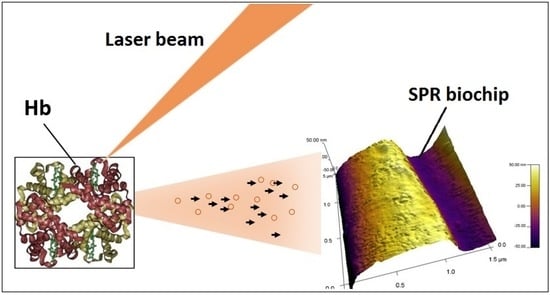
A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin
Abstract
1. Introduction
- -
- Preservation of the conformation state. This alters the ability of the molecule to transmit intramolecular messages to its various functional groups, maintaining a maximum affinity for the ligand of interest. For example, oxygen binding depends on the position of the porphyrin in relation to the plane of the heme.
- -
- The effect of dehydration on Hb/Mb activity. The structure of the heme pocket is preserved by the hydrogen bonding of water molecules with the protein surface [32]. Additionally, OH groups bind to the hydration sites of Hb/Mb and prevent conformational changes.
2. Materials and Methods
2.1. Volatile Solvent
2.2. Target
2.3. Deposition of Bioactive Ligands
2.4. Chemicals
3. Results and Discussion
3.1. Impact of Freezing Rate
3.2. Protein Molecules Take Part in the Photothermal Process: The Impact
3.2.1. Activity of the Heme Group
3.2.2. Role of Molecular Weight
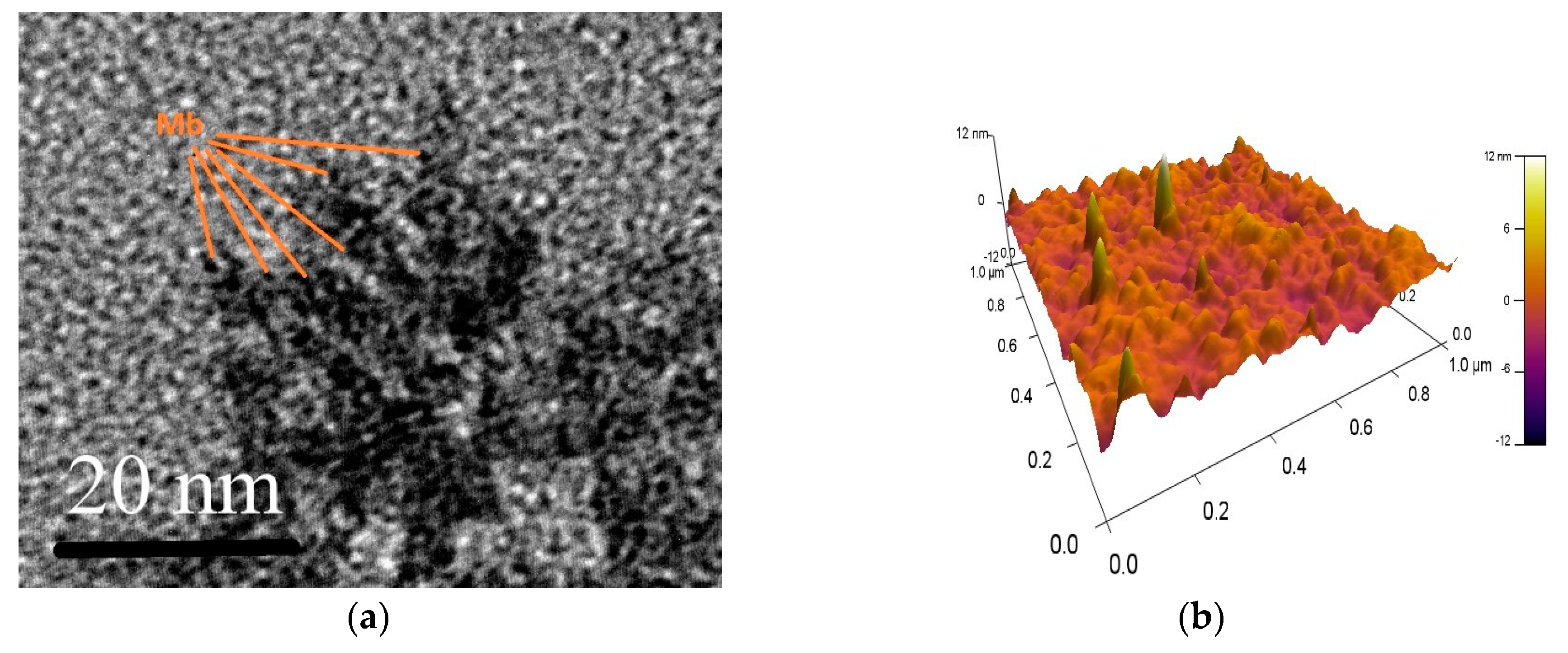
3.3. Characterization of Immobilized Proteins
3.3.1. FTIR Spectra
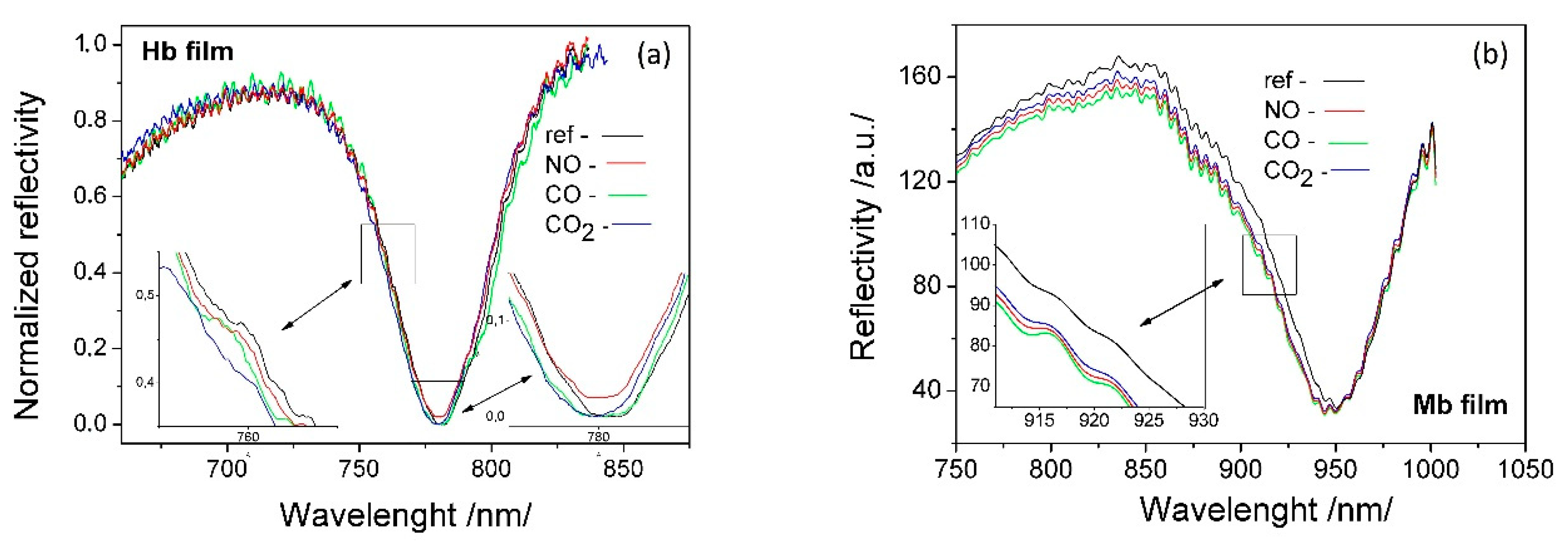
3.3.2. SPR-Detected Binding Reactions
3.4. Effectiveness of MAPLE Deposition
3.5. What Molecules are Immobilized—Fragmented or Intact?
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuators B Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, G.; Yang, B. Direct electrochemistry and electrocatalysis of hemoglobin immobilized on carbon paste electrode by silica sol–gel film. Biosens. Bioelectron. 2004, 19, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, T.; G., B.B.; Jo, J.; Oh, B.K.; Choi, J.W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 17, 14–20. [Google Scholar] [CrossRef]
- Hajian, A.; Ghodsi, J.; Afraz, A.; Yurchenko, O.; Urban, G. Nanomolar detection of methylparaben by a cost-effective hemoglobin-based biosensor. Mater. Sci. Eng. C 2016, 69, 122–127. [Google Scholar] [CrossRef]
- Murphy, M.E.; Noack, E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994, 432, 240–250. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, J.W.; Lim, J.; Mohammadniaei, M.; Bapurao, G.B.; Lee, T.; Choi, J.W. Electrochemical nitric oxide biosensor based on amine-modified MoS2/graphene oxide/myoglobin hybrid. Colloids Surf. B 2017, 159, 729–736. [Google Scholar] [CrossRef]
- Takada, Y.; Otsuka, R.; Tsukada, K. A carbon monoxide sensing film based on hemoglobin allostery. J. Biomed. Sci. Eng. 2014, 7, 173–180. [Google Scholar] [CrossRef]
- Sidhu, A.; Sundd, M. Protein Biotechnology and Its Applications. In Biotechnology Application, 1st ed.; Mishra, C.S.K., Champagne, P., Eds.; International Pvt Ltd.: New Delhi, India, 2009; pp. 213–2290. Available online: https://www.abebooks.co.uk/servlet/BookDetailsPL?bi=19756158862&cm_sp=plp-_-9789380026299-_-new (accessed on 10 December 2019).
- Boffi, A.; Rizzi, M.; Monacelli, F.; Ascenzi, P. Determination of H(2)S solubility via the reaction with ferric hemoglobin I from the bivalve mollusc Lucina pectinata. Biochim. Biophys. Acta 2000, 1523, 206–208. [Google Scholar] [CrossRef]
- Dyankov, G.; Eftimov, T.; Malinowski, N.; Belina, E.; Kisov, H.; Mikulic, P.; Bock, W. A highly efficient biosensor based on MAPLE deposited hemoglobin on LPGs around phase matching turning point. Opt. Laser Technol. 2020, 123, 105907. [Google Scholar] [CrossRef]
- Rusling, J.F.; Nassar, A.E.F. Enhanced electron transfer for myoglobin in surfactant films on electrodes. J. Am. Chem. Soc. 1993, 115, 11891–11897. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Qiao, M.; Zhang, H.; Jin, X.; Fan, S. Enhancing sensitivity of hemoglobin-based electrochemical biosensor by using protein conformational intermediate. Sens. Actuators B Chem. 2015, 221, 694–699. [Google Scholar] [CrossRef]
- Huang, H.; He, P.; Hu, N.; Zeng, Y. Electrochemical and electrocatalytic properties of myoglobin and hemoglobin incorporated in carboxymethyl cellulose films. Bioelectrochemistry 2003, 61, 29–38. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Veeramani, V.; Chen, S.M.; Madhu, R.; Kwak, C.H.; Huh, Y.S.; Han, Y.K. Immobilization of myoglobin on Au nanoparticle-decorated carbon nanotube/polytyramine composite as a mediator-free H2O2 and nitrite biosensor. Sci. Rep. 2015, 5, 18390. [Google Scholar] [CrossRef] [PubMed]
- Canbay, E.; Şahin, B.; Kıran, M.; Akyilmaz, E. MWCNT–cysteamine–Nafion modified gold electrode based on myoglobin for determination of hydrogen peroxide and nitrite. Bioelectrochemistry 2015, 101, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.S.; Xu, Q.; Xie, F.; Wang, S.F. Hydrogen peroxide biosensor based on the bioelectrocatalysis of myoglobin incorporated in multi-walled carbon nanotubes/chitosan composite film. Int. J. Electrochem. Sci. 2008, 118, 118–124. [Google Scholar]
- Palanisamy, S.; Karuppiah, C.; Chen, S.-M.; Emmanuel, R.; Muthukrishnan, P.; Prakash, P. Direct electrochemistry of myoglobin at silver nanoparticles/myoglobin biocomposite: Application for hydrogen peroxide sensing. Sens. Actuators B Chem. 2014, 202, 177–184. [Google Scholar] [CrossRef]
- Wang, M.; Sheng, Q.; Zhang, D.; He, Y.; Zheng, J. TiC nanoparticles-chitosan composite film for the direct electron transfer of myoglobin and its application in biosensing. Bioelectrochemistry 2012, 86, 46–53. [Google Scholar] [CrossRef]
- Turdean, G.; Szabo, G.-S. Nitrite detection in meat products samples by square-wave voltammetry at a new single walled carbon naonotubes—Myoglobin modified electrode. Food Chem. 2015, 179, 325–330. [Google Scholar] [CrossRef]
- Soliman, A.; Haikal, R.; Abugable, A.; Mohamed H. Hassan, M.; Stavros G. Karakalos, S.; Perry J. Pellechia, P.; Hassan, H.; Magdi H. Yacoub, M.; Alkordi., M. Tailoring the Oxygen Reduction Activity of Hemoglobin through Immobilization within Microporous Organic Polymer−Graphene Composite. ACS Appl. Mater. Interfaces 2017, 9, 27918–27926. [Google Scholar] [CrossRef]
- Liu, S.; Dai, Z.; Chen, H.; Ju, H. Immobilization of hemoglobin on zirconium dioxide nanoparticles for preparation of a novel hydrogen peroxide biosensor. Biosens. Bioelectron. 2004, 19, 963–969. [Google Scholar] [CrossRef]
- Elewi, A.S.; Al-Shammaree, S.A.W.; Al Sammarraie, A.K.M.; Wadood, S.A. Hydrogen peroxide biosensor based on hemoglobin-modified gold nanoparticles–screen printed carbon electrode. Sens. Bio-Sens. Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Richard, B.M.S. Handbook of Surface Plasmon Resonance, 2nd ed.; The Royal Society of Chemistry: Croydon, UK, 2017; pp. 202–241. [Google Scholar]
- Luches, A.; Caricato, A.P. Fundamentals and applications of MAPLE. In Laser-Surface Interactions for New Materials Production, 1st ed.; Miotello, A., Ossi, P.M., Eds.; Springer Series in Materials Science: Berlin, Germany, 2010; Volume 130, pp. 203–233. [Google Scholar]
- Caricato, A.P.; Ge, W.; Stiff-Roberts, A.D. UV- and RIR-MAPLE: Fundamentals and applications. In Advances in the Application of Lasers in Materials Science, 1st ed.; Ossi, P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Volume 274, pp. 275–308. [Google Scholar]
- Caricato, A.P.; Luches, A. Applications of the matrix-assisted pulsed laser evaporation method for the deposition of organic, biological and nanoparticle thin films: A review. Appl. Phys. A 2011, 105, 565–582. [Google Scholar] [CrossRef]
- Stamatin, L.; Cristescu, R.; Socol, G.; Moldovan, A.; Mihaiescu, D.; Stamatin, I.; Mihailescu, I.; Chrisey, D.; Mihǎilescu, I. Laser deposition of fibrinogen blood proteins thin films by matrix assisted pulsed laser evaporation. Appl. Surf. Sci. 2005, 248, 422–427. [Google Scholar] [CrossRef]
- Piqué, A.; Wu, P.; Ringeisen, B.; Bubb, D.; Melinger, J.; McGill, R.; Chrisey, D. Processing of functional polymers and organic thin films by the matrix-assisted pulsed laser evaporation (MAPLE) technique. Appl. Surf. Sci. 2002, 186, 408–415. [Google Scholar] [CrossRef]
- Belina, E.; Kisov, H.; Pavlova, E.; Borisova, E.; Dyankov, G. Thin hemoglobin layers deposited by MAPLE technology. In Proceedings of the 10th Jubilee Conference of The Balkan Physical Union, Sofia, Bulgaria, 26–30 August 2018. [Google Scholar]
- Dyankov, G.; Kisov, C.; Belina, E.; Pavlova, E.; Borisova, E.; Gisbrecht, A.; Ivanov, D. Comparative study on the bio-activity of hemoglobin and myoglobin as recognition materials in biosensors. SPIE 2019, 11047, 1104705. [Google Scholar] [CrossRef]
- Dyankov, G.; Eftimov, T.; Malinowski, N.; Belina, E.; Kisov, H.; Mikulic, P.; Bock, W. Dataset of MAPLE Parameters for Hemoglobin Deposition upon long period gratings. Data Brief 2020, 30, 105641. [Google Scholar] [CrossRef]
- Chung, J.; Takeoka, S.; Nishide, H.; Tsuchida, E. Oxygen-binding property of hemoglobin films. Polym. Adv. Technol. 1994, 5, 385–389. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser deposition of polymer and biomaterial films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef]
- Wu, P.K.; Ringeisen, B.R.; Krizman, D.B.; Frondoza, C.G.; Brooks, M.; Bubb, D.; Auyeung, R.C.Y.; Piqué, A.; Spargo, B.; McGill, R.A.; et al. Laser transfer of biomaterials: Matrix-assisted pulsed laser evaporation (MAPLE) and MAPLE direct write. Rev. Sci. Instrum. 2003, 74, 2546–2557. [Google Scholar] [CrossRef]
- Grenfell, T.C.; Perovich, D.K. Radiation absorption coefficients of polycrystalline ice from 400–1400 nm. J. Geophys. Res. Space Phys. 1981, 86, 7447–7450. [Google Scholar] [CrossRef]
- Bunn, H.; Haney, D.N.; Gabbay, K.H.; Gallop, P.M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem. Biophys. Res. Commun. 1975, 67, 103–109. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyankov, G.; Borisova, E.; Belina, E.; Kisov, H.; Angelov, I.; Gisbrecht, A.; Strijkova, V.; Malinowski, N. A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin. Sensors 2020, 20, 5572. https://doi.org/10.3390/s20195572
Dyankov G, Borisova E, Belina E, Kisov H, Angelov I, Gisbrecht A, Strijkova V, Malinowski N. A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin. Sensors. 2020; 20(19):5572. https://doi.org/10.3390/s20195572
Chicago/Turabian StyleDyankov, Georgi, Ekaterina Borisova, Evdokia Belina, Hristo Kisov, Ivan Angelov, Alexander Gisbrecht, Velichka Strijkova, and Nikola Malinowski. 2020. "A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin" Sensors 20, no. 19: 5572. https://doi.org/10.3390/s20195572
APA StyleDyankov, G., Borisova, E., Belina, E., Kisov, H., Angelov, I., Gisbrecht, A., Strijkova, V., & Malinowski, N. (2020). A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin. Sensors, 20(19), 5572. https://doi.org/10.3390/s20195572