Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring
Abstract
1. Introduction
2. Selected Nanostructures in Electrochemical Affinity Biosensing
3. Selected Nanostructures in Electrochemical Biosensing for Food Monitoring
4. Selected Nanostructures in Electrochemical Biosensing for Environmental Monitoring
5. General Considerations, Challenges and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Chen, X. Nanotechnology and nanomaterials-based no-wash electrochemical biosensors: From design to application. Nanoscale 2019, 11, 19105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Q. The role of nanomaterials in electroanalytical biosensors: A mini review. J. Electroanal. Chem. 2016, 781, 401–409. [Google Scholar] [CrossRef]
- Vasilescu, A.; Hayat, A.; Gáspár, S.; Marty, J.-L. Advantages of carbon nanomaterials in electrochemical aptasensors for food analysis. Electroanalysis 2018, 30, 2–19. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sheibani, S. Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: A review. Microchim. Acta 2018, 185, 276. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: Recent progress, applications, and future perspective. Chem. Rev. 2018, 119, 120–194. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomat. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Kirchner, E.-M.; Hirsch, T. Recent developments in carbon-based two-dimensional materials: Synthesis and modification aspects for electrochemical sensors. Microchim. Acta 2020, 187, 441. [Google Scholar] [CrossRef]
- Campuzano, S.; Gamella, M.; Serafín, V.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Biosensing and delivery of nucleic acids involving selected well-known and rising star functional nanomaterials. Nanomaterials 2019, 9, 1614. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423. [Google Scholar] [CrossRef]
- Freitas, M.; Couto, M.S.; Barroso, M.F.; Pereira, C.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J.; Delerue-Matos, C. Highly monodisperse Fe3O4@Au superparamagnetic nanoparticles as reproducible platform for genosensing genetically modified organisms. ACS Sens. 2016, 1, 1044–1053. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, E. Electrochemical biosensors based on magnetic micro/nano particles. Electrochim. Acta 2012, 84, 62–73. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Kudr, J.; Klejdus, B.; Adam, V.; Zitka, O. Magnetic solids in electrochemical analysis. Trac Trends Anal. Chem. 2018, 98, 104–113. [Google Scholar] [CrossRef]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikušová, Z.; Skládal, P. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Microchim. Acta 2019, 186, 312. [Google Scholar] [CrossRef]
- Brandão, D.; Liébana, S.; Campoy, S.; Alegret, S.; Pividori, M.I. Immunomagnetic separation of Salmonella with tailored magnetic micro and nanocarriers. A comparative study. Talanta 2015, 143, 198–204. [Google Scholar] [CrossRef]
- Serafín, V.; Torrente-Rodríguez, R.M.; Batlle, M.; García de Frutos, P.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for receptor tyrosine kinase AXL using poly(pyrrolepropionic acid)-modified disposable electrodes. Sens. Actuat. B.-Chem. 2017, 240, 1251–1256. [Google Scholar]
- Martín, M.; Salazar, P.; Jiménez, C.; Lecuona, M.; Ramos, M.J.; Ode, J.; Alcoba, J.; Roche, R.; Villalonga, R.; Campuzano, S.; et al. Rapid Legionella pneumophila determination based on a disposable core−shell Fe3O4@poly(dopamine) magnetic nanoparticles immunoplatform. Anal. Chim. Acta 2015, 887, 51–58. [Google Scholar]
- Jiang, D.; Jiang, H.; Ji, J.; Sun, X.; Qian, H.; Zhang, G.; Tang, L. Fluorescent magnetic bead-based mast cell biosensor for electrochemical detection of allergens in foodstuffs. Biosens. Bioelectron. 2015, 70, 482–490. [Google Scholar] [CrossRef]
- Zhu, F.; Zhao, G.; Dou, W. Electrochemical sandwich immunoassay for Escherichia coli O157:H7 based on the use of magnetic nanoparticles and graphene functionalized with electrocatalytically active Au@Ptcore/shell nanoparticles. Microchim. Acta. 2018, 185, 455. [Google Scholar] [CrossRef]
- Plácido, A.; Pereira, C.; Guedes, A.; Barroso, M.F.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Delerue-Matos, C. Electrochemical genoassays on gold-coated magnetic nanoparticles to quantify genetically modified organisms (GMOs) in food and feed as GMO percentage. Biosens. Bioelectron. 2018, 110, 147–154. [Google Scholar] [CrossRef]
- Plácido, A.; Pereira, C.; Barroso, M.F.; de-los-Santos-Álvarez, N.; Delerue-Matos, C. Chronoamperometric magnetogenosensing for simultaneous detection of two Roundup Ready soybean lines: GTS 40-3-2 and MON89788. Sens. Actuat B-Chem. 2019, 283, 262–268. [Google Scholar]
- Villalonga, M.L.; Borisova, B.; Arenas, C.B.; Villalonga, A.; Arévalo-Villena, M.; Sánchez, A.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical biosensors for Brettanomyces bruxellensis and total yeast content in wine based on core-shell magnetic nanoparticles. Sens. Actuat B Chem. 2019, 279, 15–21. [Google Scholar] [CrossRef]
- Sousa, J.B.; Ramos-Jesus, J.; Silva, L.C.; Pereira, C.; de-los-Santos-Alvarez, N.; Fonseca, R.A.S.; Miranda- Castro, R.; Delerue-Matos, C.; Ribeiro Santos Junior, J.; Barroso, M.F. Fe3O4@Au nanoparticles-based magnetoplatform for the HMGA maize endogenous gene electrochemical genosensing. Talanta 2020, 206, 120220. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Villalonga, A.; Martínez-García, G.; Parrado, C.; Villalonga, R. Dendrimers as soft nanomaterials for electrochemical immunosensors. Nanomaterials 2019, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Synthesis, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Zhang, X.; Shen, J.; Ma, H.; Jiang, Y.; Huang, C.; Han, E.; Yao, B.; He, Y. Optimized dendrimer-encapsulated gold nanoparticles and enhanced carbon nanotube nanoprobes for amplified electrochemical immunoassay of E. coli in dairy product based on enzymatically induced deposition of polyaniline. Biosens. Bioelectron. 2016, 80, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef] [PubMed]
- Serafín, V.; Razzino, C.A.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. Disposable immunoplatforms for the simultaneous determination of biomarkers for neurodegenerative disorders using poly(amidoamine) dendrimer/gold nanoparticles nanocomposite. Anal. Bioanal. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Pei, X.; Xu, Z.; Zhang, J.; Liu, Z.; Tian, J. Electroactive dendrimer-encapsulated silver nanoparticles for sensing low-abundance proteins with signal amplification. Anal. Methods 2013, 5, 3235–3241. [Google Scholar] [CrossRef]
- Niu, X.; Huang, L.; Zhao, J.; Yin, M.; Luo, D.; Yang, Y. An ultrasensitive aptamer biosensor for the detection of codeine based on an Au nanoparticle/polyamidoamine dendrimer-modified screen-printed carbon electrode. Anal. Methods 2016, 8, 1091–1095. [Google Scholar] [CrossRef]
- Singal, S.; Srivastava, A.K.; Kotnala, R.K.; Rajesh. Single-frequency impedance analysis of biofunctionalized dendrimer-encapsulated Pt nanoparticles-modified screen-printed electrode for biomolecular detection. J. Solid State Electrochem. 2018, 22, 2649–2657. [Google Scholar]
- Shu, J.; Qiu, Z.; Wei, Q.; Zhuang, J.; Tang, D. Cobalt-porphyrin-platinum-functionalized reduced graphene oxide hybrid nanostructures: A novel peroxidase mimetic system for improved electrochemical immunoassay. Sci. Rep. 2015, 5, 15113. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Li, J.; Ju, H. Electrochemical sensor for lead cation sensitized with a DNA functionalized porphyrinic metal–organic framework. Anal. Chem. 2015, 87, 10635–10641. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Luo, J.; Tian, Y.; Zhou, N. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Wang, Z.; Ma, H.; Wu, D.; Cheng, Q.; Wei, Q. Label-free electrochemical immunosensor based on enhanced signal amplification between Au@Pd and CoFe2O4/graphene nanohybrid. Sci. Rep. 2016, 6, 23391. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Altintas, Z. Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 2019, 12, 2189. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhan, A.F.Z.; Kashanineja, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozymes-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Wu, S.H.; Hung, Y.; Mou, C.Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef]
- Sancenón, F.; Pascual, L.; Oroval, M.; Aznar, E.; Martínez-Máñez, R. Gated silica mesoporous materials in sensing applications. Chem. Open 2015, 4, 418–437. [Google Scholar] [CrossRef]
- Castillo, R.R.; Baeza, A.; Vallet-Regí, M. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: Development of bioresponsive devices, carriers and sensors. Biomater. Sci. 2017, 5, 353–377. [Google Scholar] [CrossRef]
- Walcarius, A. Silica-based electrochemical sensors and biosensors: Recent trends. Curr. Op. Electrochem. 2018, 10, 88–97. [Google Scholar] [CrossRef]
- Jiménez-Falcao, S.; Villalonga, A.; Arévalo-Villena, M.; Briones-Pérez, A.; Martínez-Máñez, R.; Martínez-Ruiz, P.; Villalonga, R. Enzyme-controlled mesoporous nanosensor for the detection of living Saccharomyces cerevisiae. Sens. Actuat B-Chem. 2020, 303, 127197. [Google Scholar]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.-Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Wang, D.; Zeng, L.; Fang, Z. Portable and quantitative monitoring of mercury ions using DNA-gated mesoporous silica nanoparticles with a glucometer readout. Chem. Commun. 2016, 52, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Falcao, S.; Parra-Nieto, J.; Pérez-Cuadrado, H.; Martínez-Máñez, R.; Martínez-Ruiz, P.; Villalonga, R. Avidin-gated mesoporous silica nanoparticles for signal amplification in electrochemical. Electrochem. Commun. 2019, 108, 106556. [Google Scholar] [CrossRef]
- Pei, H.; Lu, N.; Wen, Y.; Song, S.; Liu, Y.; Yan, H.; Fan, C. A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv. Mater. 2010, 22, 4754. [Google Scholar] [CrossRef]
- Pei, H.; Wan, Y.; Li, J.; Hu, H.; Su, Y.; Huang, Q.; Fan, C. Regenerable electrochemical immunological sensing at DNA nanostructure-decorated gold surfaces. Chem. Commun. 2011, 47, 6254. [Google Scholar] [CrossRef]
- Goodman, R.P.; Berry, R.M.; Turberfield, A.J. The single-step synthesis of a DNA tetrahedron. Chem. Commun. 2004, 12, 1372–1373. [Google Scholar] [CrossRef]
- Li, S.; Tian, T.; Zhang, T.; Cai, X.; Lin, Y. Advances in biological applications of self-assembled DNA tetrahedral nanostructures. Mater. Today 2019, 24, 57–68. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Ji, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA biosensor based on a tetrahedral nanostructure probe for the detection of Avian Influenza A (H7N9) virus. ACS Appl. Mater. Interfaces 2015, 5, 8834–8842. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef]
- Zhao, J.; Jing, P.; Xue, S.; Xu, W. Dendritic structure DNA for specific metal ion biosensor based on catalytic hairpin assembly and a sensitive synergistic amplification strategy. Biosens. Bioelectron. 2017, 87, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Qing, M.; Yuan, Y.; Cai, W.; Xie, S.; Tang, Y.; Yuan, R.; Zhang, J. An ultrasensitive electrochemical biosensor based on multifunctional hemin/G-quadruplex nanowires simultaneously served as bienzyme and direct electron mediator for detection of lead ion. Sens. Actuat B-Chem. 2018, 263, 469–475. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Li, L.; Yang, X.; Jia, N.; Li, W.; Meng, J.; Duan, M.; Sun, X.; Liu, G. Sensitive and label-free electrochemical lead ion biosensor based on a DNAzyme triggered G-quadruplex/hemin conformation. Biosens. Bioelectron. 2018, 115, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Amine, A. Recent advances in electrochemical sensors based on molecularly imprinted polymers and nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- Azadmehr, F.; Zarei, K. An imprinted polymeric matrix containing DNA for electrochemical sensing of 2,4–dichlorophenoxyacetic acid. Microchim. Acta 2019, 186, 814. [Google Scholar] [CrossRef]
- Roushani, M.; Nezhadali, A.; Jalilian, Z. An electrochemical chlorpyrifos aptasensor based on the use of a glassy carbon electrode modified with an electropolymerized aptamer-imprinted polymer and gold nanorods. Microchim. Acta 2018, 185, 551. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Kitagawa, S. Metal-organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Meng, X.Z.; Gu, H.W.; Yi, H.C.; He, Y.Q.; Chen, Y.; Sun, W.Y. Sensitive detection of streptomycin in milk using a hybrid signal enhancement strategy of MOF-based bio-bar code and target recycling. Anal. Chim. Acta 2020, 1125, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, G.; Zhang, Y.; Wang, H.; Cao, W.; Li, T.; Wei, Q. An electrochemical aptasensor based on gold-modified MoS2/rGO nanocomposite and gold-palladium-modified Fe-MOFs for sensitive detection of lead ions. Sens. Actuat B Chem. 2020, 319, 128313. [Google Scholar] [CrossRef]
- Sun, X.; Jia, M.; Ji, J.; Guan, L.; Zhang, Y.; Tang, L.; Li, Z. Enzymatic amplification detection of peanut allergen Ara h1 using a stem-loop DNA biosensor modified with a chitosan mutiwalled carbon nanotube nanocomposite and spongy gold film. Talanta 2015, 131, 521–527. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Correr, W.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of the peanut allergen Ara h 6 in foodstuffs using a voltammetric biosensing approach. Anal. Bioanal. Chem. 2015, 407, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.; Silva, T.H.; Oliveira, M.B.P.; Delerue-Matos, C. Improving the extraction of Ara h 6 (a peanut allergen) from a chocolate based matrix for immunosensing detection: Influence of time, temperature and additives. Food Chem. 2017, 218, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Kim, S.W.; Park, C.; Lee, J. Single walled carbon nanotube based biosensor for detection of peanut allergy-inducing protein Ara h1. Korean. J. Chem. Eng. 2018, 35, 172–178. [Google Scholar]
- Khan, N.; Maddaus, A.G.; Song, K. A low-cost inkjet-printed aptamer-based electrochemical biosensor for the selective detection of lysozyme. Biosensors 2018, 8, 7. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. In Vitro Selection of DNA Aptamers Targeting β-Lactoglobulin and Their Integration in Graphene-Based Biosensor for the Detection of Milk Allergen. Biosens. Bioelectron. 2017, 91, 169–174. [Google Scholar] [CrossRef]
- Titoiu, A.M.; Porumb, R.; Fanjul-Bolado, P.; Epure, P.; Zamfir, M.; Vasilescu, A. Detection of allergenic lysozyme during winemaking with an electrochemical aptasensor. Electroanalysis 2019, 31, 2262–2273. [Google Scholar] [CrossRef]
- Manfredi, A.; Giannetto, M.; Mattarozzi, M.; Costantini, M.; Mucchino, C.; Careri, M. Competitive immunosensor based on gliadin immobilization on disposable carbon-nanogold screen-printed electrodes for rapid determination of celiotoxic prolamins. Anal. Bioanal. Chem. 2016, 408, 7289–7298. [Google Scholar] [CrossRef]
- Marín-Barroso, E.; Messina, G.A.; Bertolino, F.A.; Raba, J.; Pereira, S.V. Electrochemical immunosensor modified with carbon nanofibers coupled to a paper platform for the determination of gliadins in food samples. Anal. Methods 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, W. The determination of Ochratoxin A based on the electrochemical aptasensor by carbon aerogels and methylene blue assisted signal amplification. Chem. Centr. J. 2018, 12, 45. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuat B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Fath, R.H. Impedimetric ultrasensitive detection of chloramphenicol based on aptamer MIP using a glassy carbon electrode modified by 3-ampy-RGO and silver nanoparticle. Colloids Surf. B. 2019, 183, 110451. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. A novel aptamer- metal ions- nanoscale MOF based electrochemical biocodes for multiple antibiotics detection and signal amplification. Sens. Actuat B-Chem. 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Borisova, B.; Villalonga, M.L.; Arévalo-Villena, M.; Boujakhrout, A.; Sánchez, A.; Parrado, C.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical immunosensor for Brettanomyces bruxellensis based on nanogold-reduced graphene oxide hybrid nanomaterial. Anal. Bioanal. Chem. 2017, 409, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- Borisova, B.; Sánchez, A.; Soto-Rodríguez, P.E.D.; Boujakhrout, A.; Arévalo-Villena, M.; Pingarrón, J.M.; Briones-Pérez, A.; Parrado, C.; Villalonga, R. Disposable amperometric immunosensor for Saccharomyces cerevisiae based on carboxylated graphene oxide-modified electrodes. Anal. Bioanal. Chem. 2018, 410, 7901–7907. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Torbati, M.; Mousavi, M.-M.; de la Guardia, M.; Dolatabadi, J.E.N. Nanomaterial-based molecularly imprinted polymers for pesticides detection: Recent trends and future prospects. Trac Trends Anal. Chem. 2020, 129, 115943. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Molecularly imprinted electrochemical aptasensor for the attomolar detection of bisphenol A. Microchim. Acta 2018, 185, 265. [Google Scholar] [CrossRef]
- He, B.; Dong, X. Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sens. Actuat B Chem. 2019, 294, 192–198. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Kim, K.-H.; Deep, A. Development of an advanced electrochemical biosensing platform for E. coli using hybrid metal-organic framework/polyaniline composite. Environ. Res. 2019, 171, 395–402. [Google Scholar] [CrossRef]
- Tshikalaha, P.; Arotiba, O.A. Dendrimer supported electrochemical immunosensor for the detection of cholera toxin in water. Int. J. Electrochem. Sci. 2015, 10, 10083–10092. [Google Scholar]
- Gupta, P.K.; Gupta, A.; Dhakate, S.R.; Khan, Z.H.; Solanki, P.R. Functionalized polyacrylonitrile-nanofiber based immunosensor for Vibrio cholerae detection. J. Appl. Polym. Sci. 2016, 133, 44170. [Google Scholar] [CrossRef]
- Gupta, P.K.; Khan, Z.H.; Solanki, P.R. One-step electrodeposited porous ZnO thin film based immunosensor for detection of Vibrio cholerae toxin. J. Electrochem. Soc. 2016, 163, B309–B318. [Google Scholar] [CrossRef]
- Palomar, Q.; Gondran, C.; Holzinger, M.; Marks, R.; Cosnier, S. Controlled carbon nanotube layers for impedimetric immunosensors: High performance label free detection and quantification of anti-cholera toxin antibody. Biosens. Bioelectron. 2017, 97, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, O.C.; Maphumulo, T.; Shai, J.L.; Ozoemena, K.I. Electrospun carbon nanofibers as an electrochemical immunosensing platform for vibrio cholerae toxin: Aging effect of the redox probe. ACS Omega 2020, 5, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Shahdost-fard, F.; Roushani, M. Impedimetric detection of trinitrotoluene by using a glassy carbon electrode modified with a gold nanoparticle@fullerene composite and an aptamer-imprinted polydopamine. Microchim. Acta 2017, 184, 3997–4006. [Google Scholar] [CrossRef]
- Yarahmadi, S.; Azadbakht, A.; Derikvand, R.M. Hybrid synthetic receptor composed of molecularly imprinted polydopamine and aptamers for impedimetric biosensing of urea. Microchim. Acta 2019, 186, 71. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.-Y.; He, L.; Zhang, X.; Liu, C.S. Fe(III)-based metal–organic framework-derived core–shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, C.S.; Kim, T.H. Electrochemical detection of ultratrace lead ion through attaching and detaching DNA aptamer from electrochemically reduced graphene oxide electrode. Nanomaterials 2019, 9, 817. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Xie, X.; Liu, Y.; Wang, J.; Tang, J.; Zhang, Y.; Deng, Y. Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon–gold nanoparticles and DNAzyme catalytic beacons. Talanta 2016, 146, 641–647. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, C.; Jun, N.; Zhao, Y.; Zhang, Y.; Gao, R.; He, J. Target triggered cleavage effect of DNAzyme: Relying on Pd-Pt alloys functionalized Fe-MOFs for amplified detection of Pb2+. Biosens. Bioelectron. 2018, 101, 297–303. [Google Scholar] [CrossRef]
- Lee, I.; Kim, S.-E.; Lee, J.; Woo, D.H.; Lee, S.; Pyo, H.; Song, C.-S.; Lee, J. A self-calibrating electrochemical aptasensing platform: Correcting external interference errors for the reliable and stable detection of avian influenza viruses. Biosens. Bioelectron. 2020, 152, 112010. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef] [PubMed]

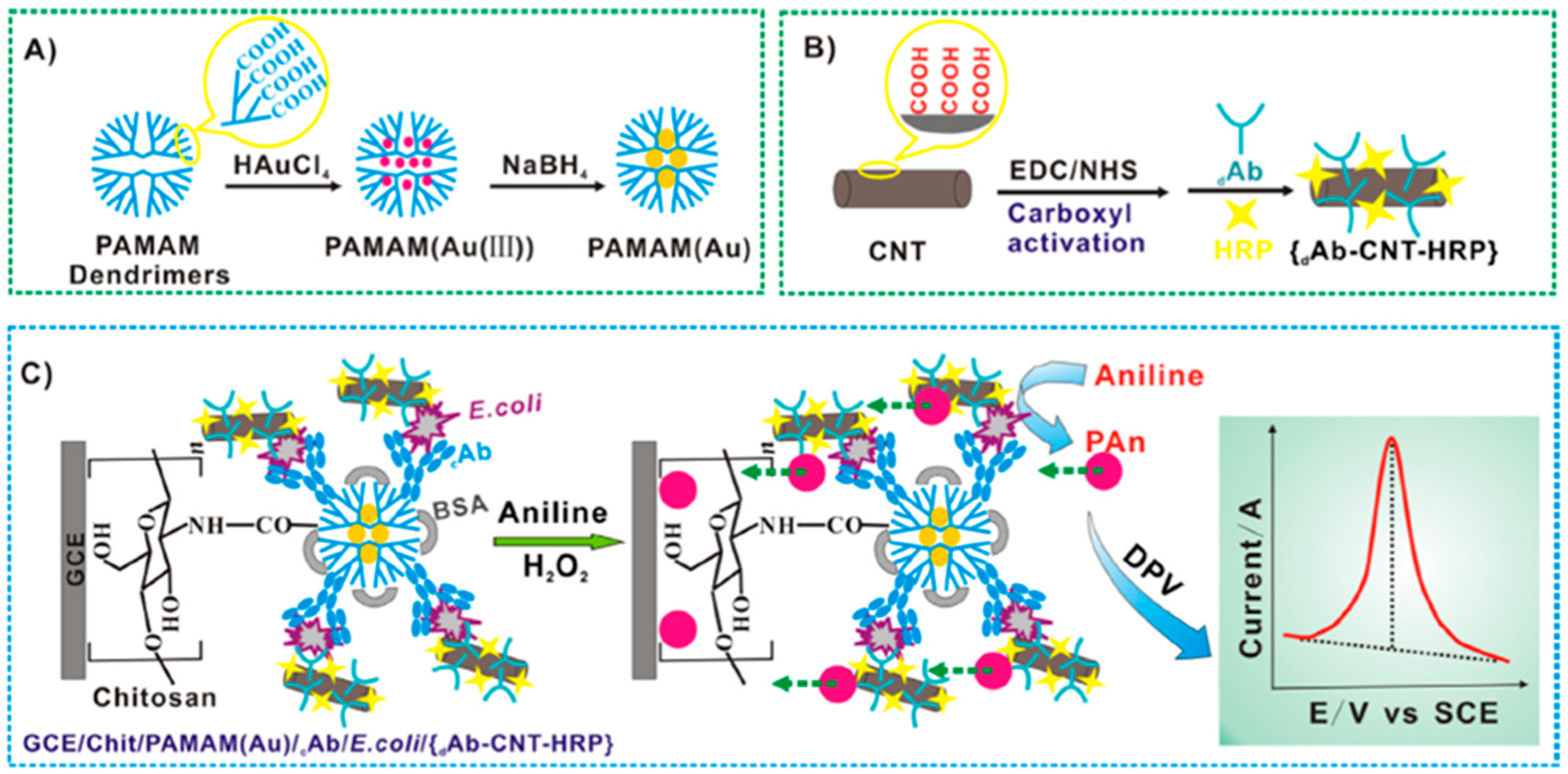
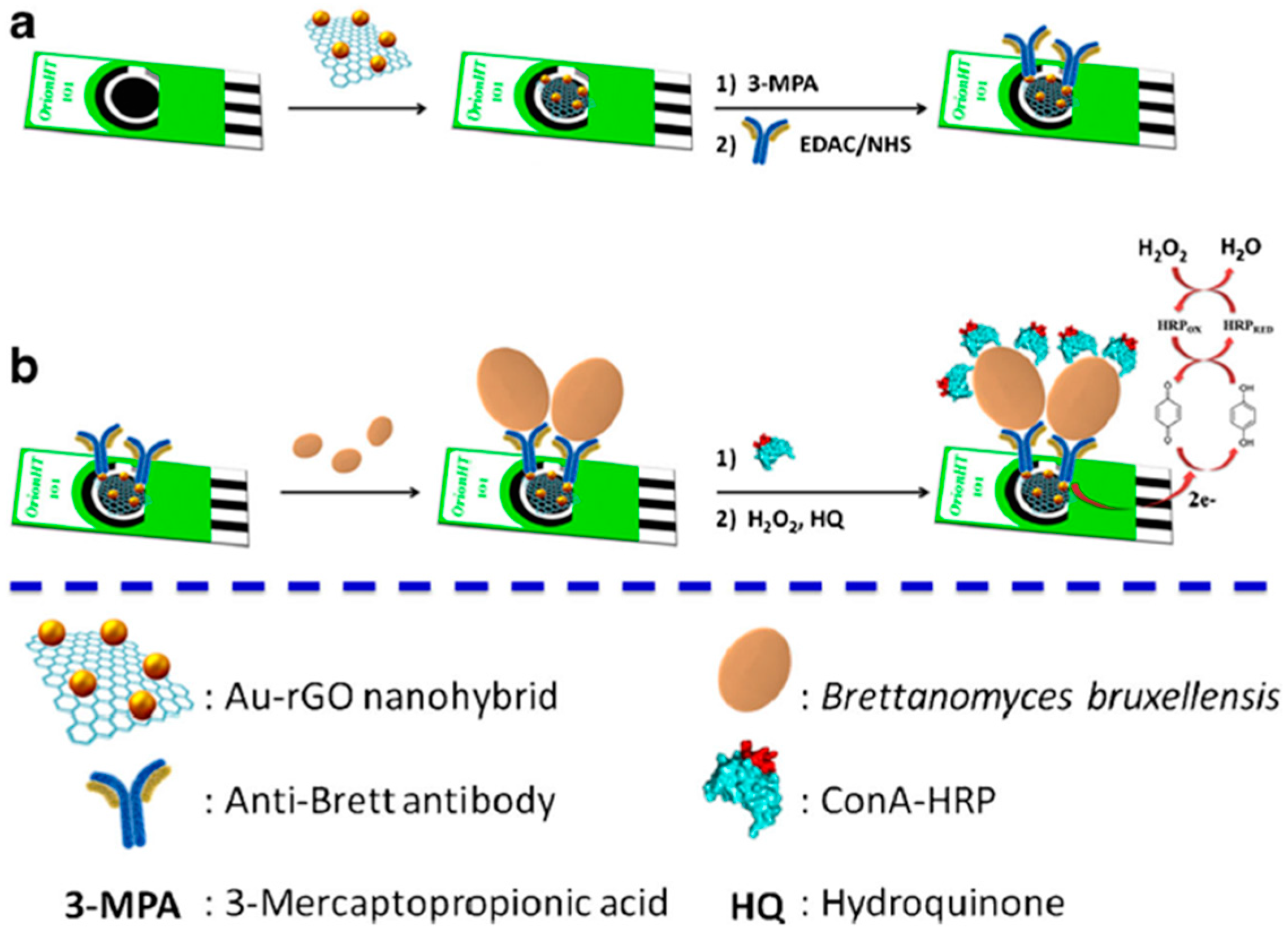
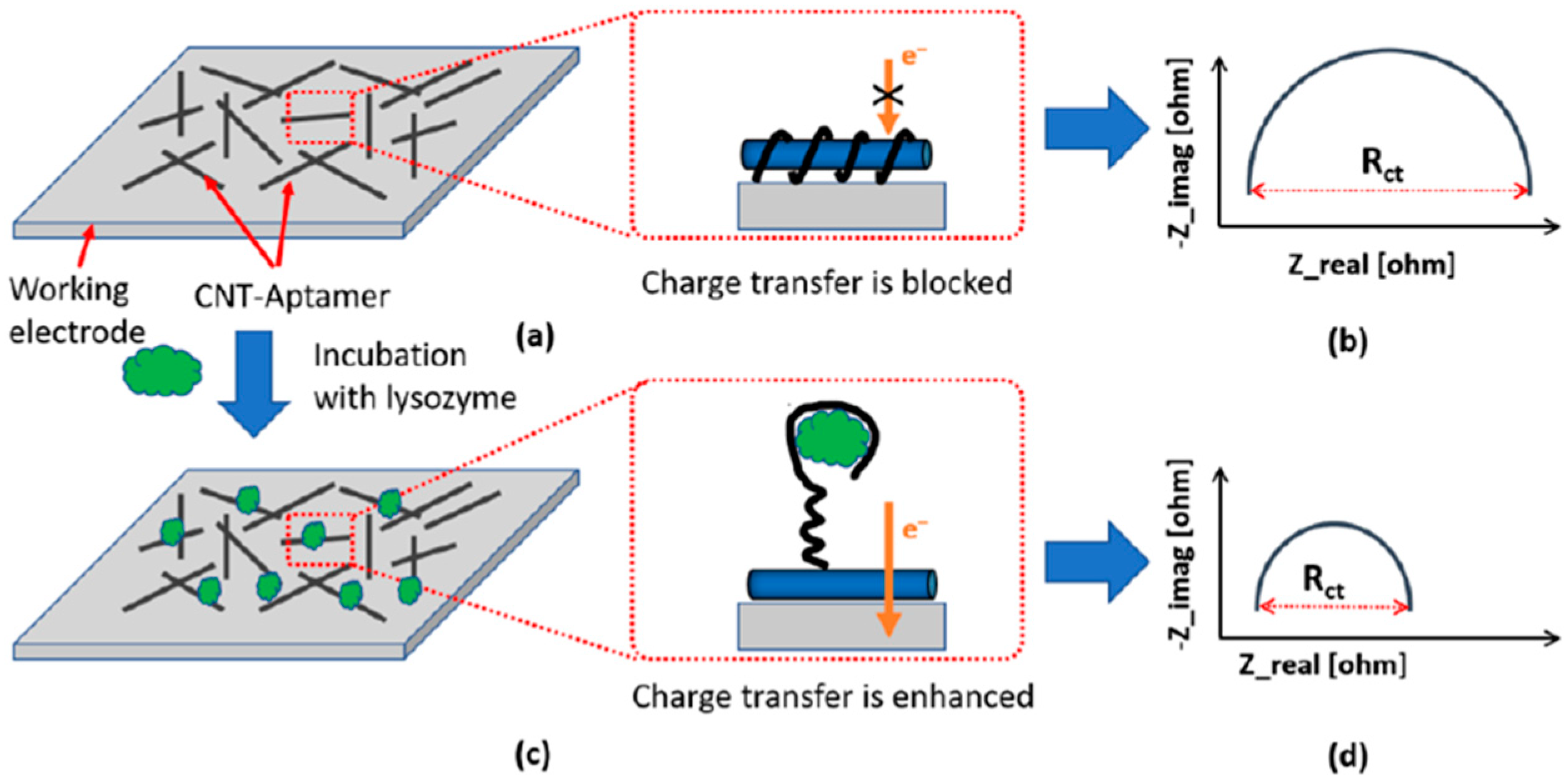
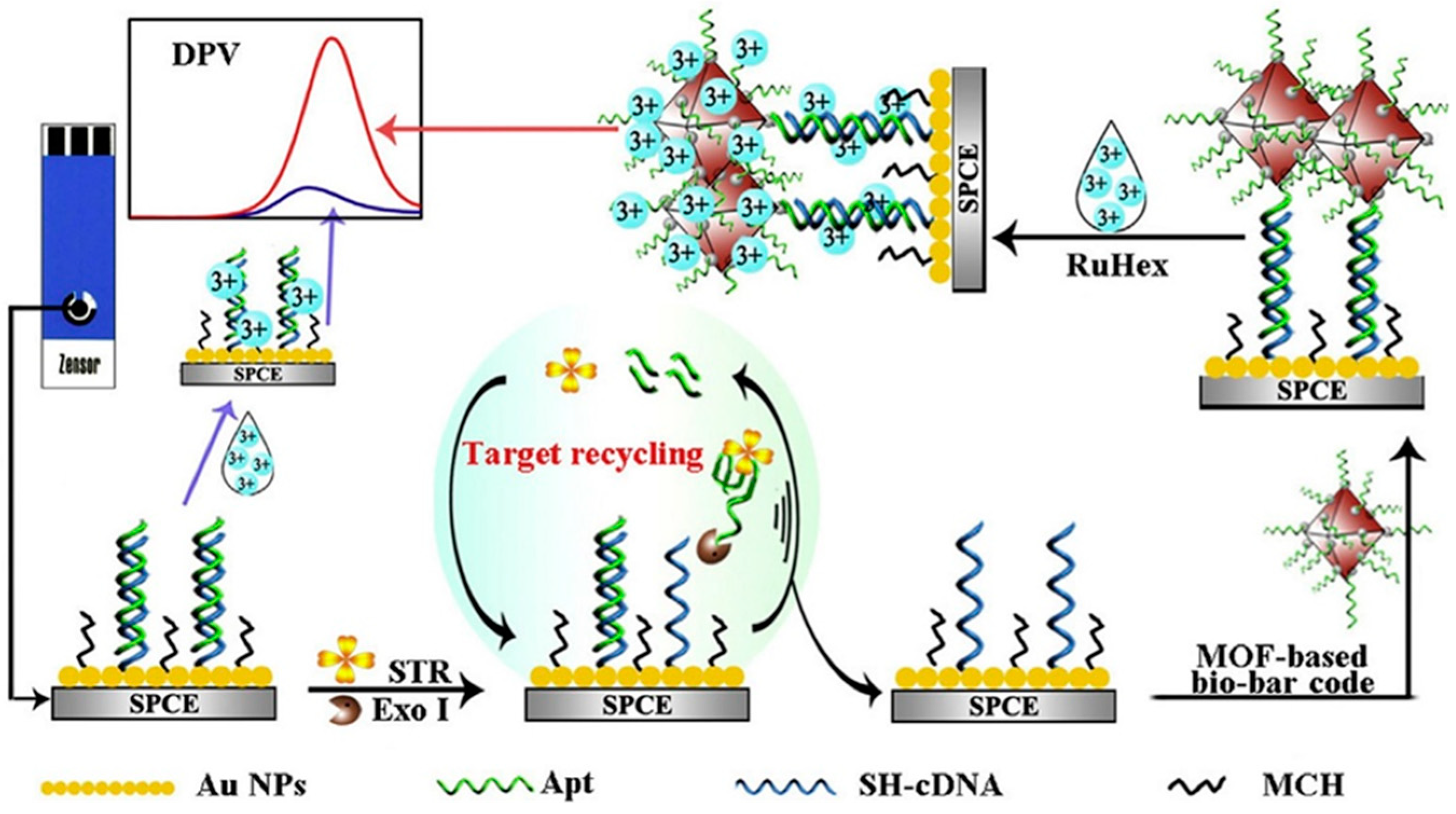


| Electrode | Bioassay Format | Nanomaterial//Role | Detection Technique | Target Analyte | Analytical Characteristics | Demonstrated Applicability | Ref. | |
|---|---|---|---|---|---|---|---|---|
| IMMUNOSENSORS | SPCE | Sandwich immunosensor (b-DAb+Strep-AP) | AuNPs//Electrode modifier | DPV (3-IP/Ag+) | Ara h 1 | L.R. = 12.6–2,000 ng mL−1 LOD = 3.8 ng mL−1 | Spiked cookies and chocolate samples | [62] |
| SPCE | Sandwich immunosensor (b-DAb+Strep-AP) | AuNPs//Electrode modifier | DPV (3-IP/Ag+) | Ara h 6 | L.R. = 1–100 ng mL−1 LOD = 0.27 ng mL−1 | Spiked cookies and chocolate samples | [63,64] | |
| AuNPs-SPCEs | Indirect competitive immunosensor (AP-secondary antibody) | AuNPs//Electrode modifier | DPV (HQDP) | Gliadin | LOD = 8 ng mL−1 | Commercial flours (mile, chestnut, chickpeas, quinoa and potato), samples of durum wheat pasta, breadcrumb, crackers and biscuits | [69] | |
| SPGE | Direct immunosensor | AuNPs//Electrode modifier | CV ([Fe(CN)6]4−/3−) | Lysozyme | L.R. = 1–10 µg mL−1 LOD = 0.32 µg mL−1 | Spiked white wine samples | [68] | |
| SPCE | Direct immunosensor | SWCNTs//Electrode modifier | FET | Ara h 1 | L.R. = 1–1,000 ng mL−1 LOD = 100 ng mL−1 | — | [65] | |
| GCE | Indirect competitive immunosensor | PtNPs/CoTPP/rGO//Nanocarriers +nanozymes | DPV (TMB/H2O2) | AFB1 | L.R. = 0.005–5.0 ng mL−1 LOD = 5.0 pg mL−1 | Naturally contaminated or spiked peanut samples | [32] | |
| GCE | Sandwich immunosensor (CNTs decorated with DAb+HRP) | PAMAM(Au)//Electrode modifier and CNTs/Nanocarriers | DPV (Aniline/H2O2) | E. coli | L.R. = 1.0 × 102−1.0 × 106 cfu mL−1 LOD = 50 cfu mL−1 | Spiked dairy product (pure fresh milk, infant milk powder, yogurt in shelf-life and expired yogurt) | [26] | |
| SPCE | Sandwich immunosensor | Fe3O4@SiO2MNPs// Solid support and rGO-NR-Au@Pt//nanocarriers+nanozymes | CV (Thi/H2O2) | E. coli O157:H7 | L.R. = 4.0 × 102–4.0 × 108 cfu mL−1 LOD = 91 cfu mL−1 | Spiked pork and milk samples | [19] | |
| SPCE | Direct immunosensor and enzymatic labeling with ConA-HRP | AuNPs-rGO//Electrode modifier | Amperometry (HQ/H2O2) | Brett | L.R. = 10–106 cfu mL−1 (buffered solutions); 102–106 cfu mL−1 (red wine samples) LOD = 8 cfu mL−1 (buffered solutions); 56 cfu mL−1 (red wine samples) | Spiked red wine samples | [75] | |
| SPCE | Direct immunosensor and enzymatic labeling with ConA-HRP | GO//Electrode modifier | Amperometry (HQ/H2O2) | S. cerevisiae | L.R. = 10–107 cfu mL−1 (buffered solutions and red wine samples) LOD = 4 cfu mL−1 (buffered solutions); 6 cfu mL−1 (white wine samples) | Spiked white wine samples | [76] | |
| AuE | Direct immunosensor | GQDs//Electrode modifier+nanozyme | Chrono-amperometry (H2O2) | Y. enterocolitica | L.R. = 6.23 × 102−6.23 × 108 cfu mL−1 LOD = 5 cfu mL−1 (milk) | Milk samples | [36] | |
| SPCE | Sandwich immunosensor (HRP-DAb) | CNFs//Electrode modifier | Chrono-amperometry (HQ/H2O2) | Gliadin | L.R. = up to 80 μg kg−1 LOD = 0.005 mg kg−1 | Manioc, rice, gluten-free, and wheat flours | [69] | |
| APTASENSORS | AuE | Direct aptasensor | AuNPs//Electrode modifier+nanozyme | DPV (Thi/H2O2) | KANA | L.R. = 0.1–60 nM LOD = 0.06 nM | Honey samples | [34] |
| SPCE | Inkjet-Printed, direct aptasensor | CNTs//Electrode modifier | EIS ([Fe(CN)6]4−/3−) | Lysozyme | LOD = 90 ng mL−1 | — | [66] | |
| SPCE | Direct aptasensor | G//Electrode modifier | SWV ([Fe(CN)6]4−/3−) | β-LG | L.R. = 100 pg mL−1–100 ng mL−1 LOD = 20 pg mL−1 | Spiked food extracts | [67] | |
| AuE | Direct hybridization of a specific aptamer with a complementary DNA (cDNA) and displacement of cDNA in the presence of the target molecule | CAs//Nanocarriers | DPV (MB) | OTA | L.R. = 0.10–10 ng mL−1 LOD = 1.0 × 10−4 ng mL−1 | Spiked corn samples | [71] | |
| GCE | Direct aptasensor-MIP hybrid | 3-ampy-rGO/MIP//Electrode modifier | EIS ([Fe(CN)6]4−/3−) | CAP | L.R. = 1.0 pM–1.0 nM LOD = 0.3 pM | Spiked milk | [73] | |
| GCE | Direct aptasensor-MIP hybrid | (o-PD/o-DB) / AuNRs/MIP//Electrodo modifier | DPV ([Fe(CN)6]4−/3−) | CPS | L.R. = 1.0 fM–0.4 pM LOD = 0.35 fM. | Apples, lettuce | [57] | |
| GCE | anti-ssDNA Ab immobilized onto MBs and MOFs labelled with metal ions and the aptamers | nanometal ions (Cd2+ Pb2+ )/MOF//signal tags | SWV (Cd2+, Pb2+) | KANA, CAP | L.R. = 0.002 nM–100 nM. LOD = 0.16 pM (KANA); 0.19 pM (CAP) | Milk | [74] | |
| SPCE | MOF-based bio-bar code with dsDNA and MCH immobilized and enzyme-assisted target recycling | AuNPs//Electrode modifiers and F//Nanocarriers | DPV (Ru(NH3)63+) | STR | L.R. = 0.005–150 ng mL−1 LOD = 2.6 pg mL−1 | Milk | [59] | |
| AuE | DNA H1 immobilized onto CoSe2/AuNRs and Thi-labelled 3 ds-DNA immobilized onto PtNi@Co/MOF networks | CoSe2/AuNRs//electrode modifier and PtNi@Co/MOF//nanocarriers | DPV (Thi) | ZEN | L.R. = 10 fg mL−1 –10 ng mL−1 LOD = 1.37 fg mL−1 | Maize | [72] | |
| AuE | Modified electrode with ss-DNA and self- assembled signal tags; dissociation of Apt- cDNA in presence of PAT | AuNPs/Chit/ZnO// Electrode modifier and MB@MOF// Nanocarrier | DPV (MB) | PAT | L.R. = 5 × 10−8–0.5 μg mL−1 LOD = 1.46 × 10−8 μg mL−1 | Apple juice | [79] | |
| DNA SENSORS | GCE | Direct hybridization at a stem-loop DNA probe dually labelled with 5′-SH and 3′-biotin (Strep-HRP) | CS-MWCNTs modified with a spongy gold film/Electrode modifier | Chrono-amperometry (HQ/H2O2) | Ara h 1 | L.R.: 3.91 × 10−17–1.25 × 10−15 M LOD: 1.3 × 10−17 M | Peanut DNA extracts | [61] |
| SPCE | Sandwich hybridization and enzymatic labelling with anti-FITC-HRP Fab fragment conjugate | Fe3O4@Au MNPs/Solid support | Chrono-amperometry (HQ/H2O2) | GMO (a specific fragment of the trans- genic MON 810 maize) | L.R.: 0.25–2.5 nM LOD = 0.15 ng mL−1 | Certified samples containing the transgenic event (PCR amplicons) | [10] | |
| SPCE | Sandwich hybridization and enzymatic labeling with HRP-anti-FITC Fab fragments | Fe3O4@Au MNPs/Solid support | Chrono- amperometry (HQ/H2O2) | GMO (specific frag- ments from the insertion point of the trans- genic construct of RR GTS 40- 3 -2 soy-bean and of the taxon-specific soybean gene, lectin) | L.R.: 0.1−10.0 nM (RR); 0.1−5.0 nM (lectin) LOD = 0.02 nM (RR); 0.05 nM (lectin) | Soybean seeds and cat feed (PCR amplicons) | [20] | |
| SPdCEs | Sandwich hybridization and enzymatic labeling with HRP-anti-FITC- and anti-DIG Fab fragments | Fe3O4@Au MNPs/Solid support | Chrono-amperometry (HQ/H2O2) | GMO (RR soybean lines GTS 40-3-2 and MON89788) | L.R.: 0.1−2.5 nM (GTS 40-3-2); 0.1−1.0 nM (MON89788) LOD = 0.1 nM (GTS 40-3-2 and MON89788) | — | [21] | |
| AuE (homemade) | Sandwich hybridization and enzymatic labeling with HRP-anti-FITC- Fab fragments | Fe3O4@Au MNPs/Solid support | Chronoamperometry (HQ/H2O2) | GMO (specific fragment from maize taxon-specific HMGA gene) | L.R.: 0.5–5 nM LOD = 90 pM | Maize flour (PCR amplicons) | [23] | |
| GCE | Label-free DNA-MIP hybrid sensor | AuNPs/pPy/MIP// Electrode modifier | EIS ([Fe(CN)6]4−/3−) | BPA | L.R. = 0.5 fM–5 pM LOD = 80 aM | Spiked milk, milk powder, water | [78] | |
| OTHERS | MGCE | Mast RBL-2H3 cell biosensor | Fe3O4@SiO2@FITC encapsulated with lipidosome/Solid support +Transfection | EIS ([Fe(CN)6]4−/3−) | Shrimp Pen a 1 and fish PV | LOD = 0.03 μg mL−1 (shrimp Pen a 1); 0.16 ng mL−1 (fish PV) | Crucian carp and brown shrimp crude extracts | [18] |
| SPCE | Direct Affinity sensors and enzymatic labeling with ConA-HRP | Fe3O4@SiO2 MNPs/Solid support | Amperometry (HQ/H2O2) | Brett (CAb-Fe3O4@SiO2 MNPs) and total yeast content (ConA- Fe3O4@SiO2 MNPs) | Brettanomyces bruxellensis: L.R.: 10–106 cfu mL−1 (buffered solutions and red wine samples) LOD = 6 cfu mL−1 (buffered solutions) and 8 cfu mL−1 (red wine samples) Total yeast content: L.R.: 10–106 cfu mL−1 (buffered solutions and red wine samples) LOD = 5 cfu mL−1 (buffered solutions and red wine samples) | Spiked red wine samples | [22] |
| Electrode | Biosensor Type | Nanomaterial//Role | Detection Technique | Target Analyte | Analytical Characteristics | Application | Ref. | |
|---|---|---|---|---|---|---|---|---|
| IMMUNOSENSORS | SPCE | Sandwich immunosensor (HRP-DAb) | Fe3O4@pDA MNPs //Solid support | Chrono-amperometry (H2O2/HQ) | Legionella pneumophila | L.R. = 104–108 cfu mL−1 and 104–108 cfu mL−1 (with preconcentration step) LOD = 104 cfu mL−1 and 10 cfu mL−1 (with preconcentration step) | Inoculated water samples | [17] |
| GCE | Direct immunosensor | CoFe2O4/rGO +Au@PdNRs// Electrode modifier + nanozyme | Amperometry (H2O2) | Estradiol | L.R. = 0.01−18.0 ng mL−1 LOD = 3.3 pg mL−1 | Spiked river water | [35] | |
| GCE | Direct immunosensor | PPI-AuNP nanocomposite// Electrode modifier | SWV and EIS ([Fe(CN)6]4−/3−) | Cholera toxin | L.R. = 10−7–10−12 g mL−1 (SWV and EIS) LOD = 7.2 × 10−13 (SWV) and 4.2 × 10−13 g mL−1 (EIS) | — | [81] | |
| ITO | Direct immunosensor | PANnf’s// Electrode modifier | DPV ([Fe(CN)6]4−/3−) | Vibrio cholerae | L.R. = 6.25–500 ng mL−1 LOD = 0.22 ng mL−1 | — | [82] | |
| ITO | Direct immunosensor | ZnO NPs// Electrode modifier | DPV ([Fe(CN)6]4−/3−) | Vibrio cholerae toxin B | L.R. = 12.5–500 ng mL−1 LOD = 0.16 ng mL−1 | — | [83] | |
| GCE | Direct immunosensor | CNTs// Electrode modifier | EIS ([Fe(CN)6]4−/3−) | Anti-cholera toxin antibodies | L.R. = 10−13−10−5 g mL−1 LOD = 10−13 g mL−1 | — | [84] | |
| GCE | Direct immunosensor | CNFs// Electrode modifier | EIS ([Fe(CN)6]4−/3−) | Vibrio cholerae toxin | L.R. = 10−13−10−5 g mL−1 LOD = 1.2 × 10−13 g mL-1 | — | [85] | |
| ITO | Label-free immunosensor with immobilized anti-E-coli onto PANI/MOF modified electrode | Cu3(BTC)2/ PANI//MOF | EIS ([Fe(CN)6]3−/4−) | E. coli | L.R.: 2.0−2.0 × 108 cfu mL−1 LOD: 2.0 cfu mL−1 | Spiked lake water | [80] | |
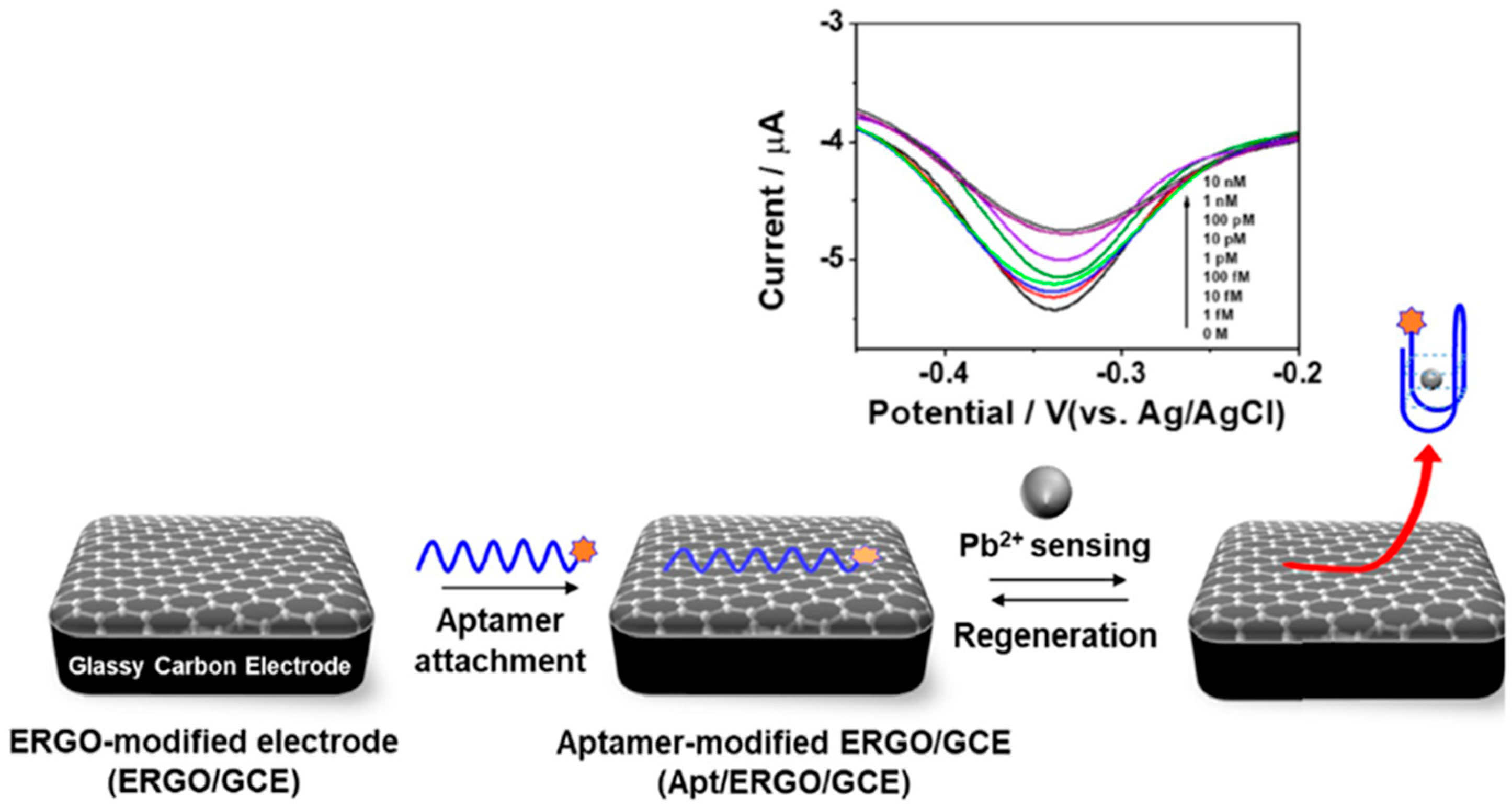
| APTASENSORS | GCE | Direct aptasensor (MB-tagged aptamer) | ERGO//Electrode modifier | DPV (MB) | Pb2+ | L.R. = 10−15–10−9 M LOD = 0.51 fM | Spiked tap water, valley water, and secondary treated wastewater | [89] |
| GCE | Label-free MIP-aptasensor prepared by DA electropolymerization with Apt-TNT complex onto modified electrode | AuNPs@C60/ aptamer-MIP biosensor | EIS ([Fe(CN)6]3−/4−) | TNT | L.R. = 0.01 fM–1.5 μM LOD = 3.5 aM | Spiked soil and river water | [86] | |
| GCE | Label-free MIP aptasensor prepared by DA electropolymerization with Apt-urea complex onto modified electrode | AuNPs/CNTs/ aptamer-MIP biosensor | EIS ([Fe(CN)6]3−/4−) | Urea | L.R. = up to 500 nM LOD = 900 fM | Spiked soil and tap water | [87] | |
| AuE | Label-free MOF apta-sensor combining confor-mational transition inter-action caused by G-qua-druplex formed between a ssDNA aptamer and adsorbed metal ions | Fe-MOF@ mFe3O4@mC | EIS ([Fe(CN)6]3−/4−) | Pb2+, As3+ | L.R.: 0.01–10.0 nM LOD: 2.27 pM (Pb2+); 6.73 pM (As3+) | River waters, serum | [88] | |
| AuE | Label-free aptasensor prepared by aptamer immobilization onto modified electrode | Co-MOF@TPN-COF | EIS ([Fe(CN)6]3−/4−) | AMP | L.R.: 1.0 fg mL−1–2.0 ng mL−1 LOD: 0.217 fg mL−1 | waters, serum, milk | [93] | |
| AuE | Label-free aptasensor prepared by aptamer immobilization onto modified electrode | Ce-MOF@MCA | EIS ([Fe(CN)6]3−/4−) | OTC | L.R.: 0.1–0.5 ng mL−1 LOD: 17.4 fg mL−1 | Wastewater, urine, milk | [90] | |
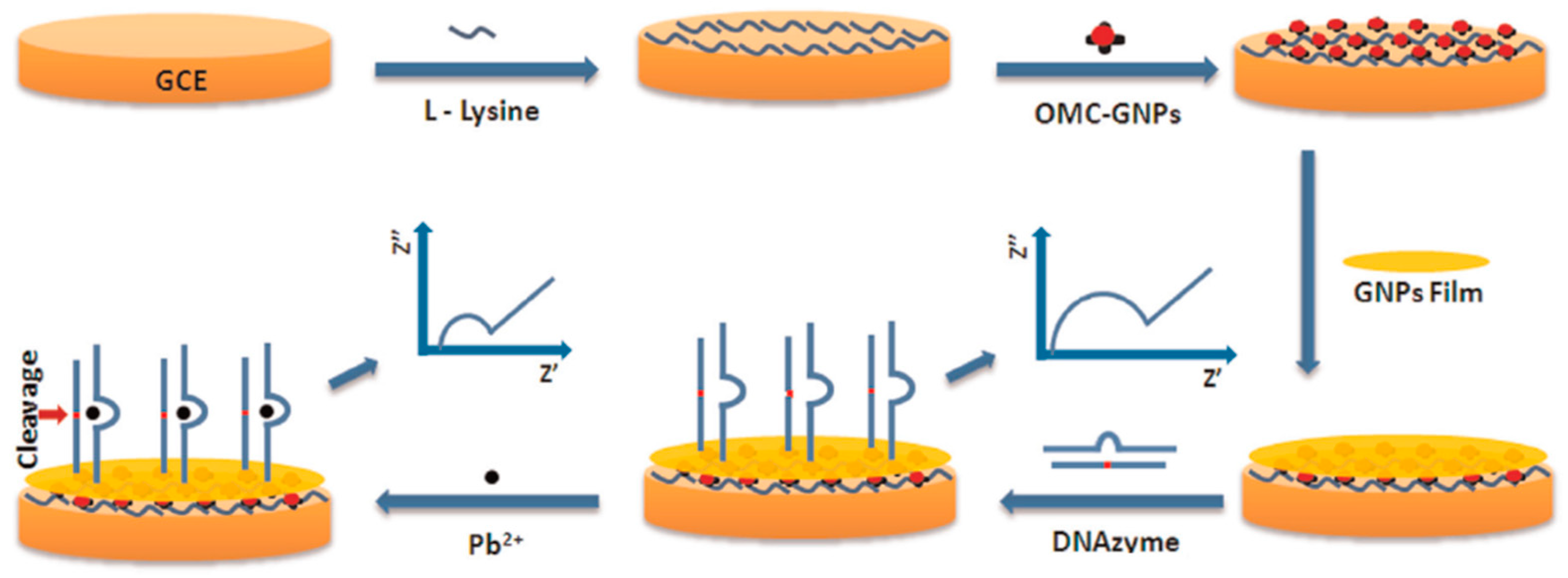
| DNA SENSORS | GCE | Direct hybridization approach of DNAzyme catalytic beacons at a surface tethered thiolated DNA probe and selective cleavage in the presence of Pb2+ | OMC–AuNPs and AuNPs//Electrode modifier | EIS ([Fe(CN)6]4−/3−) | Pb2+ | L.R. = 5 × 10−10–5 × 10−5 M LOD = 2 × 10−10 M | Environmental water samples (tap water, lake and fresh river water) | [88] |
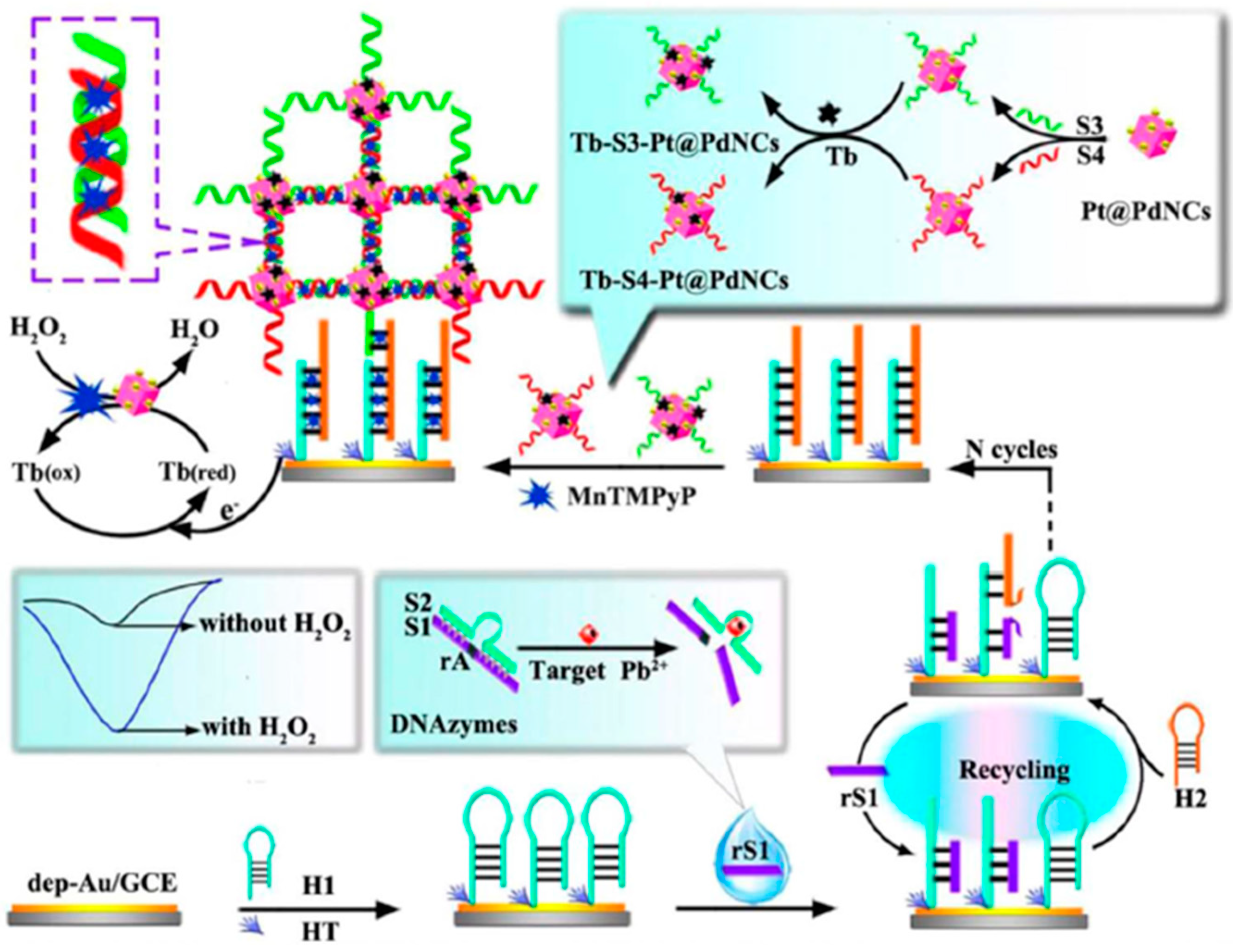
| GCE | Biosensing approach which combine the use of specific DNAzymes, nanomaterials Pt@PdNCs and mimicking enzyme MnTMPyP assisted by DSDNA and CHA | AuNPs//Electrode modifier and Pt@PdNCs//Nanocarriers of signaling elements | DPV (TB) | Pb2+ | L.R. = 0.1 pM–200 nM LOD = 0.033 pM | Tap and lake water samples | [52] | |
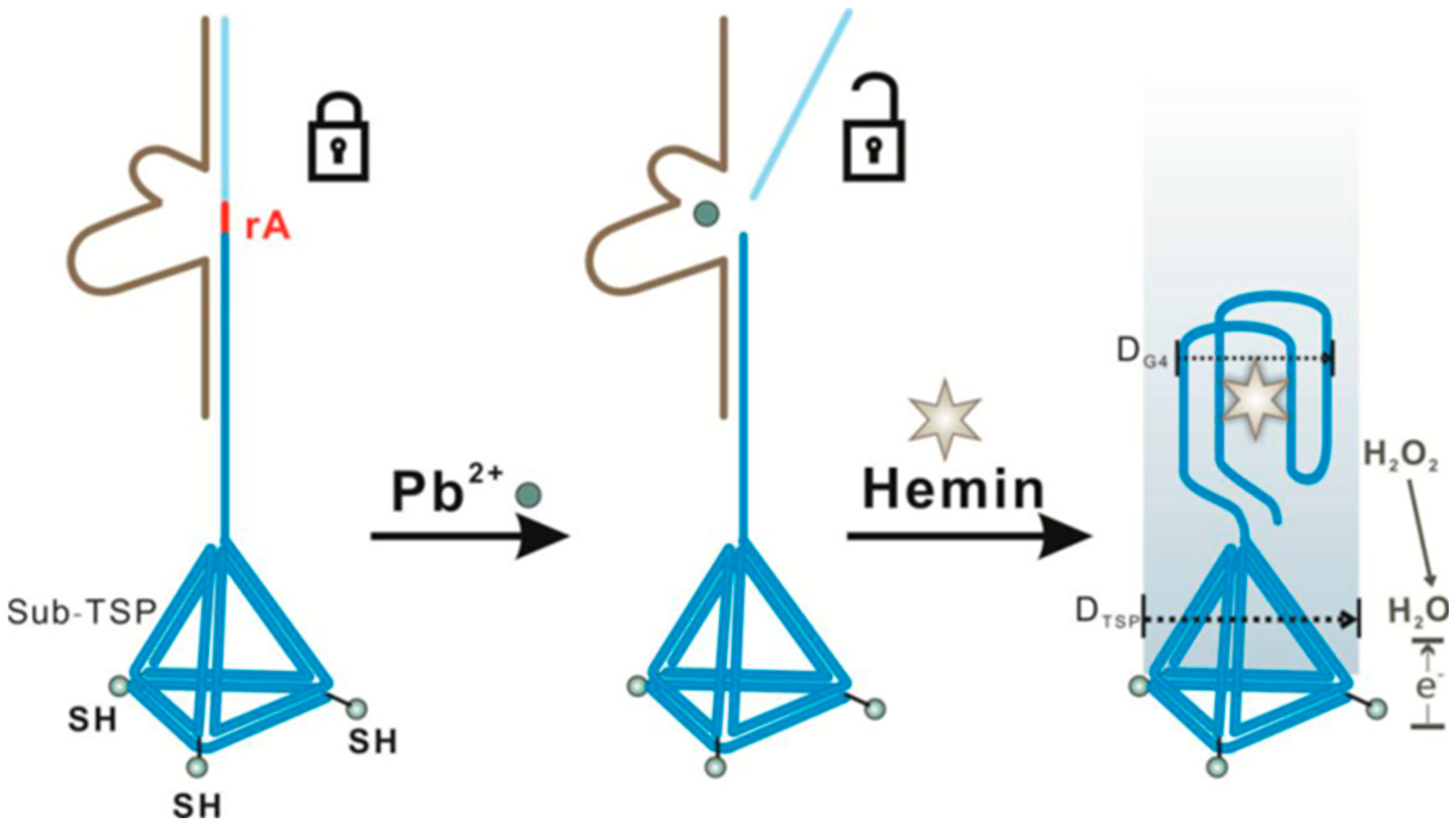
| AuE | Specific DNAzyme (composed of a DNAzyme strand and a substrate strand) immobilized on a TSP. In the presence of Pb2+ the substrate strand is cleaved and released a “G-rich” oligo able to form a G-quadruplex/hemin complex | TSP//Electrode modifiers | CV (H2O2) | Pb2+ | LOD = 0.008 nM | Spiked tap and pool water samples | [54] | |
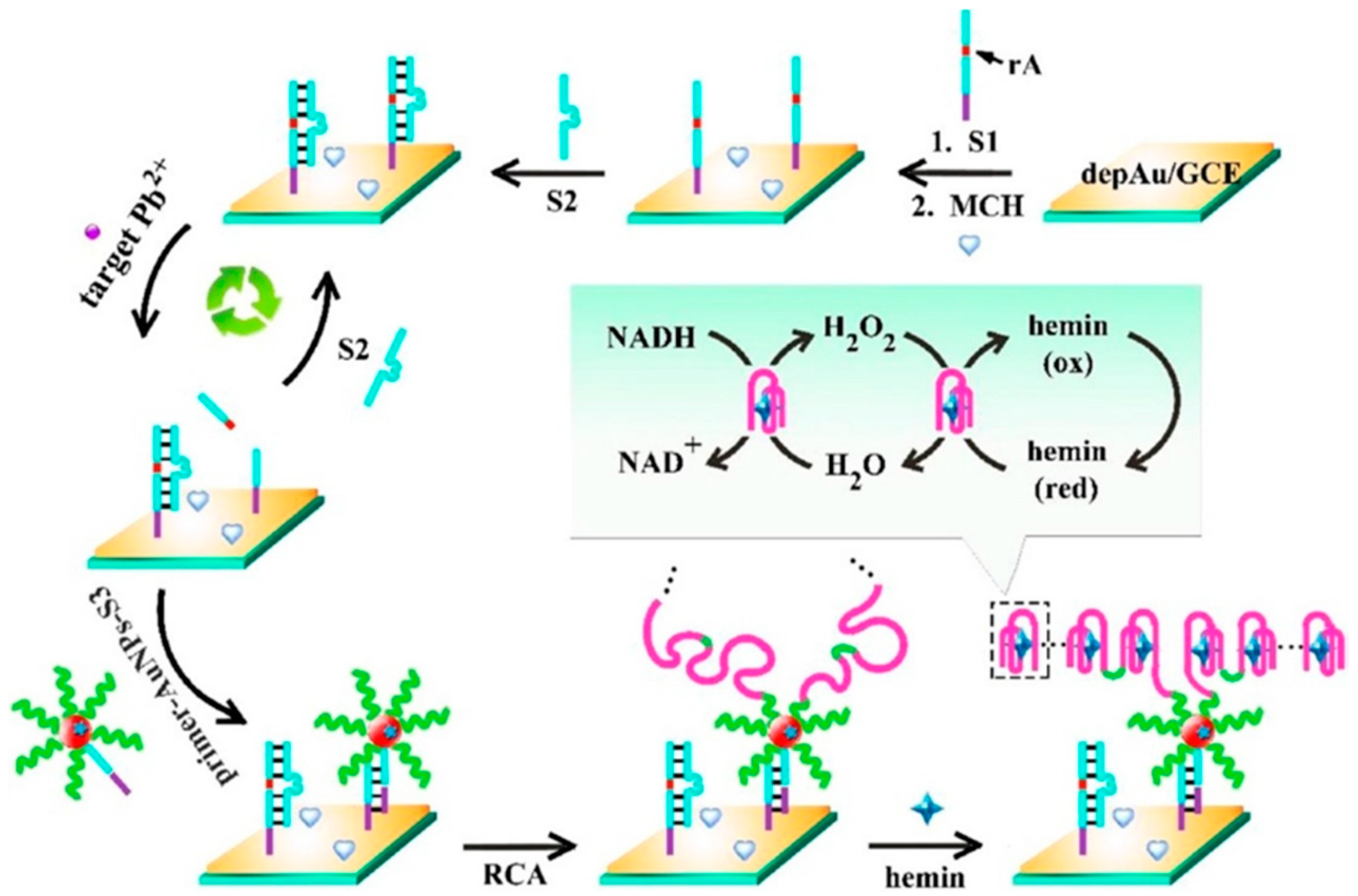
| GCE | Biosensing approach based on pseudo bienzyme cascade amplification and direct electron transfer of multifunctional hemin/ G- quadruplex nanowires formed by RCA reaction | AuNPs//Electrode modifiers and multifunctional hemin/G-quadruplex nanowires/Bienzyme and direct electron transfer | DPV (NADH) | Pb2+ | L.R. = 10 fM–200 nM LOD = 3.3 fM | Spiked tap water samples | [53] | |
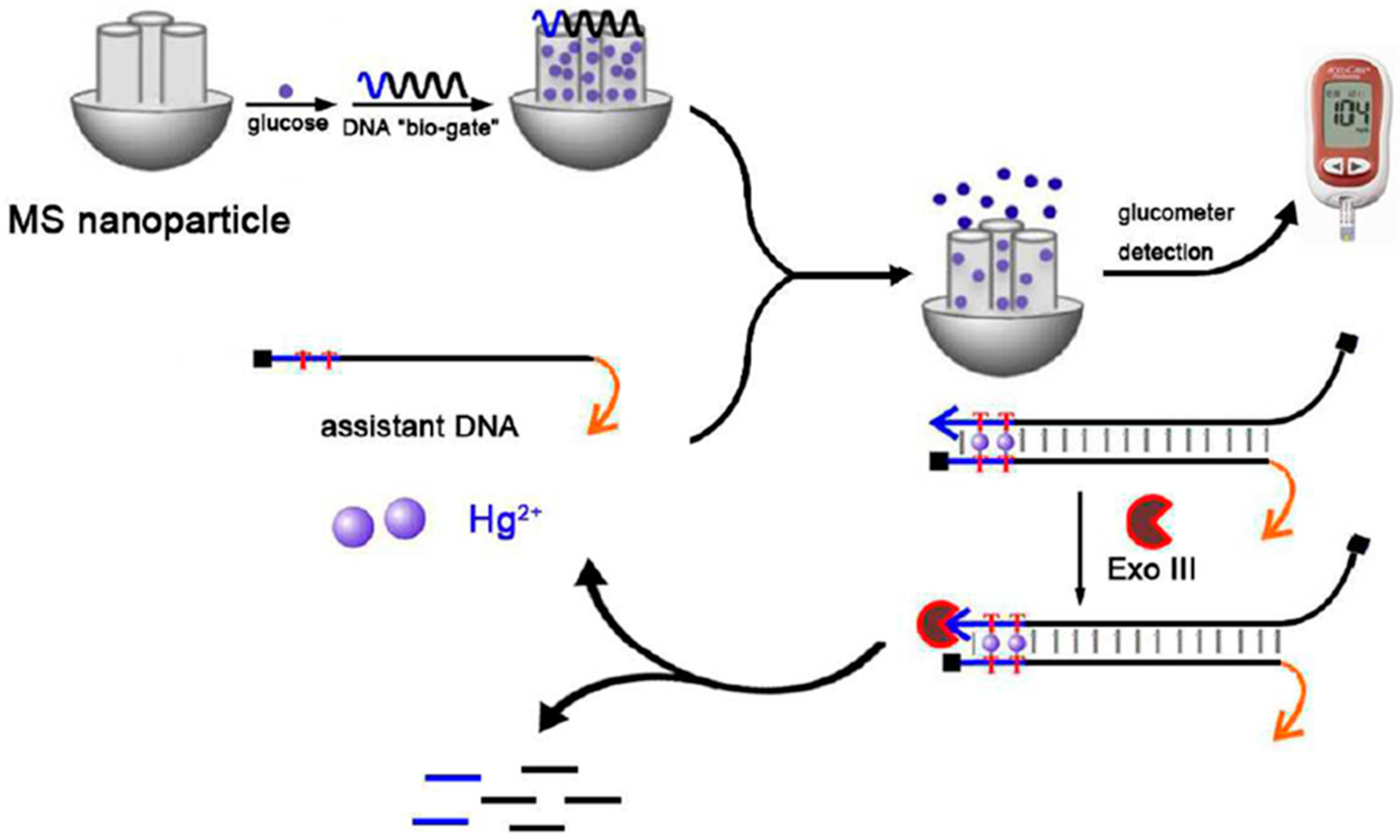
| SPCE | Target-responsive release of glucose from single-strand wrapping DNA sealed MSNs | MSNs/Molecular gate | Amperometry (glucose, glucometer) | Hg2+ | L.R. = 0.1–80 nM LOD = 0.1 nM | Spiked environmental water samples (tap water and lake water) | [44] | |
| PGE | Label-free MIP-DNA biosensor with electro-polymerized oPD in presence of DNA and 2,4-D onto modified electrode | MWCNTs/Electrode modifier | DPV ([Fe(CN)6]3−/4−) | 2,4-D | L.R. = 0.01–10 pM LOD = 4.0 fM | Spiked environmental water and soil samples | [56] | |
| SPCE | Catalytic cleavage of the DNA (GR-5) functionalized (Fe−P)n -MOF in the presence of Pb2+ and direct hybridization with a SPCE modified with an hairpin capture probe | (Fe−P)n-MOF/ Nanozyme | Chrono-amperometry (H2O2/TMB) | Pb2+ | L.R. = 0.05–200 nM LOD = 0.034 nM | Spiked soil samples | [33] | |
| AuE | DNAzyme sensor with amplified detection based on a target triggered nuclear acid cleavage of DNAzyme | rGO-TEPA/PdPt NPs/Fe-MOF/signal tag | Amperometry H2O2 | Pb2+ | L.R.: 0.005–1000 nM LOD: 2 pM | Sewage, spiked water | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors 2020, 20, 5125. https://doi.org/10.3390/s20185125
Campuzano S, Yáñez-Sedeño P, Pingarrón JM. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors. 2020; 20(18):5125. https://doi.org/10.3390/s20185125
Chicago/Turabian StyleCampuzano, Susana, Paloma Yáñez-Sedeño, and José M. Pingarrón. 2020. "Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring" Sensors 20, no. 18: 5125. https://doi.org/10.3390/s20185125
APA StyleCampuzano, S., Yáñez-Sedeño, P., & Pingarrón, J. M. (2020). Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors, 20(18), 5125. https://doi.org/10.3390/s20185125






