Optimization of Sensors to be Used in a Voltammetric Electronic Tongue Based on Clustering Metrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Sensor Array
2.3. Samples Preparation and Voltammetric Measurements
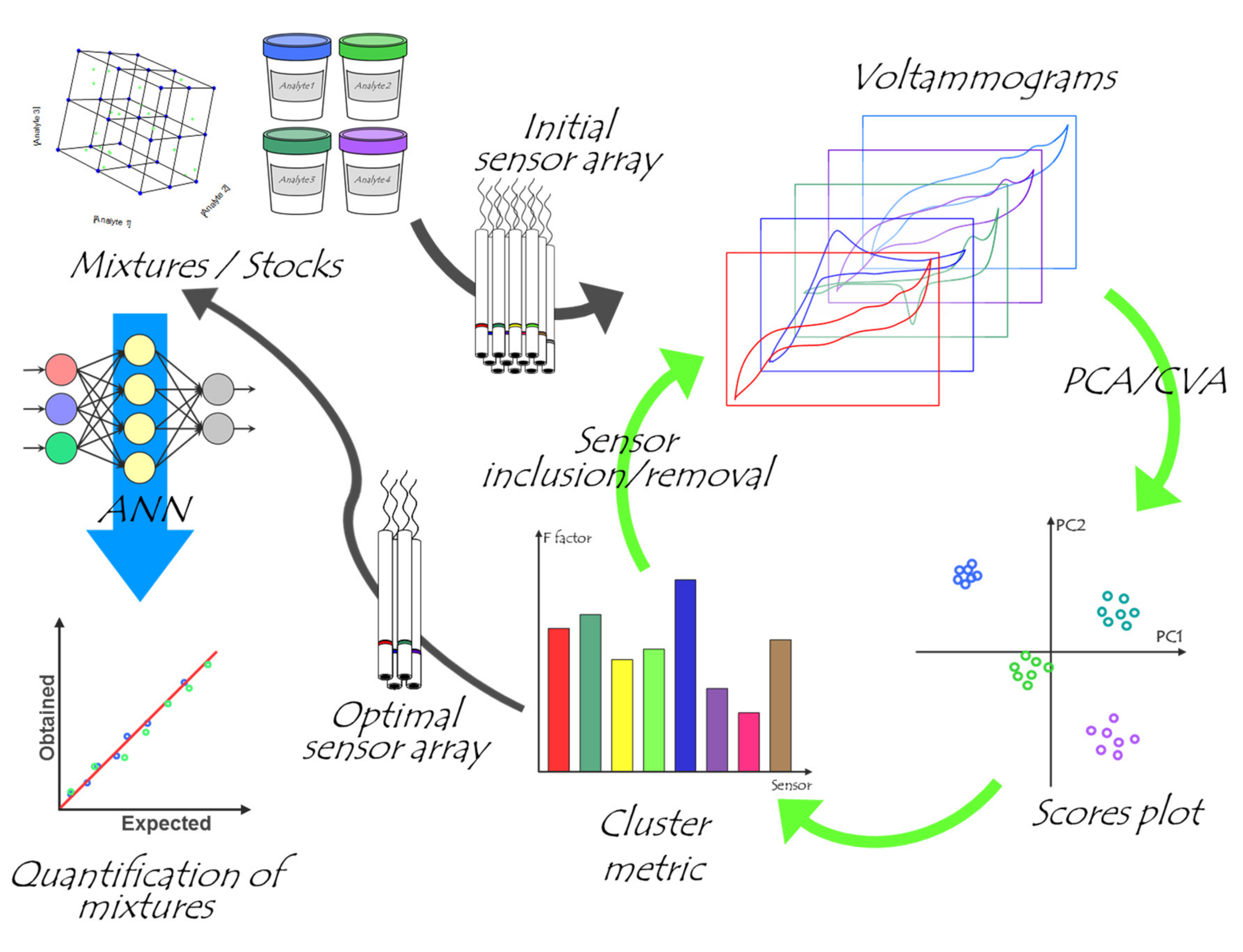
2.4. Chemometric Analysis
3. Results and Discussion
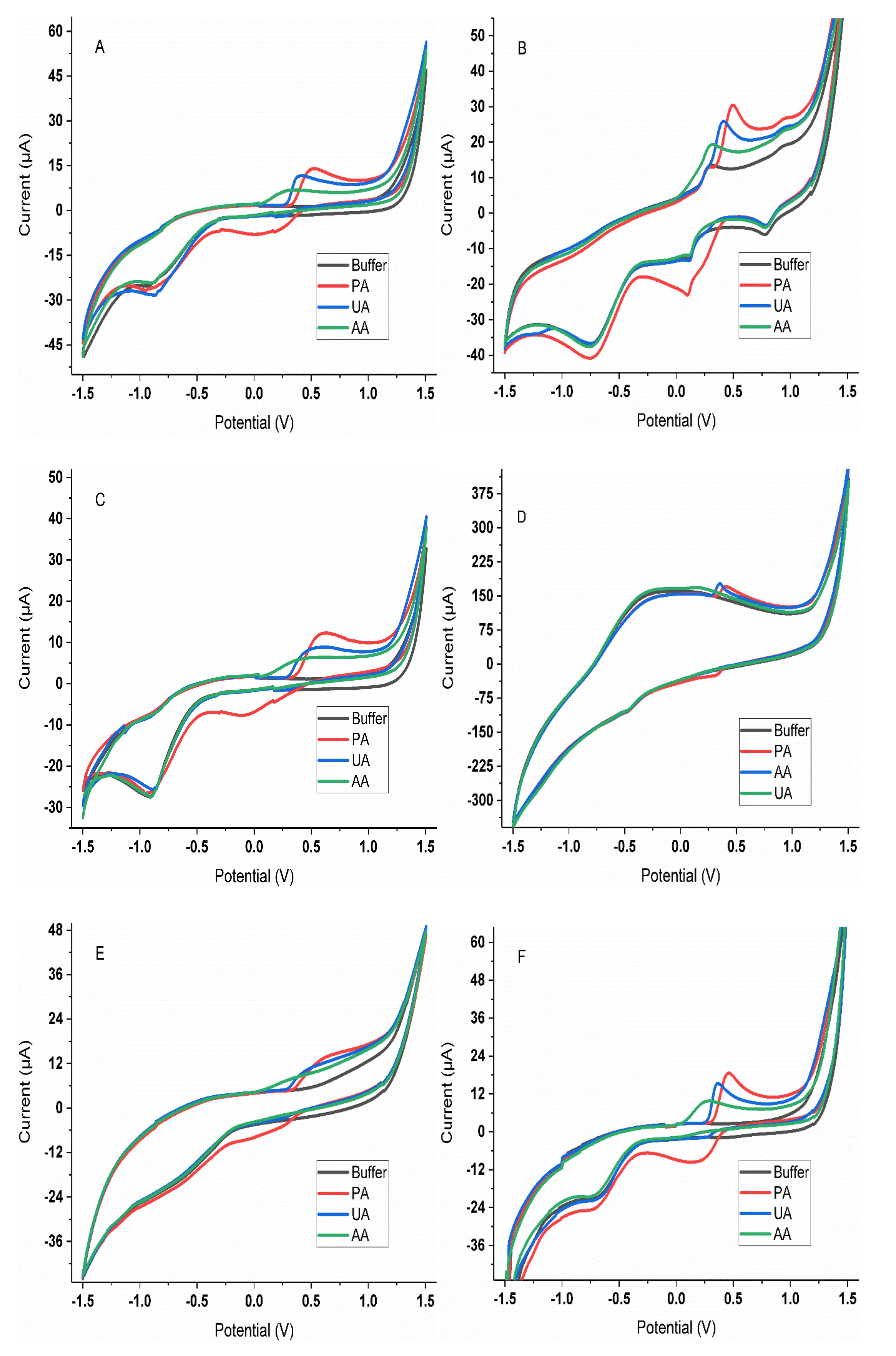
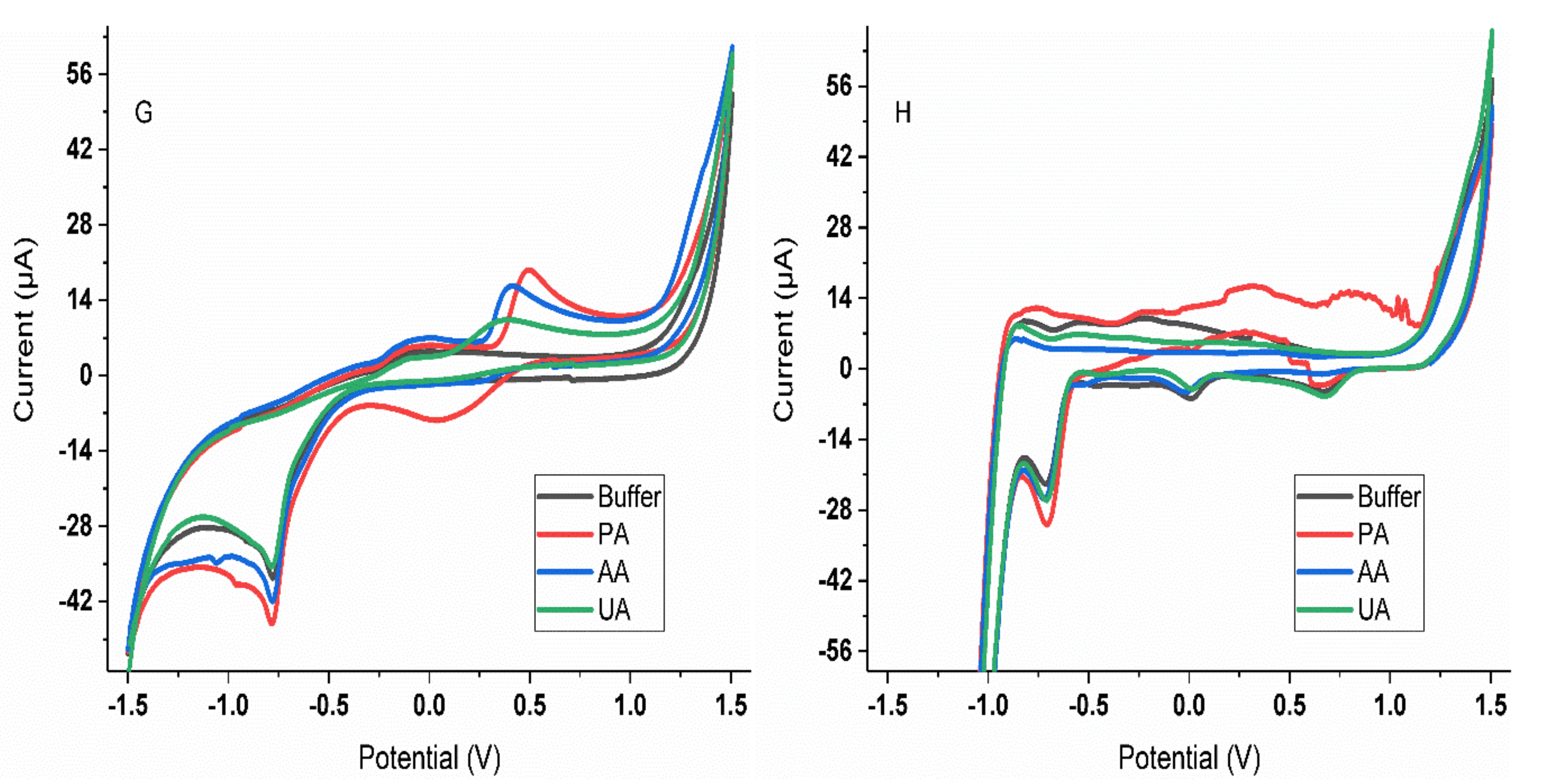
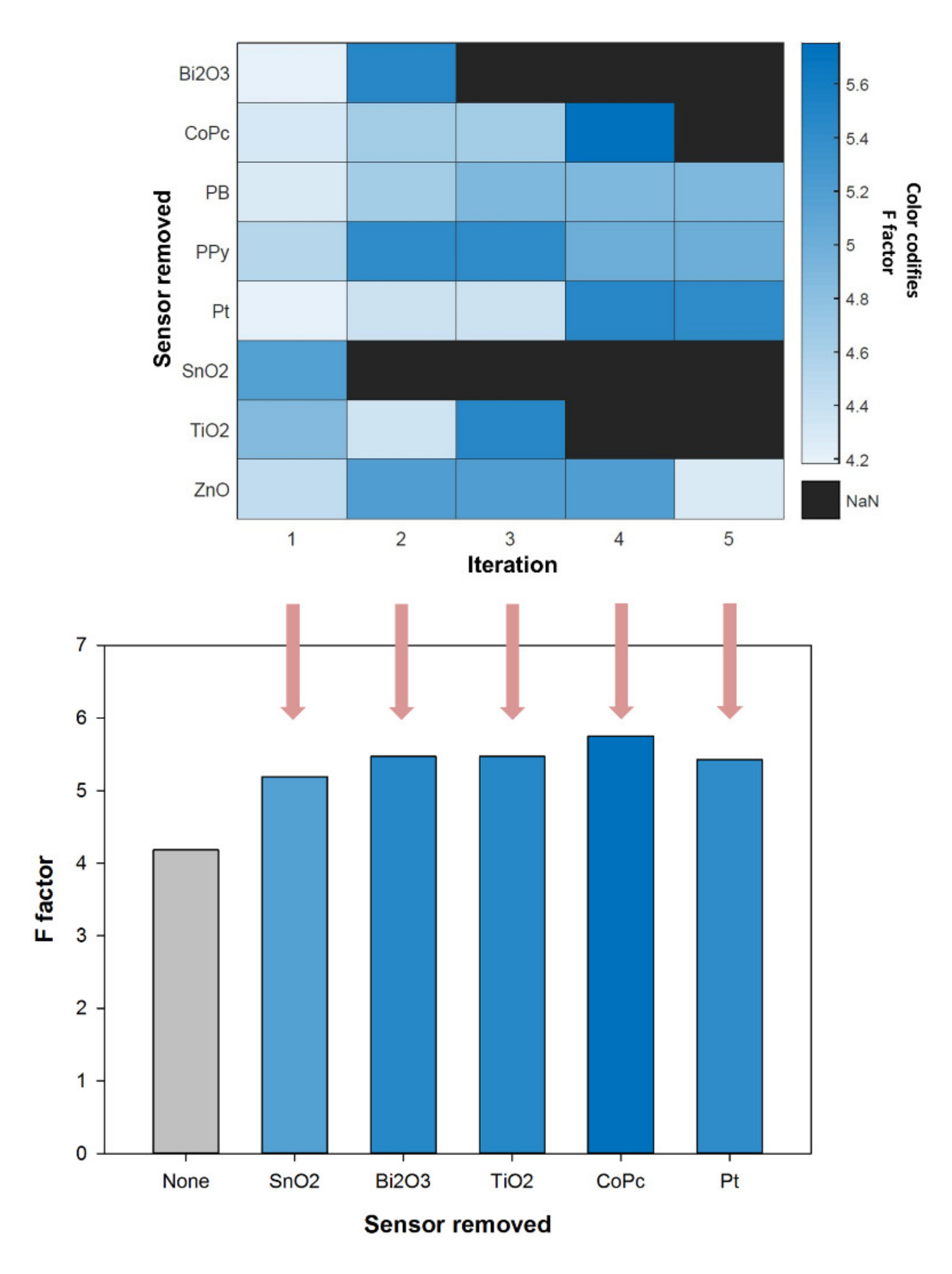
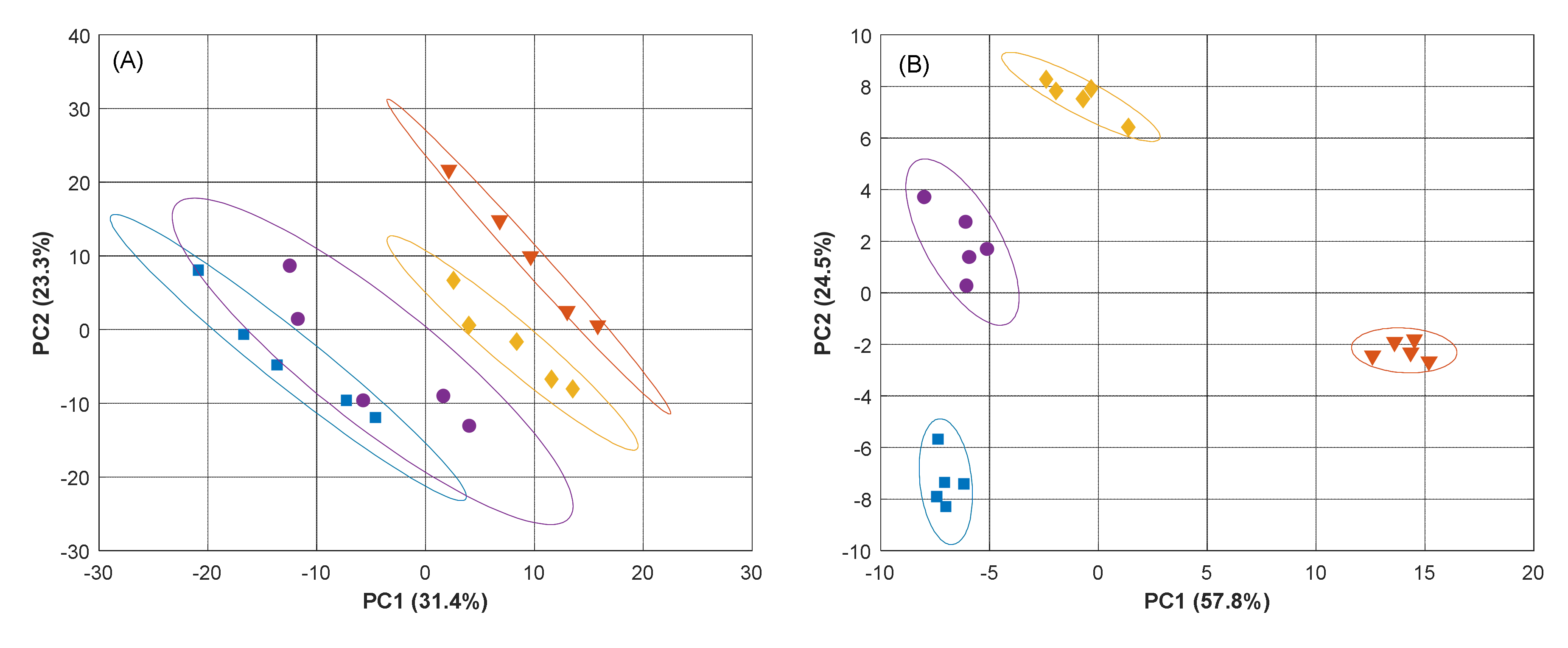
3.1. Selection of Sensors
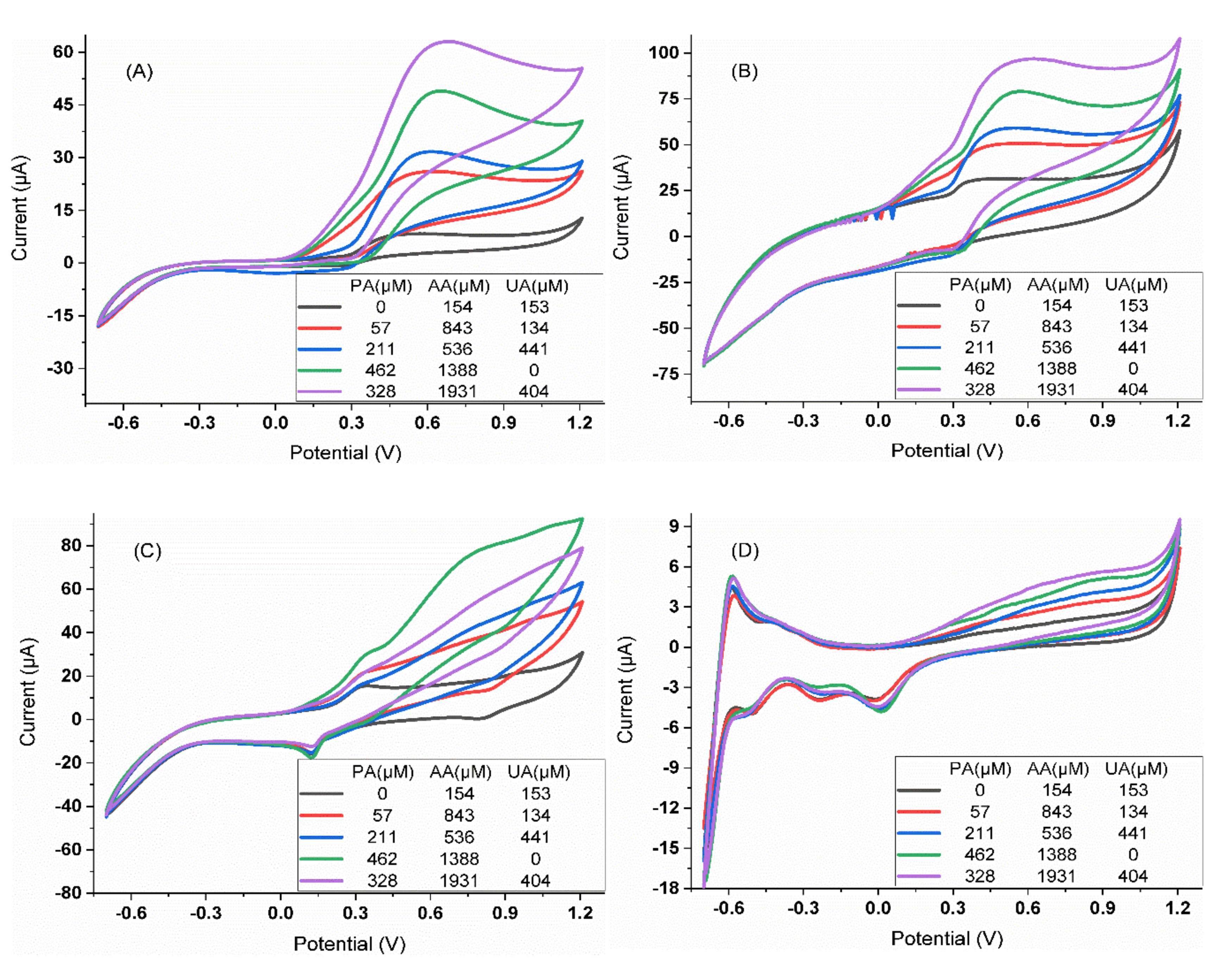
3.2. Characterization of the Analytical Response
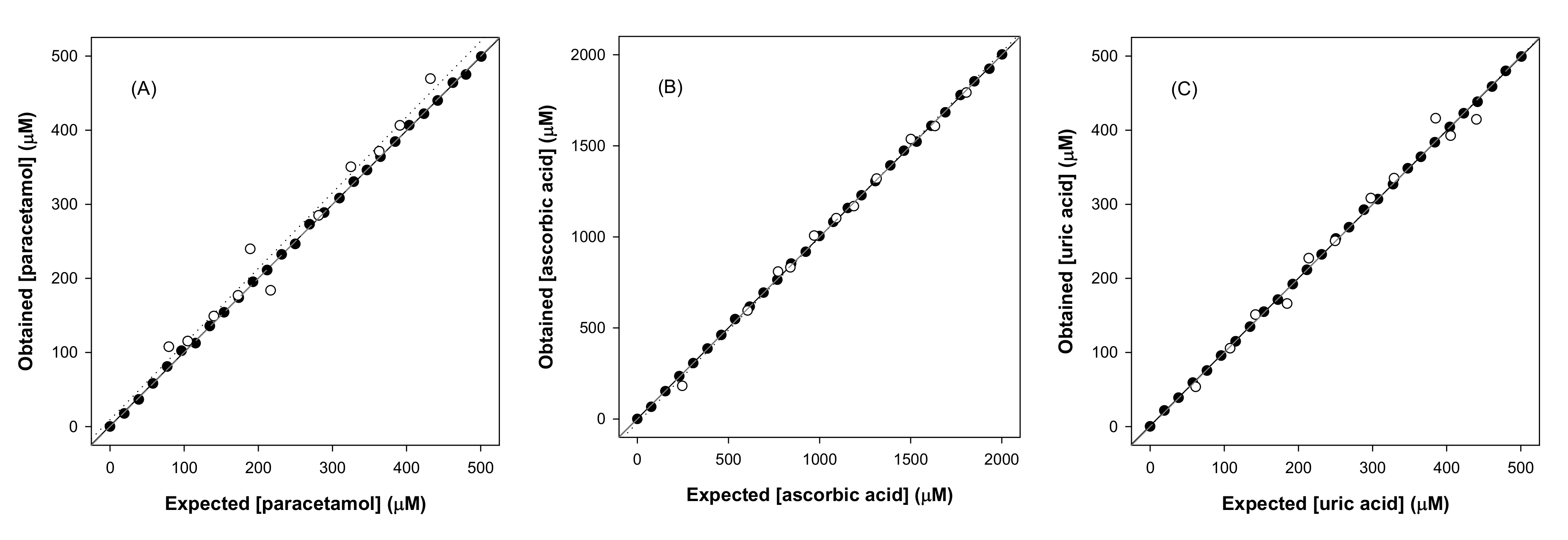
3.2.1. Calibration Curves
3.2.2. Stability Measurements
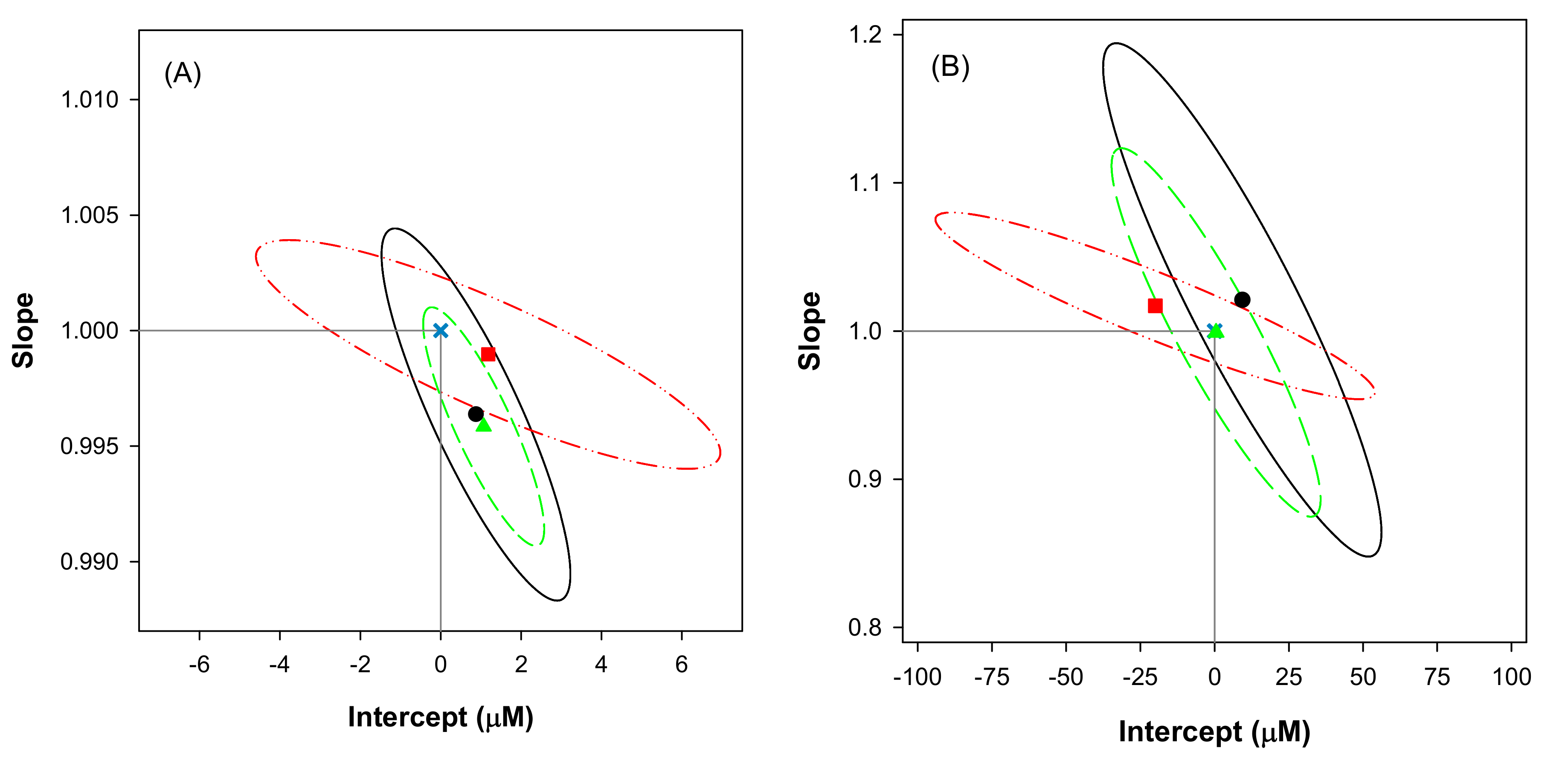
3.3. Quantitative Analysis of APIs Mixtures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Del Valle, M. Electronic Tongues Employing Electrochemical Sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Ciosek, P.; Wroblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.; Kirsanov, D.; del Valle, M. Avoiding nonsense in electronic taste sensing. TrAC Trend. Anal. Chem. 2019, 121, 115675. [Google Scholar] [CrossRef]
- Mimendia, A.; Legin, A.; Merkoçi, A.; del Valle, M. Use of Sequential Injection Analysis to construct a potentiometric electronic tongue: Application to the multidetermination of heavy metals. Sensor Actuat. B Chem. 2010, 146, 420–426. [Google Scholar] [CrossRef]
- Dias, L.G.; Veloso, A.C.A.; Sousa, M.E.B.C.; Estevinho, L.; Machado, A.A.S.C.; Peres, A.M. A novel approach for honey pollen profile assessment using an electronic tongue and chemometric tools. Anal. Chim. Acta 2015, 900, 36–45. [Google Scholar] [CrossRef]
- Cetó, X.; Apetrei, C.; del Valle, M.; Rodríguez-Méndez, M.L. Evaluation of red wines antioxidant capacity by means of a voltammetric e-tongue with an optimized sensor array. Electrochim. Acta 2014, 120, 180–186. [Google Scholar] [CrossRef]
- Cetó, X.; González-Calabuig, A.; Capdevila, J.; Puig-Pujol, A.; del Valle, M. Instrumental measurement of wine sensory descriptors using a voltammetric electronic tongue. Sens. Actuators B Chem. 2015, 207 Pt B, 1053–1059. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Shimizu, F.M.; Carr, O.; Oliveira, O.N. A Guiding Method to Select and Reduce the Number of Sensing Units in Electronic Tongues. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Huang, H.-Y.; Appel, L.J.; Choi, M.J.; Gelber, A.C.; Charleston, J.; Norkus, E.P.; Miller, E.R., III. The effects of vitamin C supplementation on serum concentrations of uric acid: Results of a randomized controlled trial. Arthritis Rheum. 2005, 52, 1843–1847. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller, E.R., III; Gelber, A.C. Effect of oral vitamin C supplementation on serum uric acid: A meta-analysis of randomized controlled trials. Arthrit. Care Res. 2011, 63, 1295–1306. [Google Scholar] [CrossRef]
- Alegret, S.; Alonso, J.; Bartrolí, J.; Cespedes, F.; Martínez Fàbregas, E.; del Valle, M. Amperometric biosensors based on bulk-modified epoxy graphite biocomposites. Sens. Mater. 1996, 8, 147–153. [Google Scholar]
- Ortiz-Aguayo, D.; Bonet-San-Emeterio, M.; del Valle, M. Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools. Sensors 2019, 19, 3286. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; García-Hernández, C.; de Saja, J.A.; Fernández-Escudero, J.A.; Barajas, E.; Medrano, G.; García-Cabezón, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L. The advantages of disposable screen-printed biosensors in a bioelectronic tongue for the analysis of grapes. LWT Food Sci. Technol. 2015, 62, 940–947. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Bertotti, M. Fabrication of disposable voltammetric electronic tongues by using Prussian Blue films electrodeposited onto CD-R gold surfaces and recognition of milk adulteration. Sensor Actuat. B Chem. 2009, 137, 266–273. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Sarma, M.; del Valle, M. Improved Sensing of Capsaicin with TiO2 Nanoparticles Modified Epoxy Graphite Electrode. Electroanalysis 2020, 32, 230–237. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Apetrei, C.; Rodriguez-Mendez, M.L.; de Saja, J.A. Voltammetric sensor array based on conducting polymer-modified electrodes for the discrimination of liquids. Electrochim. Acta 2004, 49, 4543–4551. [Google Scholar] [CrossRef]
- Tsakova, V.; Seeber, R. Conducting polymers in electrochemical sensing: Factors influencing the electroanalytical signal. Anal. Bioanal. Chem. 2016, 408, 7231–7241. [Google Scholar] [CrossRef]
- Domínguez, R.B.; Moreno-Barón, L.; Muñoz, R.; Gutiérrez, J.M. Voltammetric electronic tongue and support vector machines for identification of selected features in Mexican coffee. Sensors 2014, 14, 17770–17785. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; García-Hernandez, C.; Medina-Plaza, C.; García-Cabezón, C.; Saja, J.A.d. Multisensor systems based on phthalocyanines for monitoring the quality of grapes. J. Porphyr. Phthalocya. 2016, 20, 889–894. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; de Saja, J.A.; Rodriguez-Mendez, M.L. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens. Bioelectron. 2014, 57, 276–283. [Google Scholar] [CrossRef]
- Winquist, F.; Wide, P.; Lundström, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–31. [Google Scholar] [CrossRef]
- Gutés, A.; Calvo, D.; Céspedes, F.; del Valle, M. Automatic sequential injection analysis electronic tongue with integrated reference electrode for the determination of ascorbic acid, uric acid and paracetamol. Microchim. Acta 2007, 157, 1–6. [Google Scholar] [CrossRef]
- Cetó, X.; Pérez, S. Voltammetric electronic tongue for vinegar fingerprinting. Talanta 2020, 219, 121253. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, J.M.; del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst 2012, 137, 349–356. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; del Valle, M. Comparison of methods for the processing of voltammetric electronic tongues data. Microchim. Acta 2013, 180, 319–330. [Google Scholar] [CrossRef]
- The MathWorks Inc. Statistics and Machine Learning Toolbox Toolbox: User’s Guide; MathWorks: Natick, MA, USA, 2020. [Google Scholar]
- Beale, M.H.; Hagan, M.T.; Demuth, H.B. Neural Network Toolbox: User’s Guide; MathWorks: Natick, MA, USA, 2012. [Google Scholar]
- Ciosek, P.; Brzózka, Z.; Wróblewski, W. Classification of beverages using a reduced sensor array. Sensor Actuat. B Chem. 2004, 103, 76–83. [Google Scholar] [CrossRef]
- Kutyła-Olesiuk, A.; Zaborowski, M.; Prokaryn, P.; Ciosek, P. Monitoring of beer fermentation based on hybrid electronic tongue. Bioelectrochemistry 2012, 87, 104–113. [Google Scholar] [CrossRef]
- Sorvin, M.; Belyakova, S.; Stoikov, I.; Shamagsumova, R.; Evtugyn, G. Solid-Contact Potentiometric Sensors and Multisensors Based on Polyaniline and Thiacalixarene Receptors for the Analysis of Some Beverages and Alcoholic Drinks. Front. Chem. 2018, 6, 134. [Google Scholar] [CrossRef]
- Despagne, F.; Massart, D.L. Neural networks in multivariate calibration. Analyst 1998, 123, 157R–178R. [Google Scholar] [CrossRef]
- Cetó, X.; Voelcker, N.H.; Prieto-Simón, B. Bioelectronic tongues: New trends and applications in water and food analysis. Biosens. Bioelectron. 2016, 79, 608–626. [Google Scholar] [CrossRef]
- Cetó, X.; Gutés, A.; del Valle, M. Simple data preprocessing method for voltammetric electronic tongues using artificial neural networks. Acta Manilana 2013, 61, 39–49. [Google Scholar]
| Electrode modifier | ZnO | Prussian Blue | Polypyrrole | Metallic Pt | |
|---|---|---|---|---|---|
| Paracetamol | Equation | y = 0.0598x + 0.613 | y = 0.0629x + 10.2 | y = 0.0857x + 29.4 | y = 0.0027x + 1.08 |
| R2 | 0.9971 | 0.9993 | 0.9989 | 0.9589 | |
| LOD1 (μM) | 28.4 | 13.9 | 17.2 | 59.4 | |
| Ascorbic acid | Equation | y = 0.0152x + 0.989 | y = 0.0261x + 6.93 | y = 0.0304x + 21.0 | y = 0.0015x + 0.634 |
| R2 | 0.9977 | 0.9990 | 0.9987 | 0.9624 | |
| LOD1 (μM) | 59.3 | 68.5 | 77.5 | 417 | |
| Uric acid | Equation | y = 0.0452x + 1.12 | y = 0.0499x + 10.8 | y = 0.0668x + 31.0 | y = 0.0028x + 1.02 |
| R2 | 0.9988 | 0.9983 | 0.9995 | 0.9967 | |
| LOD1 (μM) | 17.9 | 23.5 | 9.35 | 28.3 |
| Compound | Slope | Intercept (μM) | R2 | RMSE1 (μM) | Total NRMSE1 | Sensor Array | Ref. |
|---|---|---|---|---|---|---|---|
| training subset (n = 33) | Bare GEC plus metallic Pt and Au electrodes | [23] | |||||
| Paracetamol | 0.942 ± 0.031 | 32 ± 21 | 0.968 | 2 | 2 | ||
| Ascorbic acid | 0.933 ± 0.040 | 36 ± 25 | 0.947 | 2 | |||
| Uric acid | 0.873 ± 0.046 | 58 ± 25 | 0.923 | 2 | |||
| testing subset (n = 15) | |||||||
| Paracetamol | 0.895 ± 0.105 | 82 ± 71 | 0.848 | 2 | 2 | ||
| Ascorbic acid | 0.919 ± 0.081 | 65 ± 41 | 0.908 | 2 | |||
| Uric acid | 0.871 ± 0.138 | −8 ± 86 | 0.753 | 2 | |||
| training subset (n = 33) | Bare GEC plus metallic Pt and Au electrodes | [34] | |||||
| Paracetamol | 0.981 ± 0.032 | 13 ± 24 | 0.992 | 29 | 0.0257 | ||
| Ascorbic acid | 0.990 ± 0.031 | 6 ± 17 | 0.993 | 25 | |||
| Uric acid | 0.981 ± 0.027 | 9 ± 16 | 0.994 | 23 | |||
| testing subset (n = 15) | |||||||
| Paracetamol | 0.990 ± 0.143 | −2 ± 80 | 0.945 | 97 | 0.101 | ||
| Ascorbic acid | 1.009 ± 0.136 | −28 ± 78 | 0.952 | 66 | |||
| Uric acid | 0.992 ± 0.208 | 36 ± 125 | 0.891 | 73 | |||
| training subset (n = 27) | SPCEs modified with CoPc, PB, graphite and CuO | [12] | |||||
| Paracetamol | 1.000 ± 0.082 | 0 ± 25 | 0.962 | 29 | 1.00 | ||
| Ascorbic acid | 1.000 ± 0.089 | 0 ± 25 | 0.955 | 31 | |||
| Uric acid | 1.000 ± 0.104 | 0 ± 31 | 0.940 | 36 | |||
| testing subset (n = 12) | |||||||
| Paracetamol | 1.021 ± 0.219 | −13 ± 28 | 0.915 | 32 | 1.03 | ||
| Ascorbic acid | 1.073 ± 0.422 | −3 ± 54 | 0.762 | 71 | |||
| Uric acid | 1.044 ± 0.334 | −32 ± 36 | 0.829 | 44 | |||
| training subset (n = 27) | GECs modified with ZnO, PB, and PPy plus Pt metallic electrode | This work | |||||
| Paracetamol | 0.996 ± 0.006 | 0.9 ± 1.9 | 0.9998 | 2.43 | 0.00378 | ||
| Ascorbic acid | 0.999 ± 0.004 | 1.1 ± 4.6 | 0.9999 | 5.86 | |||
| Uric acid | 0.996 ± 0.004 | 1.1 ± 1.2 | 0.9999 | 1.64 | |||
| testing subset (n = 11) | |||||||
| Paracetamol | 1.021 ± 0.134 | 9 ± 36 | 0.971 | 26.4 | 0.0368 | ||
| Ascorbic acid | 1.017 ± 0.049 | −20 ± 57 | 0.996 | 31.2 | |||
| Uric acid | 0.999 ± 0.096 | 1 ± 27 | 0.984 | 16.2 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, M.; Romero, N.; Cetó, X.; Valle, M.d. Optimization of Sensors to be Used in a Voltammetric Electronic Tongue Based on Clustering Metrics. Sensors 2020, 20, 4798. https://doi.org/10.3390/s20174798
Sarma M, Romero N, Cetó X, Valle Md. Optimization of Sensors to be Used in a Voltammetric Electronic Tongue Based on Clustering Metrics. Sensors. 2020; 20(17):4798. https://doi.org/10.3390/s20174798
Chicago/Turabian StyleSarma, Munmi, Noelia Romero, Xavier Cetó, and Manel del Valle. 2020. "Optimization of Sensors to be Used in a Voltammetric Electronic Tongue Based on Clustering Metrics" Sensors 20, no. 17: 4798. https://doi.org/10.3390/s20174798
APA StyleSarma, M., Romero, N., Cetó, X., & Valle, M. d. (2020). Optimization of Sensors to be Used in a Voltammetric Electronic Tongue Based on Clustering Metrics. Sensors, 20(17), 4798. https://doi.org/10.3390/s20174798







