Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles
Abstract
1. Introduction
2. Materials and Methods
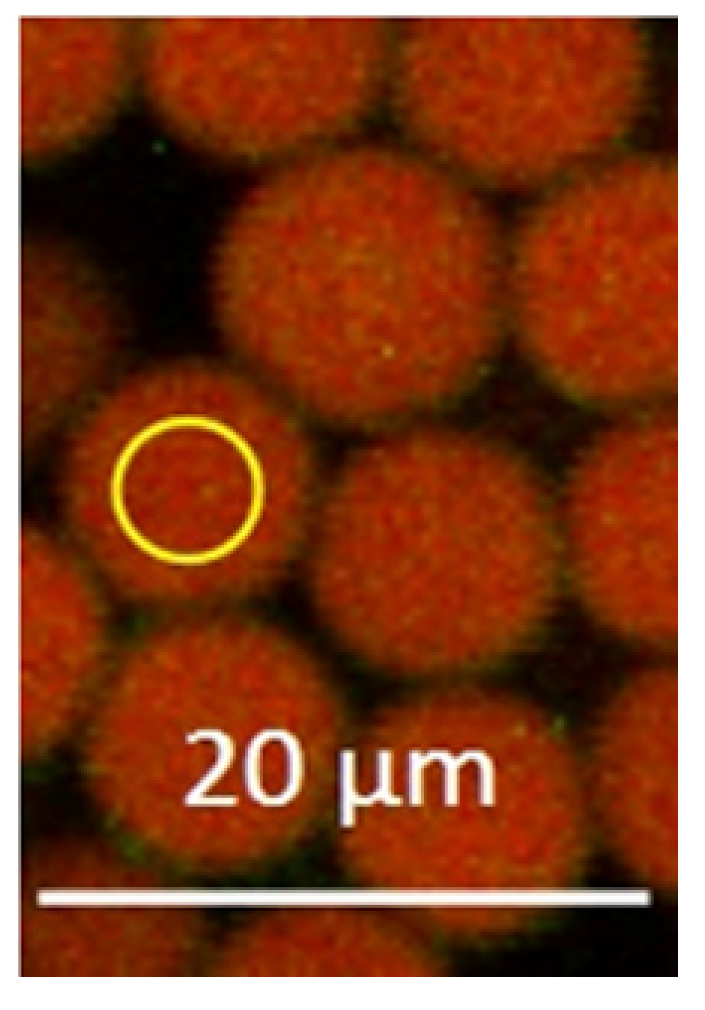
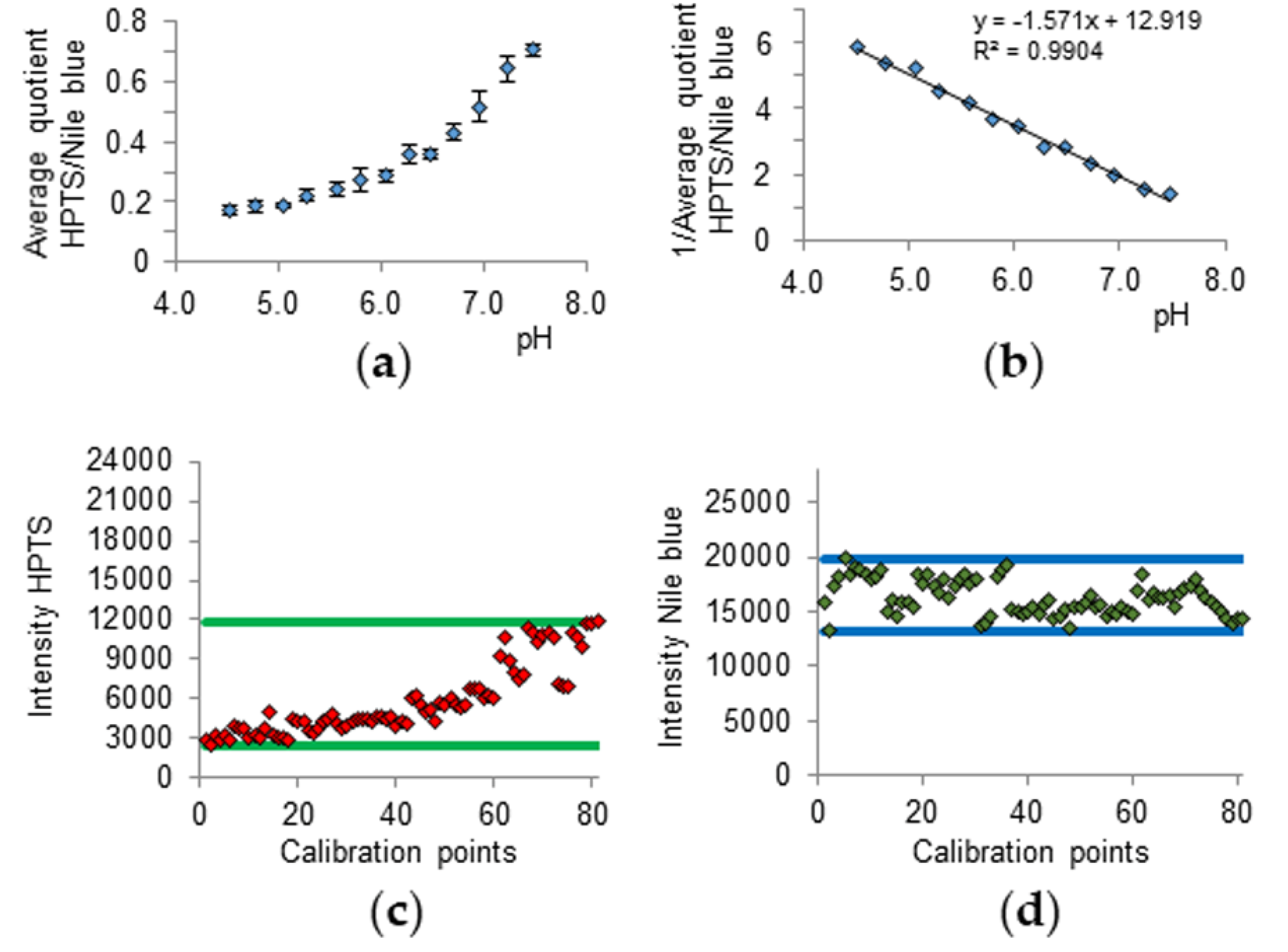
2.1. pH Sensing Melamine Formaldehyde Particles via Fluorescence Measurements of Immobilized Pyranine and Nile Blue
2.2. Subjects
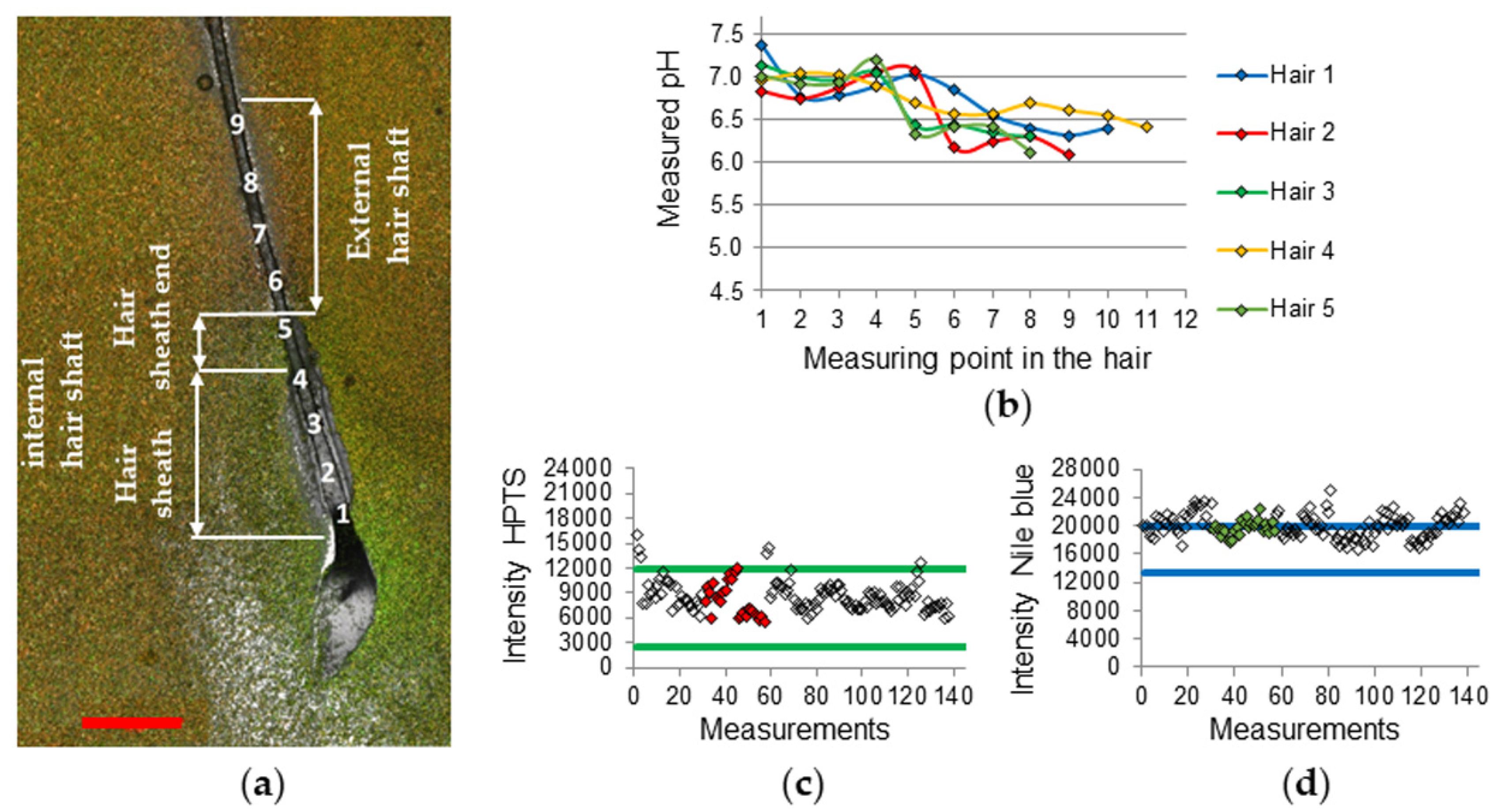
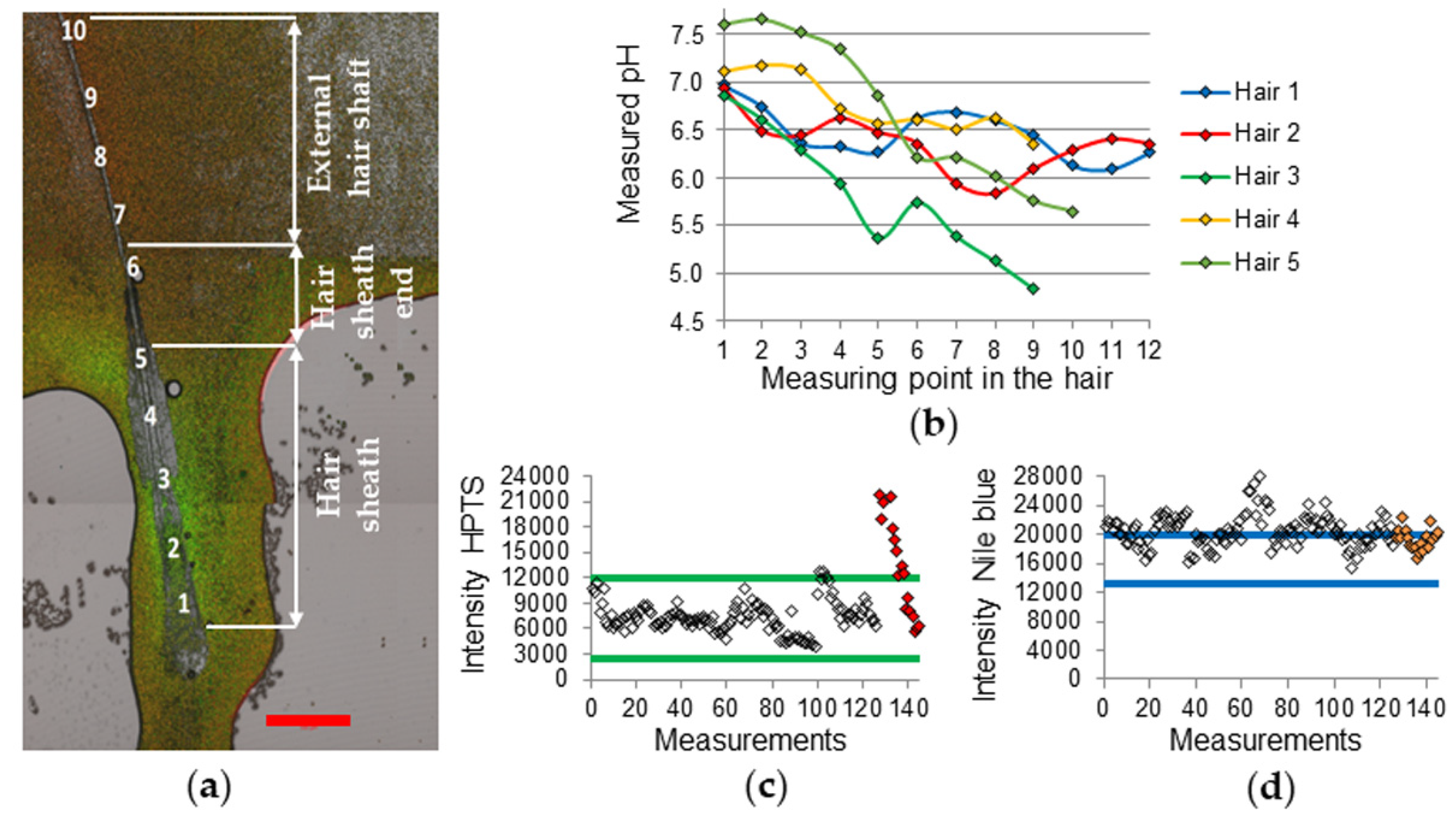
2.3. CLSM Parameters, Calibration, and Hair Measurement
2.4. Statistical Analysis
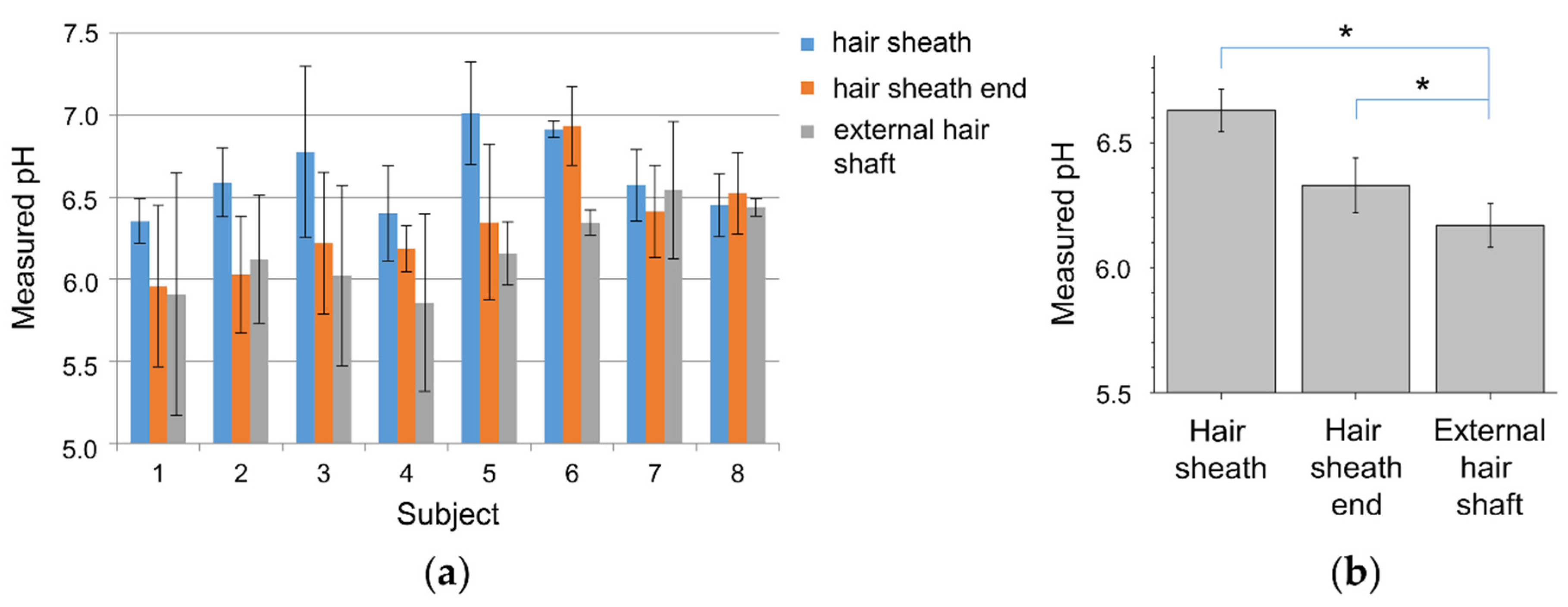
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statements
References
- Knorr, F.; Patzelt, A.; Darvin, M.E.; Lehr, C.; Schäfer, U.; Gruber, A.D.; Ostrowski, A.; Lademann, J. Penetration of topically applied nanocarriers into the hair follicles of dog and rat dorsal skin and porcine ear skin. Vet. Dermatol. 2016, 27, 256-e60. [Google Scholar] [CrossRef] [PubMed]
- Dokka, S.; Cooper, S.R.; Kelly, S.; Hardee, G.E.; Karras, J.G. Dermal delivery of topically applied oligonucleotides via follicular transport in mouse skin. J. Investig. Dermatol. 2005, 124, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Shiraki, T.; Okajima, K.; Tanino, T.; Iwaki, M.; Wada, T. Transfollicular drug delivery: Penetration of drugs through human scalp skin and comparison of penetration between scalp and abdominal skins In Vitro. J. Drug Target. 2002, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Erdmenger, U.; Patzelt, A.; Beyer, M.; Meinke, M.C.; Darvin, M.E.; Koscielny, J.; Lademann, J. Significance of the follicular pathway for dermal substance penetration quantified by laser Doppler flowmetry. J. Biophotonics 2015, 9, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lademann, H.J.; Richter, S.; Schanzer, M.C.; Meinke, M.E.; Darvin, J.; Schleusener, V.; Carrer, P.; Breuckmann, A. Follicular penetration of nanocarriers is an important penetration pathway for topically applied drugs. Hautarzt 2019, 70, 85–192. [Google Scholar]
- Hueber, F.; Wepierre, J.; Schaefer, H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: In Vivo study in the hairless rat. Skin Pharmacol. Physiol. 1992, 5, 99–107. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Massoudy, L.; Patzelt, A.; Lademann, J.; Dietz, E.; Rasulev, U.; Bartels, N.G. Follicular and percutaneous penetration pathways of topically applied minoxidil foam. Eur. J. Pharm. Biopharm. 2010, 76, 450–453. [Google Scholar] [CrossRef]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2007, 65, 488–492. [Google Scholar] [CrossRef]
- Baroli, B.M. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Darvin, M.E.; König, K.; Kellner-Hoefer, M.; Breunig, H.; Werncke, W.; Meinke, M.; Patzelt, A.; Sterry, W.; Lademann, J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacol. Physiol. 2012, 25, 219–226. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin. Drug Deliv. 2019, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.; Patzelt, A.; Knorr, F.; Lademann, J.; Netz, R.R. Ratchet effect for nanoparticle transport in hair follicles. Eur. J. Pharm. Biopharm. 2017, 116, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin–Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Sahle, F.F.; Lohan, S.B.; Saeidpour, S.; Albrecht, S.; Teutloff, C.; Bodmeier, R.; Unbehauen, M.; Wolff, C.; Haag, R.; et al. pH-sensitive Eudragit® L 100 nanoparticles promote cutaneous penetration and drug release on the skin. J. Control. Release 2019, 295, 214–222. [Google Scholar] [CrossRef]
- Choi, E.-H.; Man, M.-Q.; Xu, P.; Xin, S.; Liu, Z.; Crumrine, D.A.; Jiang, Y.J.; Fluhr, J.W.; Feingold, K.R.; Elias, P.M.; et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J. Investig. Dermatol. 2007, 127, 2847–2856. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M. Stratum corneum pH: Formation and function of the ‘acid mantle’. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Rippke, F.; Schreiner, V.; Schwanitz, H.-J. The acidic milieu of the horny layer: New findings on the physiology and pathophysiology of skin pH. Am. J. Clin. Dermatol. 2002, 3, 261–272. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Dimde, M.; Sahle, F.F.; Wycisk, V.; Steinhilber, D.; Camacho, L.C.; Licha, K.; Lademann, J.; Haag, R. Synthesis and validation of functional nanogels as pH-sensors in the hair follicle. Macromol. Biosci. 2017, 17, 1600505. [Google Scholar] [CrossRef]
- Sahle, F.F.; Balzus, B.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur. J. Pharm. Sci. 2016, 92, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, Y.; Du, Y.; Chen, J.; Hou, X.; Meng, J. Preparation and application of melamine-formaldehyde photochromic microcapsules. Sens. Actuators B Chem. 2013, 188, 502–512. [Google Scholar] [CrossRef]
- Wolfbeis, E.O.S.; Furlinger, H.; Kroneis, H. Fluorimetric Analysis 1. A study on fluorescent indicators for measuring near neutral (physiological) Ph-values. Fresen Z. Anal. Chem. 1983, 314, 119–124. [Google Scholar] [CrossRef]
- Offenbacher, H.; Wolfbeis, O.S.; Fürlinger, E. Fluorescence optical sensors for continuous determination of near-neutral pH values. Sens. Actuators 1986, 9, 73–84. [Google Scholar] [CrossRef]
- Neurauter, G.; Klimant, I.; Wolfbeis, O.S. Fiber-optic microsensor for high resolution pCO2 sensing in marine environment. Anal. Bioanal. Chem. 2000, 366, 481–487. [Google Scholar] [CrossRef]
- Lutty, G.A. The acute intravenous toxicity of biological stains, dyes, and other fluorescent substances. Toxicol. Appl. Pharmacol. 1978, 44, 225–249. [Google Scholar] [CrossRef]
- Woislawski, S. The spectrophotometric determination of ionization constants of basic dyes1. J. Am. Chem. Soc. 1953, 75, 5201–5203. [Google Scholar] [CrossRef]
- Ping, S.; Xingquan, Z. Relationship between the elemental content and pH of human hair. Sci. Total. Environ. 1993, 128, 151–156. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, S.Y.; Byun, H.J.; Huh, C.H.; Park, K.C.; Patel, R.A.; Shinn, A.H.; Youn, S.W. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch. Dermatol. Res. 2006, 298, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Paus, R.; Plonka, P.M.; Chakraborty, A.; Maurer, M.; Pruski, D.; Lukiewicz, S. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J. Investig. Dermatol. 1994, 102, 862–869. [Google Scholar] [CrossRef] [PubMed]
| Subject | pH Decrease | pH Increase | pH Stagnate |
|---|---|---|---|
| 1 | 40% | 20% | 40% |
| 2 | 60% | None | 40% |
| 3 | 60% | None | 40% |
| 4 | 60% | None | 40% |
| 5 | 80% | None | 20% |
| 6 | 100% | None | None |
| 7 | 20% | 60% | 20% |
| 8 | None | 20% | 80% |
| Overall | 52.5% | 12.5% | 35% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaden, D.; Dähne, L.; Knorr, F.; Richter, H.; Lademann, J.; Meinke, M.C.; Patzelt, A.; Darvin, M.E.; Jung, S. Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles. Sensors 2020, 20, 5243. https://doi.org/10.3390/s20185243
Kaden D, Dähne L, Knorr F, Richter H, Lademann J, Meinke MC, Patzelt A, Darvin ME, Jung S. Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles. Sensors. 2020; 20(18):5243. https://doi.org/10.3390/s20185243
Chicago/Turabian StyleKaden, Dennis, Lars Dähne, Fanny Knorr, Heike Richter, Jürgen Lademann, Martina C. Meinke, Alexa Patzelt, Maxim E. Darvin, and Sora Jung. 2020. "Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles" Sensors 20, no. 18: 5243. https://doi.org/10.3390/s20185243
APA StyleKaden, D., Dähne, L., Knorr, F., Richter, H., Lademann, J., Meinke, M. C., Patzelt, A., Darvin, M. E., & Jung, S. (2020). Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles. Sensors, 20(18), 5243. https://doi.org/10.3390/s20185243








