]
- Rice, K.R.; Joschtel, B.; Trost, S.G. Validity of family child care providers’ proxy reports on children’s physical activity. Child. Obes. 2013, 9, 393–398. [Google Scholar] [CrossRef]
- Lobenius-Palmer, K.; Sjoqvist, B.; Hurtig-Wennlof, A.; Lundqvist, L.O. Accelerometer-Assessed Physical Activity and Sedentary Time in Youth with Disabilities. Adapt. Phys. Activ. Q. 2018, 35, 1–19. [Google Scholar] [CrossRef]
- King-Dowling, S.; Rodriguez, C.; Missiuna, C.; Timmons, B.W.; Cairney, J. Health-related Fitness in Preschool Children with and without Motor Delays. Med. Sci. Sports Exerc. 2018, 50, 1442–1448. [Google Scholar] [CrossRef]
- Wood, A.C.; Asherson, P.; Rijsdijk, F.; Kuntsi, J. Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 1023–1030. [Google Scholar] [CrossRef]
- Pan, C.Y.; Tsai, C.L.; Chu, C.H.; Sung, M.C.; Ma, W.Y.; Huang, C.Y. Objectively Measured Physical Activity and Health-Related Physical Fitness in Secondary School-Aged Male Students with Autism Spectrum Disorders. Phys. Ther. 2016, 96, 511–520. [Google Scholar] [CrossRef]
- Brewis, A. Social and biological measures of hyperactivity and inattention: Are they describing similar underlying constructs of child behavior? Soc. Biol. 2002, 49, 99–115. [Google Scholar] [CrossRef]
- Uebel, H.; Albrecht, B.; Kirov, R.; Heise, A.; Dopfner, M.; Freisleder, F.J.; Gerber, W.D.; Gunter, M.; Hassler, F.; Ose, C.; et al. What can actigraphy add to the concept of labschool design in clinical trials? Curr. Pharm. Des. 2010, 16, 2434–2442. [Google Scholar] [CrossRef]
- Lea, S.E.; Matt Alderson, R.; Patros, C.H.G.; Tarle, S.J.; Arrington, E.F.; Grant, D.M. Working Memory and Motor Activity: A Comparison Across Attention-Deficit/Hyperactivity Disorder, Generalized Anxiety Disorder, and Healthy Control Groups. Behav. Ther. 2018, 49, 419–434. [Google Scholar] [CrossRef]
- Gapin, J.; Etnier, J.L. The relationship between physical activity and executive function performance in children with attention-deficit hyperactivity disorder. J. Sport Exerc. Psychol. 2010, 32, 753–763. [Google Scholar] [CrossRef]
- Heathers, J.A.J.; Gilchrist, K.H.; Hegarty-Craver, M.; Grego, S.; Goodwin, M.S. An analysis of stereotypical motor movements and cardiovascular coupling in individuals on the autism spectrum. Biol. Psychol. 2019, 142, 90–99. [Google Scholar] [CrossRef]
- Garcia-Pastor, T.; Salinero, J.J.; Theirs, C.I.; Ruiz-Vicente, D. Obesity Status and Physical Activity Level in Children and Adults with Autism Spectrum Disorders: A Pilot Study. J. Autism. Dev. Disord. 2019, 49, 165–172. [Google Scholar] [CrossRef]
- Benson, S.; Bender, A.M.; Wickenheiser, H.; Naylor, A.; Clarke, M.; Samuels, C.H.; Werthner, P. Differences in sleep patterns, sleepiness, and physical activity levels between young adults with autism spectrum disorder and typically developing controls. Dev. Neurorehabil. 2019, 22, 164–173. [Google Scholar] [CrossRef]
- Goldman, S.E.; Alder, M.L.; Burgess, H.J.; Corbett, B.A.; Hundley, R.; Wofford, D.; Fawkes, D.B.; Wang, L.; Laudenslager, M.L.; Malow, B.A. Characterizing Sleep in Adolescents and Adults with Autism Spectrum Disorders. J. Autism. Dev. Disord. 2017, 47, 1682–1695. [Google Scholar] [CrossRef]
- Abrishami, M.S.; Nocera, L.; Mert, M.; Trujillo-Priego, I.A.; Purushotham, S.; Shahabi, C.; Smith, B.A. Identification of Developmental Delay in Infants Using Wearable Sensors: Full-Day Leg Movement Statistical Feature Analysis. IEEE J. Transl. Eng. Health Med. 2019, 7, 2800207. [Google Scholar] [CrossRef]
- Heinze, F.; Hesels, K.; Breitbach-Faller, N.; Schmitz-Rode, T.; Disselhorst-Klug, C. Movement analysis by accelerometry of newborns and infants for the early detection of movement disorders due to infantile cerebral palsy. Med. Biol. Eng. Comput. 2010, 48, 765–772. [Google Scholar] [CrossRef]
- Jiang, C.; Lane, C.J.; Perkins, E.; Schiesel, D.; Smith, B.A. Determining if wearable sensors affect infant leg movement frequency. Dev. Neurorehabil. 2018, 21, 133–136. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, T.; Li, G.; Li, T.; Inoue, Y. Wearable sensor systems for infants. Sensors 2015, 15, 3721–3749. [Google Scholar] [CrossRef] [PubMed]
- Kirste, T.; Hoffmeyer, A.; Koldrack, P.; Bauer, A.; Schubert, S.; Schroder, S.; Teipel, S. Detecting the effect of Alzheimer’s disease on everyday motion behavior. J. Alzheimers. Dis. 2014, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.; Hankey, C.; Penpraze, V.; Boyle, S.; Macmillan, S.; Miller, S.; Murray, H.; Pert, C.; Spanos, D.; Robinson, N.; et al. Agreement of accelerometer and a physical activity questionnaire in adults with intellectual disabilities. Prev. Med. 2011, 52, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Block, V.A.; Pitsch, E.; Tahir, P.; Cree, B.A.; Allen, D.D.; Gelfand, J.M. Remote Physical Activity Monitoring in Neurological Disease: A Systematic Review. PLoS ONE 2016, 11, e0154335. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Sanchez-Vazquez, A.; Ivory, C. Social, Organizational, and Technological Factors Impacting Clinicians’ Adoption of Mobile Health Tools: Systematic Literature Review. JMIR Mhealth Uhealth 2020, 8, e15935. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.; Kelly, C.; Quinby, E.; Mac, A.; Parmanto, B.; Dicianno, B.E. Systematic Review of Mobile Health Applications in Rehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, 115–127. [Google Scholar] [CrossRef]


| Variable Name | Explored In: | Evaluation in Health Condition: | |||
|---|---|---|---|---|---|
| Absence of Health Condition | Health Condition | Reliability | Validity | Responsiveness | |
| Lower Limb [16,18,21,30,34,40,41,49,53,57,64,65,66,67,68,69,70] | |||||
| Time-based variables | |||||
| % time inactive | ● | ● | ● | ● | ● |
| Walking duration | ● | ● | ● | ● | ● |
| Amount-based variables | |||||
| Steps/day | ● | ● | ● | ● | ● |
| Bouts/day | ● | ● | ● | ● | ● |
| Steps/bout | ● | ● | ● | ● | ● |
| Intensity-based variables | |||||
| Stepping intensity | ● | ● | ● | ● | ● |
| Maximum output | ● | ● | ● | ● | ● |
| Mod. intensity minutes | ● | ● | ● | ● | ● |
| Peak activity index | ● | ● | ● | ● | ● |
| Other variables | |||||
| Step length variability | ● | ● | ● | ● | ● |
| Upper Limb [21,32,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] | |||||
| Time-based variables | |||||
| Hours/duration of use | ● | ● | ● | ● | ● |
| Use/activity ratio | ● | ● | ● | ● | ● |
| Amount-based variables | |||||
| Acceleration area | ● | ● | ● | ● | ● |
| Activity counts | ● | ● | ● | ● | ● |
| Mono-arm use index | ● | ● | ● | ● | ● |
| Intensity-based variables | |||||
| Acceleration variability | ● | ● | ● | ● | ● |
| Acceleration magnitude | ● | ● | ● | ● | ● |
| Acceleration asymmetry | ● | ● | ● | ● | ● |
| Laterality index | ● | ● | ● | ● | ● |
| Magnitude ratio | ● | ● | ● | ● | ● |
| Bilateral magnitude | ● | ● | ● | ● | ● |
| Other variables | |||||
| Variation ratio | ● | ● | ● | ● | ● |
| Jerk asymmetry | ● | ● | ● | ● | ● |
| Spectral arc length | ● | ● | ● | ● | ● |
| Benchmark | |
|---|---|
| Convenience for purchase and use | Commercially-available, consumer-grade device system that can be easily used by clinicians and consumers; comprehensive, accessible tech support. |
| Initial set-up time for clinician | 5–6 min for first time with new patient. |
| Routine set-up time for clinician | ≤1 min in subsequent times with same patient. |
| Time to extract data and generate output or report | ≤5 min |
| Ease of donning/doffing for patient | ≤2 min; without assistance from another person if intended for home use. |
| Comfort for extended wear | Soft plastic or other flexible strapping that can be tolerated 12–24 h/day; no hard edges on device that push into skin; water resistant so does not have to be removed for bathing, dishwashing, etc. |
| Device operations | ≥95% of the time, device collects, stores, and/or uploads data as programmed and does not malfunction. |
| Algorithms for extracting data and generating variables of interest | ≥90% accuracy to measure intended construct; must be accurate across a broad range of movement abilities typically seen in physical rehabilitation clinics. |
| Standardization of variables of interest | Reliability: consistently captures construct with reliability coefficients of ≥0.80. |
| Validity: comprehensively captures construct that has known relevance to clinical decision-making and management. | |
| Responsiveness: detects changes of ≥5%; changes of 5–10% or higher provide relevant information for clinical decision-making and management. | |
| Values can be computed & reported in sensor-independent units. | |
| Report to clinician and patient | Consumer friendly, targeting audience with ≤ secondary school education; 1–3 key outcome variables presented; simple graphics with colors to make accessible across languages and language and/or cognitive deficits; ability to integrate into electronic medical record. |
| Progress toward Benchmark | |
|---|---|
| Convenience for purchase and use | Commercial-availability achieved. Can be easily purchased. Consumer-grade not achieved. Marketed and sold as research-grade device. Technology support helpful for researchers but would be too difficult for clinician or patient consumers. |
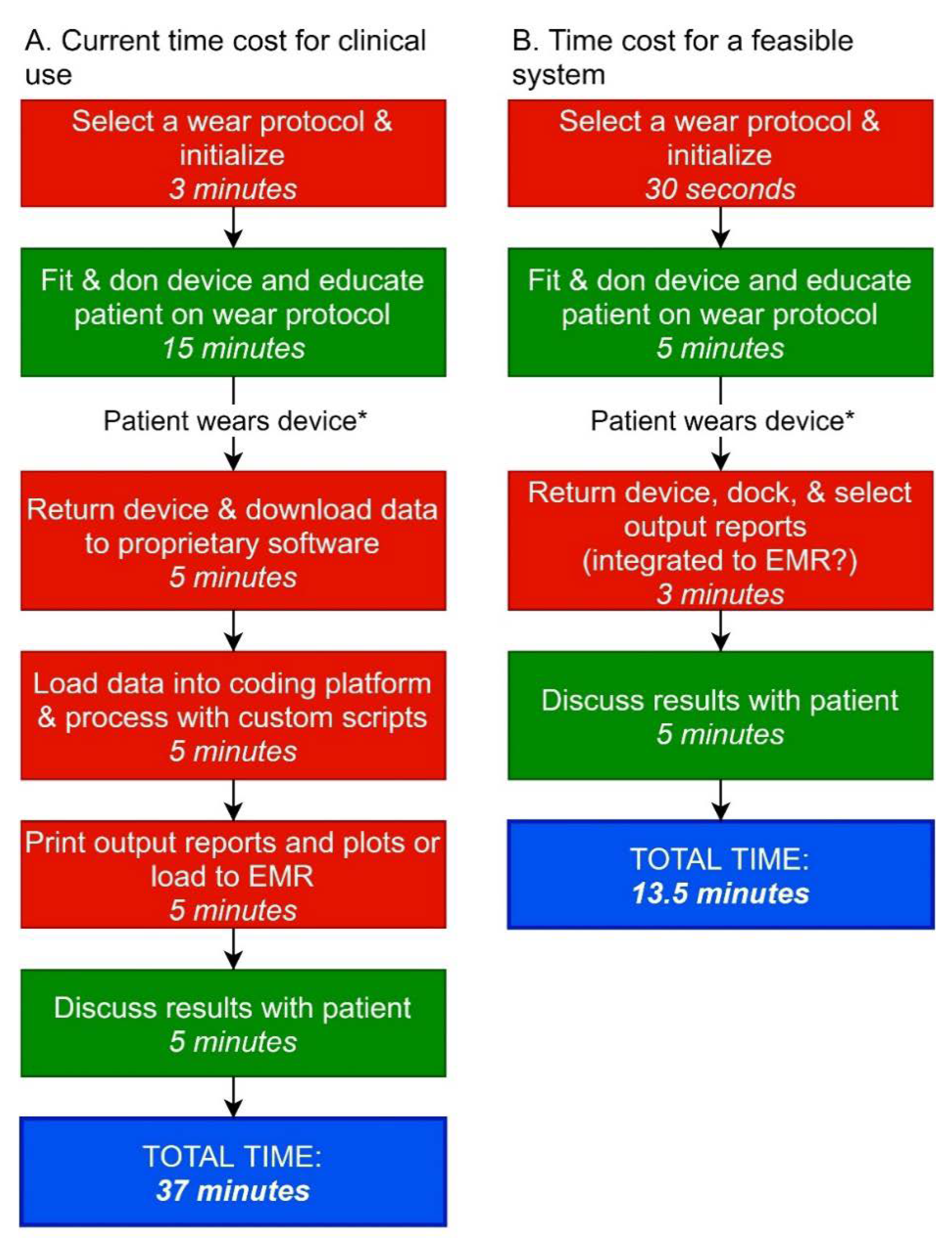
| Initial set-up time for clinician | Not achieved. Current time estimate is 18 min. |
| Routine set-up time for clinician | Not achieved. Current time estimate is 8–10 min. |
| Time to extract data and generate output or report | Not achieved. Current time estimate, using ActiLife + custom-written software in MATLAB or R is 15 min |
| Ease of donning/doffing for patient | Achieved. Can be done at home for most patients without assistance from another person. |
| Comfort for extended wear | Achieved. Allows for variety of strapping options and has been worn 12–24 h by hundreds of patients, with many wearing it for 24 hrs 1x/wk or 1x/month. Water resistant. |
| Device operations | Achieved. Have lost data <2% of the time. |
| Algorithms for extracting data and generating variables of interest | Achieved for use ratio. Algorithm is stable across a range of movement abilities in typical adults and children, and persons with stroke. |
| Standardization of variable of interest: Use ratio | Reliability achieved. Test-retest reliability coefficient = 0.86 [79] |
| Validity achieved for adult stroke population, but not other populations [99]. Captures relative use of the upper limbs, which is stable and narrowly distributed in referent populations [83,88], but wide-ranging post stroke. | |
| Responsive to change achieved. Can detect changes of ≤5% [32,79]. Clinical relevance of change not achieved. Currently unknown how much change is clinically meaningful. | |
| Sensor-independent units achieved. Values are a ratio, making differences across sensors irrelevant. | |
| Report to clinician and patient | Not achieved. Current output can be consumed by trained researchers but is not clinician-, patient-, or family friendly. Output is not integrated with electronic medical record. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, C.E.; Barth, J.; Holleran, C.L.; Konrad, J.D.; Bland, M.D. Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field. Sensors 2020, 20, 5744. https://doi.org/10.3390/s20205744
Lang CE, Barth J, Holleran CL, Konrad JD, Bland MD. Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field. Sensors. 2020; 20(20):5744. https://doi.org/10.3390/s20205744
Chicago/Turabian StyleLang, Catherine E., Jessica Barth, Carey L. Holleran, Jeff D. Konrad, and Marghuretta D. Bland. 2020. "Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field" Sensors 20, no. 20: 5744. https://doi.org/10.3390/s20205744
APA StyleLang, C. E., Barth, J., Holleran, C. L., Konrad, J. D., & Bland, M. D. (2020). Implementation of Wearable Sensing Technology for Movement: Pushing Forward into the Routine Physical Rehabilitation Care Field. Sensors, 20(20), 5744. https://doi.org/10.3390/s20205744






