Application of Linear Gradient Magnetic Field in Arterial Profile Scanning Imaging
Abstract
1. Introduction
2. Methods and Models
2.1. Basic Electromagnetic Theory
2.1.1. Magnetoelectric Effects of Blood Flow
2.1.2. Reciprocity Theorem Integral Equation
2.2. Combined Coil for Arterial Profile Imaging
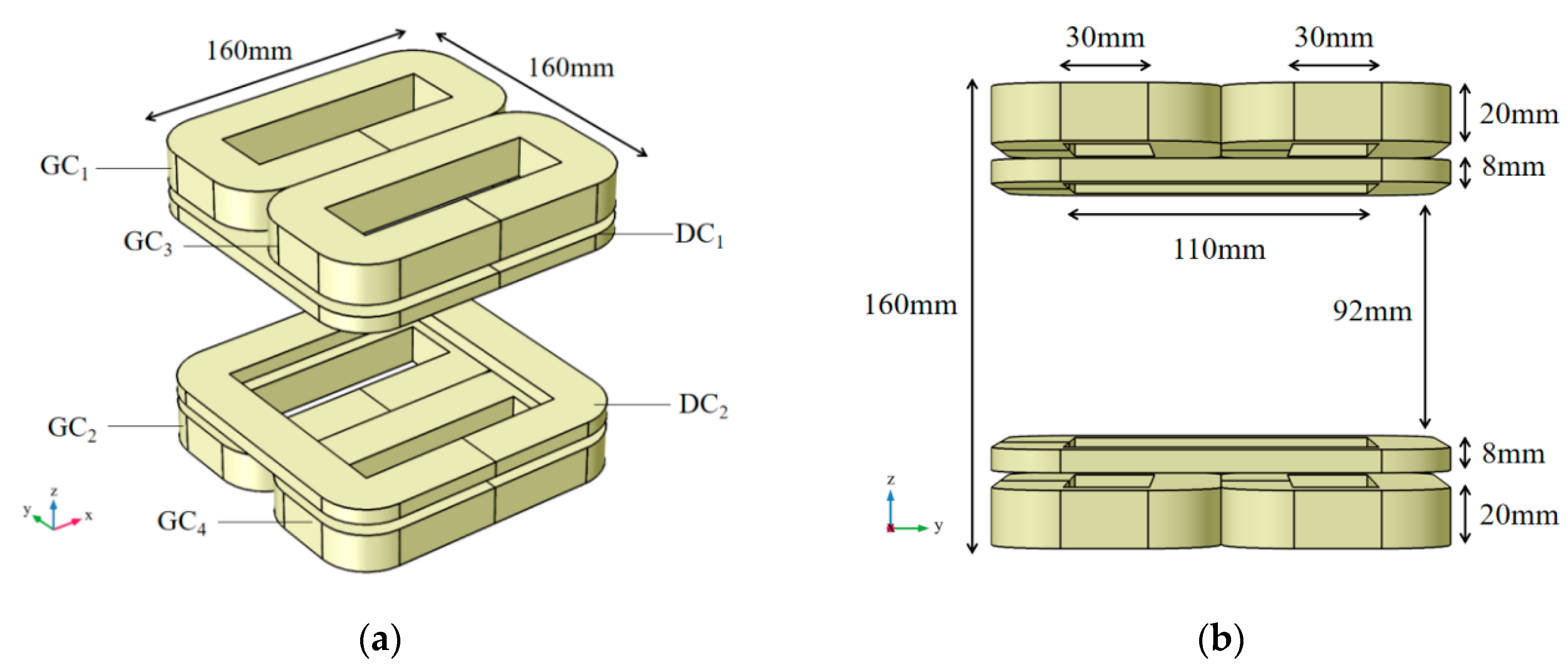
2.2.1. Combined Coil Structure
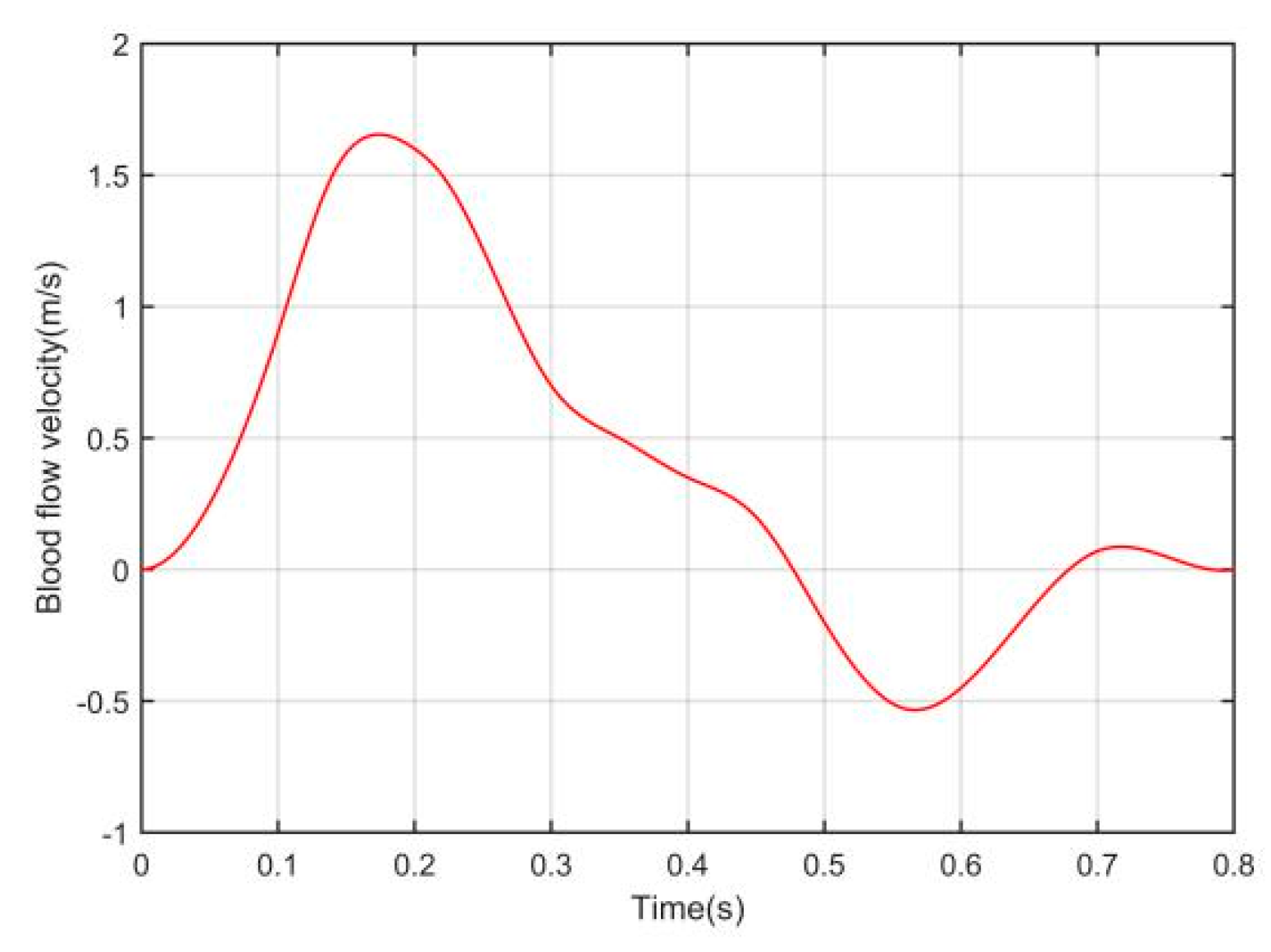
2.2.2. Time–Space Relationship
2.3. Relationship between Surface Potential Signal and Arterial Radius
2.4. Principle of Arterial Profile Scanning Imaging
3. Results
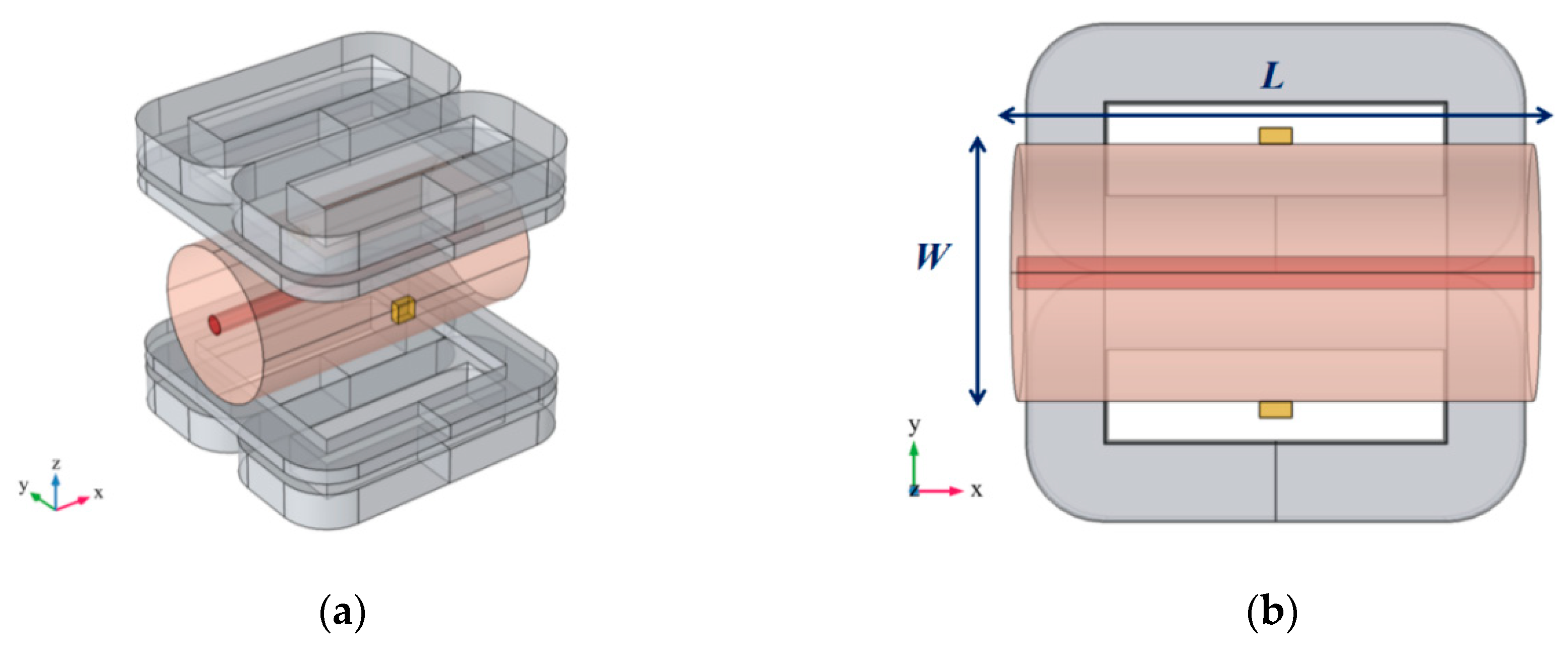
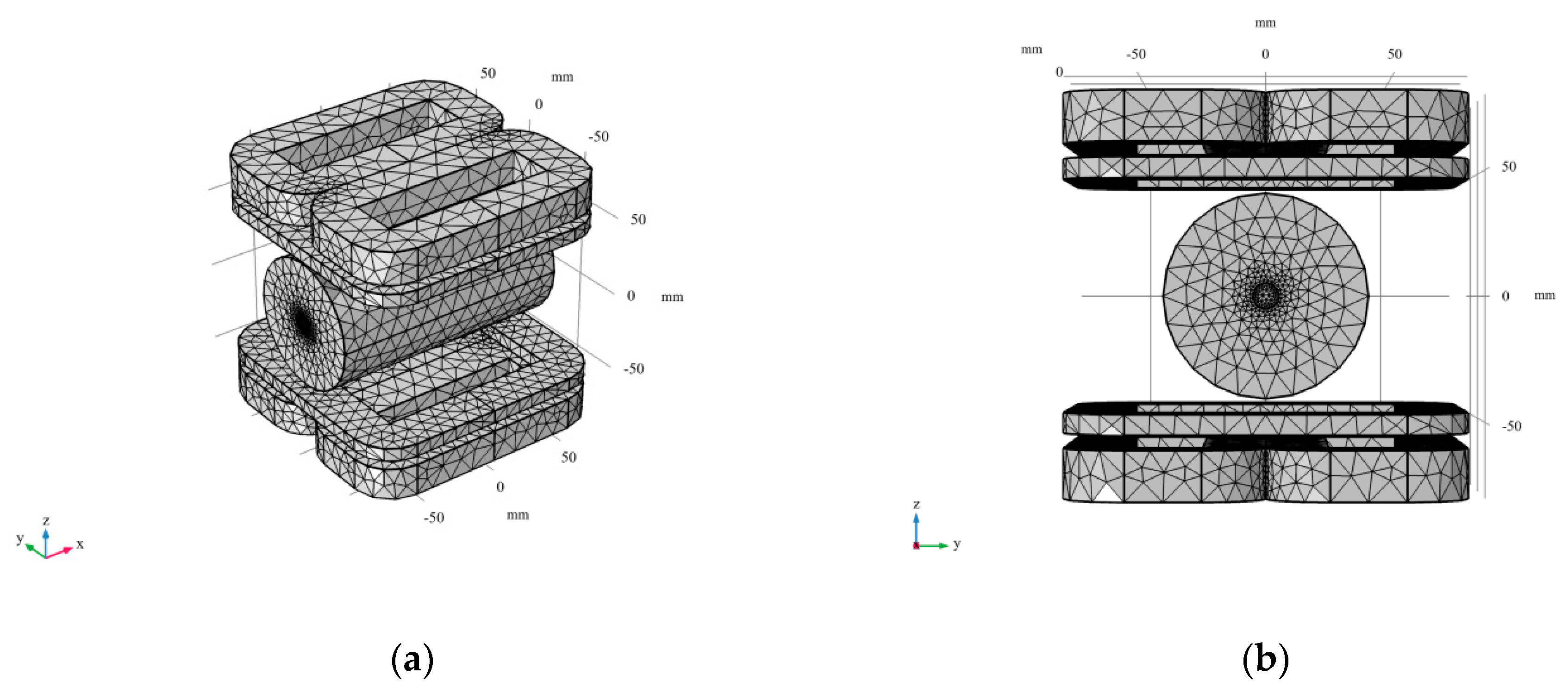
3.1. Finite Element Modeling
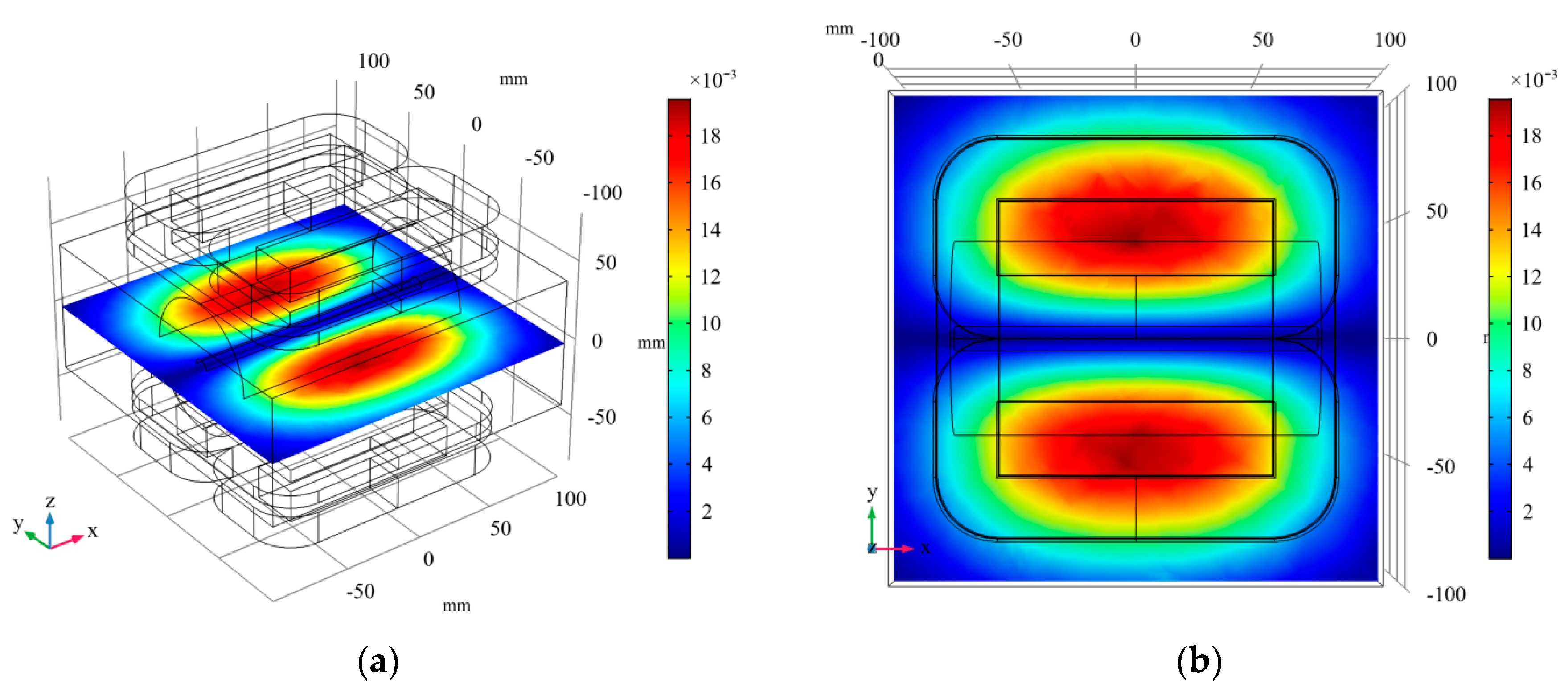
3.2. Magnetic Field Analysis
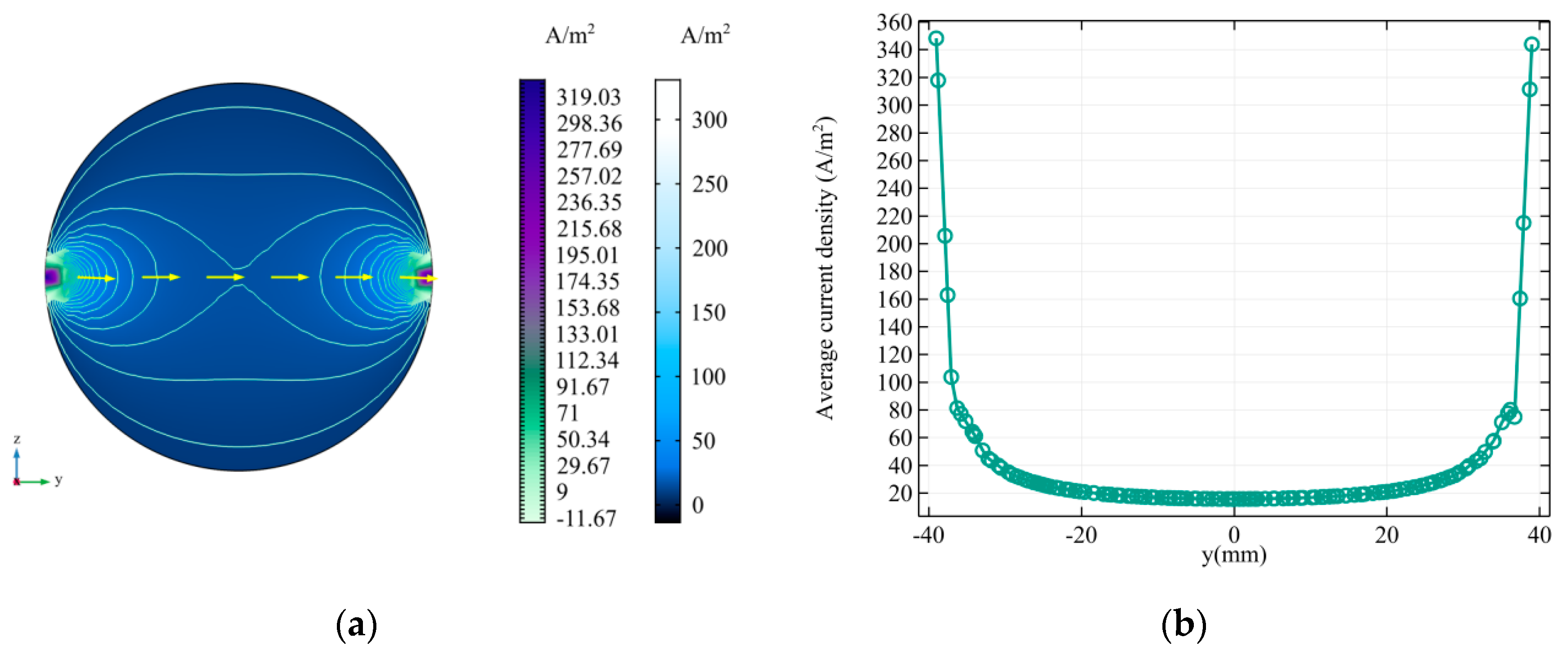
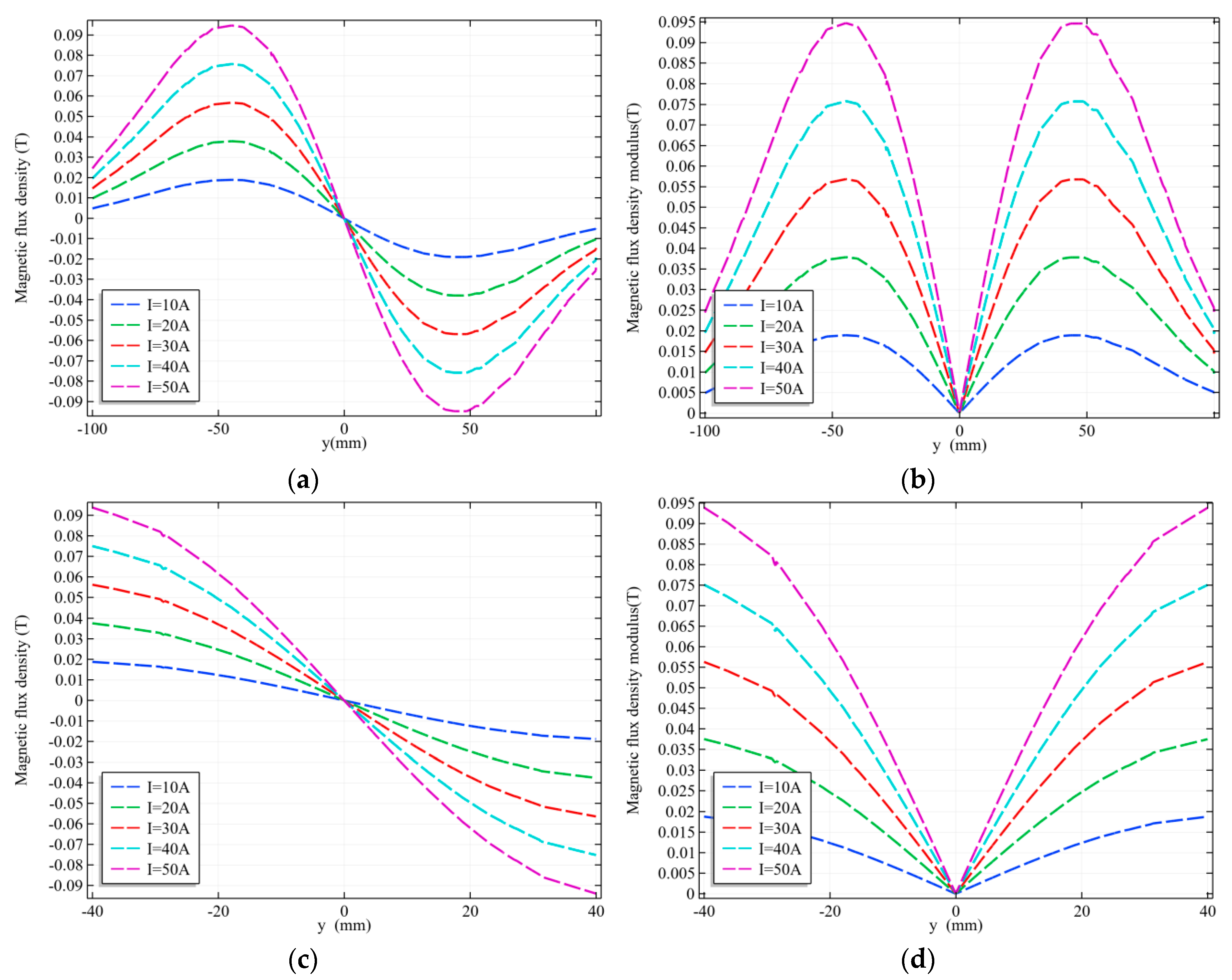
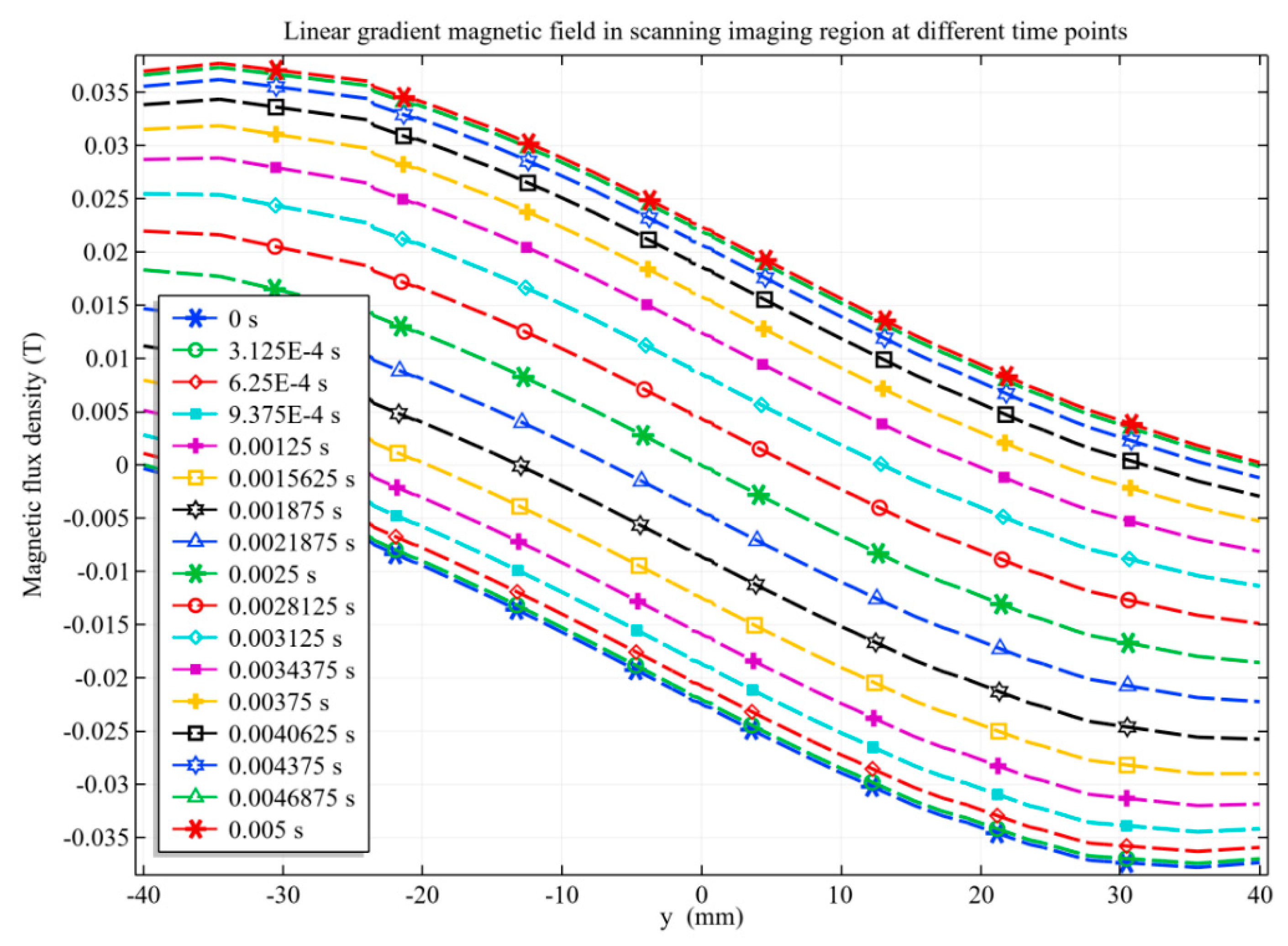
3.2.1. Linear Gradient Magnetic Field
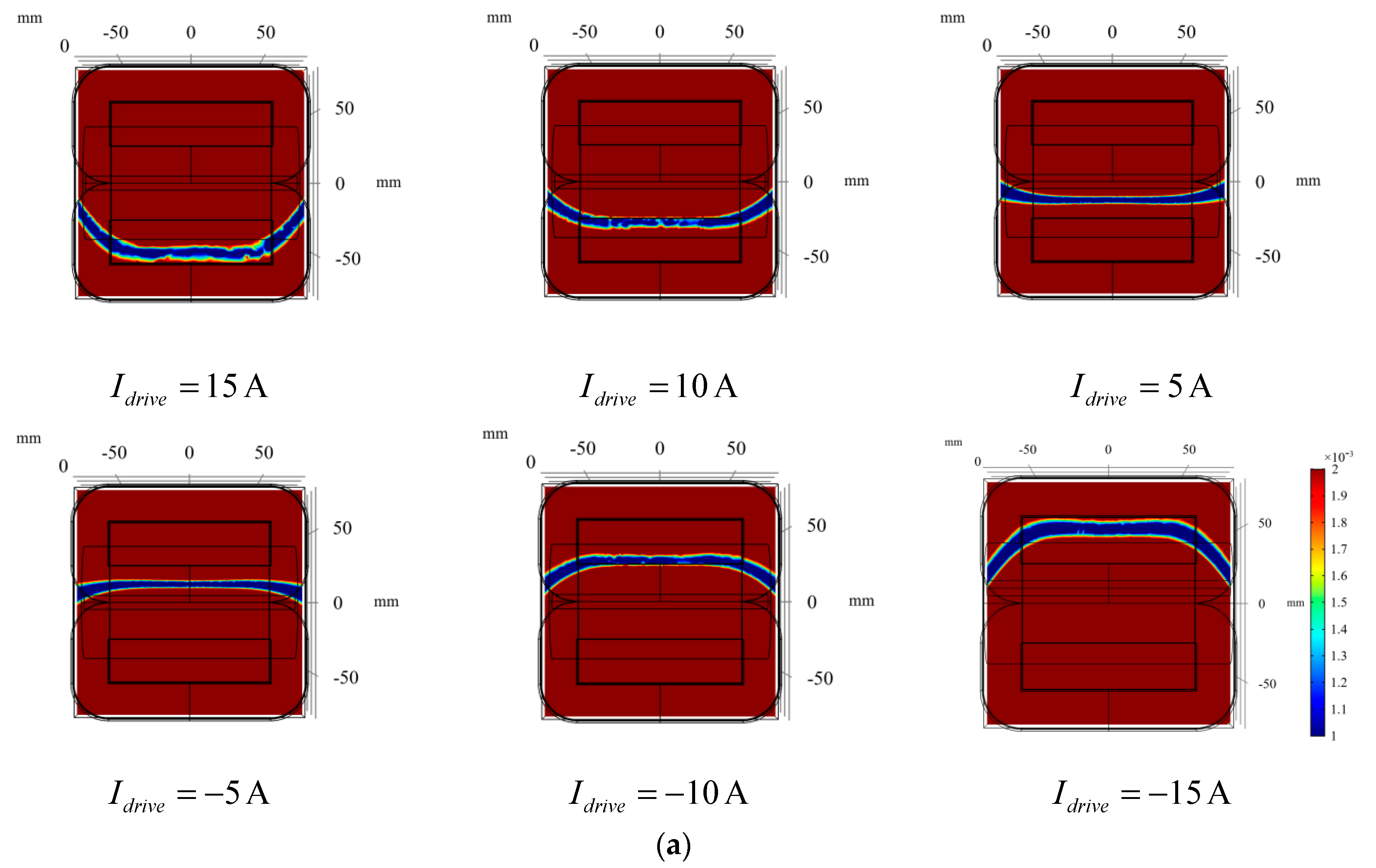
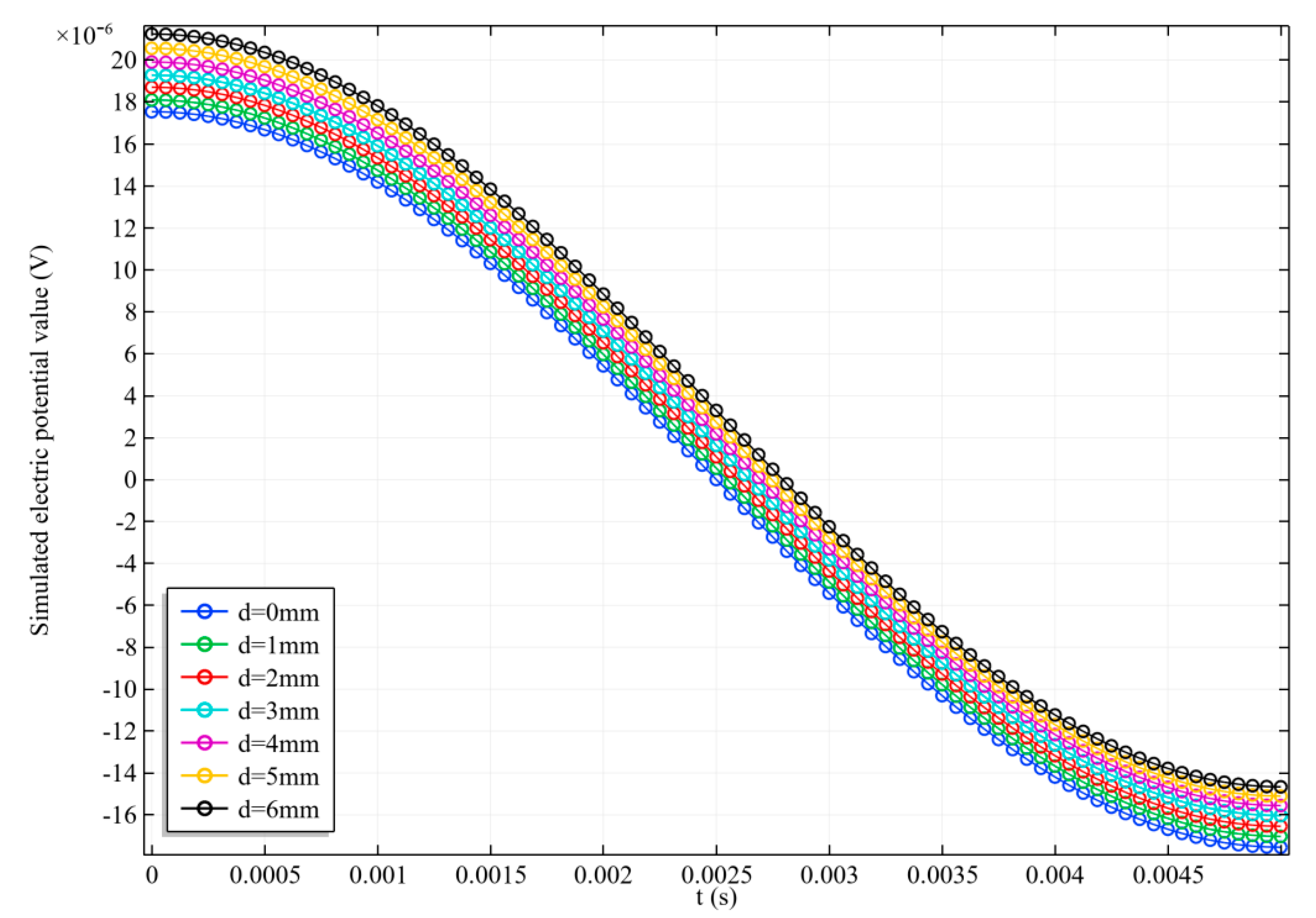
3.2.2. Zero Magnetic Field Scanning
3.3. Scanning Imaging Results
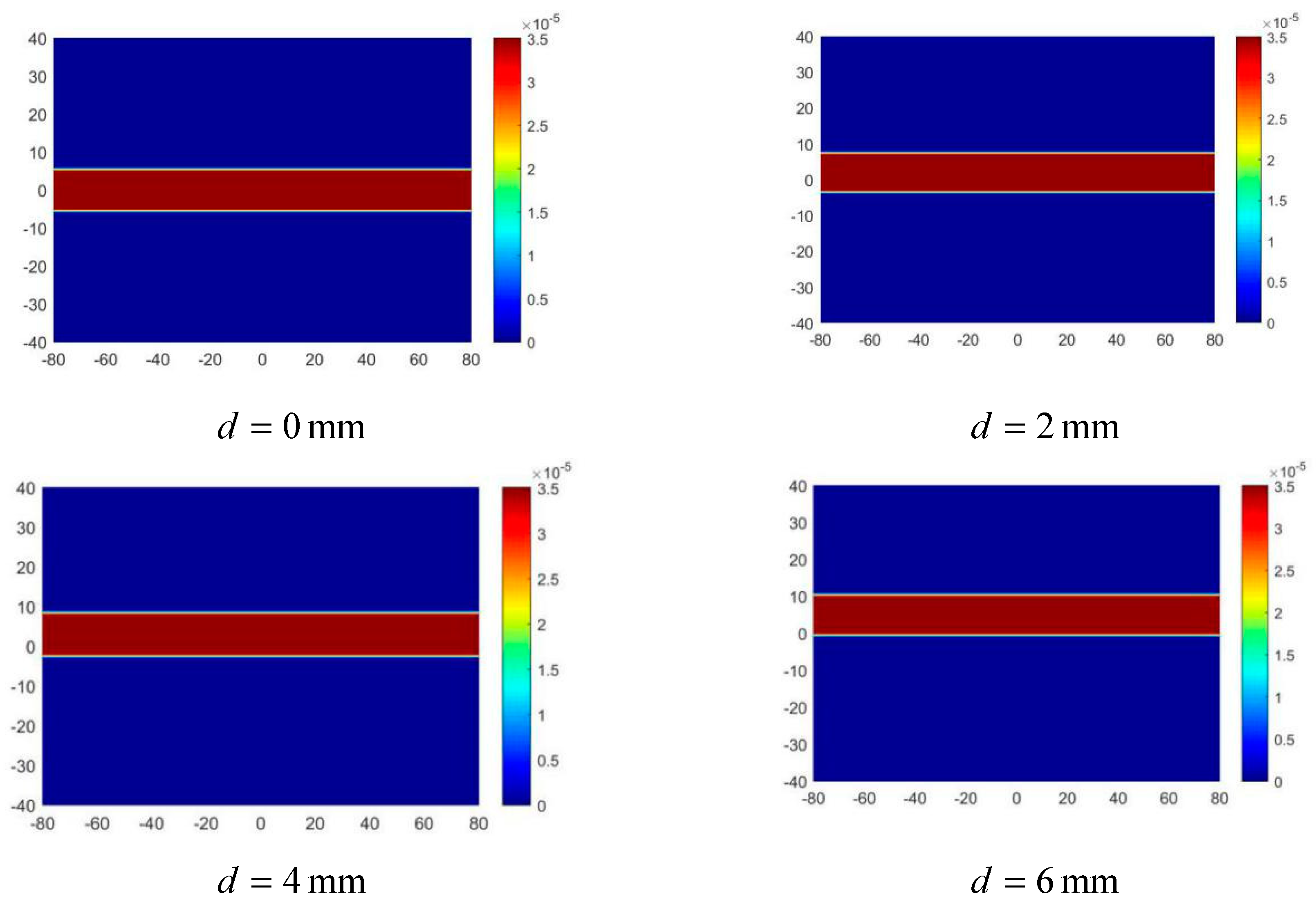
3.3.1. Arterial Position Imaging
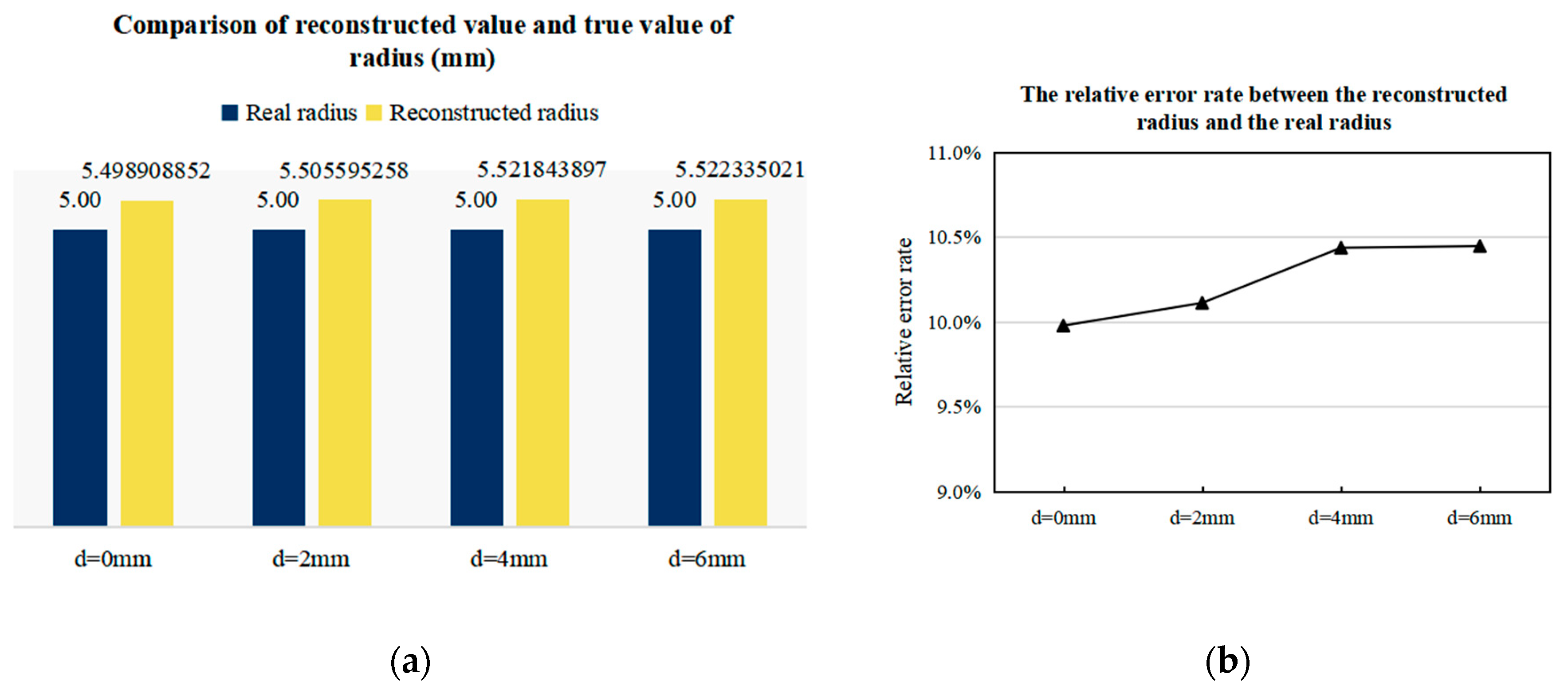
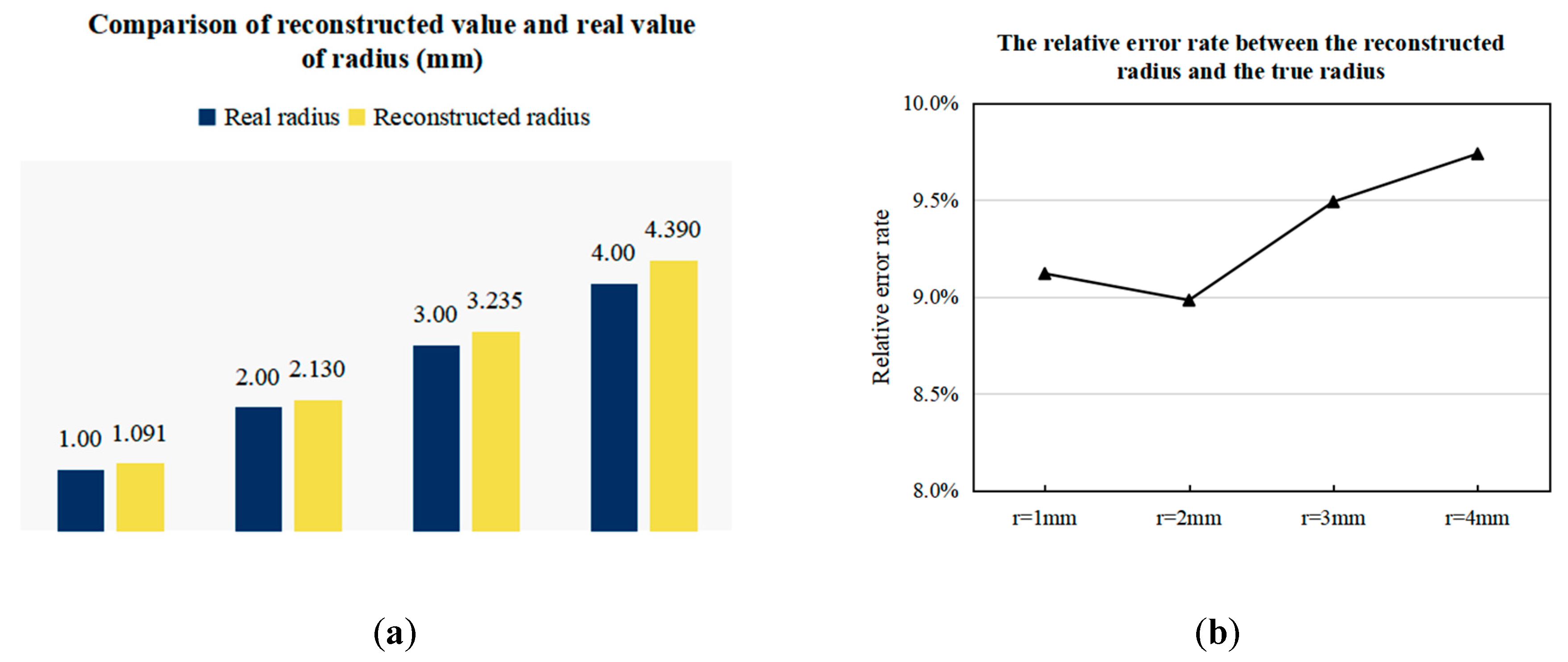
3.3.2. Imaging Results of Arterial Profile with Different Radii
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 22 June 2020).
- Anxionnat, R.; Bracard, S.; Ducrocq, X.; Trousset, Y.; Launay, L.; Kerrien, E.; Braun, M.; Vaillant, R.; Scomazzoni, F.; Lebedinsky, A.; et al. Intracranial Aneurysms: Clinical Value of 3D Digital Subtraction Angiography in the Therapeutic Decision and Endovascular Treatment1. Radiology 2001, 218, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Imamura, H.; Matsuyama, Y.; Kitamura, H.; Miwa, S.; Kobayashi, A.; Miyagawa, S.; Kawasaki, S. Value of Lipiodol Computed Tomography and Digital Subtraction Angiography in the Era of Helical Biphasic Computed Tomography as Preoperative Assessment of Hepatocellular Carcinoma. Ann. Surg. 2001, 234, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Choo, G.H.; Ng, K.H. Coronary CT angiography: Current status and continuing challenges. Br. J. Radiol. 2012, 85, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Husmann, L.; Valenta, I.; Gaemperli, O.; Adda, O.; Treyer, V.; Wyss, C.A.; Veit-Haibach, P.; Tatsugami, F.; von Schulthess, G.K.; Kaufmann, P.A. Feasibility of low-dose coronary CT angiography: First experience with prospective ECG-gating. Eur. Heart J. 2007, 29, 191–197. [Google Scholar] [CrossRef]
- Uchino, A.; Kato, A.; Takase, Y.; Kudo, S. Middle cerebral artery variations detected by magnetic resonance angiography. Eur. Radiol. 2000, 10, 560–563. [Google Scholar] [CrossRef]
- Hundley, W.G.; Clarke, G.D.; Landau, C.; Lange, R.A.; Willard, J.E.; Hillis, L.D.; Peshock, R.M. Noninvasive determination of infarct artery patency by cine magnetic resonance angiography. Circulation 1995, 91, 1347. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.J.; Edwards, W.S.; Mack, L.A.; Cyr, D.R.; Kim, Y. Three-dimensional ultrasonic angiography using power-mode Doppler. Ultrasound Med. Biol. 1996, 22, 277–286. [Google Scholar] [CrossRef]
- Nagai, S.; Abe, S.; Sato, T.; Hozawa, K.; Yuki, K.; Hanashima, K.; Tomoike, H. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am. J. Cardiol. 1999, 83, 180–186. [Google Scholar] [CrossRef]
- Wang, H.; Xu, G.; Zhang, S.; Geng, D.; Yang, Q.; Yan, W. Implementation of Generalized Back Projection Algorithm in 3-D EIT. IEEE Trans. Magn. 2011, 47, 1466–1469. [Google Scholar] [CrossRef]
- Griffiths, H.; Stewart, W.R.; Gough, W. Magnetic Induction Tomography: A Measuring System for Biological Tissues. Ann. N. Y. Acad. Sci. 1999, 873, 335–345. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X. Application of particle filtering algorithm in image reconstruction of EMT. Meas. Sci. Technol. 2015, 26, 7. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; He, B. Imaging Electrical Impedance From Acoustic Measurements by Means of Magnetoacoustic Tomography With Magnetic Induction (MAT-MI). IEEE Trans. Biomed. Eng. 2007, 54, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, G.; Xia, H. Magneto-Acousto-Electrical Tomography with Magnetic Induction for Conductivity Reconstruction. IEEE Trans. Biomed. Eng. 2015, 62, 2114–2124. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Xia, H.; Xia, Z.; Yang, Y. Conductivity Reconstruction and Numerical Simulation for Magnetically Mediated Thermoacoustic Imaging. IEEE Trans. Magn. 2017, 53, 1–4. [Google Scholar] [CrossRef]
- Zanet, D.D.; Battiston, M.; Lombardi, E.; Specogna, R.; Trevisan, F.; Marco, L.D.; Affanni, A.; Mazzucato, M. Impedance biosensor for real-time monitoring and prediction of thrombotic individual profile in flowing blood. PLoS ONE 2017, 12, e0184941. [Google Scholar] [CrossRef] [PubMed]
- Affanni, A.; Chiorboli, G.; Codecasa, L.; Cozzi, M.; Marco, L.D.; Mazzucato, M.; Morandi, C.; Specogna, R.; Tartagni, M.; Trevisan, F. A novel inversion technique for imaging thrombus volume in microchannels fusing optical and impedance data. IEEE Trans. Magn. 2014, 50, 1021–1024. [Google Scholar] [CrossRef]
- Maythem, A. Development of an Electromagnetic Induction Method for NonInvasive Blood Flow Measurement. Ph.D. Thesis, University of Huddersfield, Queensgate, Huddersfield, UK, 2016. [Google Scholar]
- Andrzej, P.; Michal, P.; Tomasz, W.; Stefanczyk, L.; Strzelecki, M. Computational Fluid Dynamics as an Engineering Tool for the Reconstruction of Hemodynamics after Carotid Artery Stenosis Operation: A Case Study. Medicina 2018, 54, 42. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Liu, G.; Wang, W.; Xu, B. Numerical model and finite element simulation of arterial blood flow profile reconstruction in a uniform magnetic field. J. Phys. D Appl. Phys. 2020, 53, 11. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.-J.; Xu, B.; Duo, Y.-H. A Blood Flow Volume Linear Inversion Model Based on Electromagnetic Sensor for Predicting the Rate of Arterial Stenosis. Sensors 2019, 19, 3006. [Google Scholar] [CrossRef]
- Top, C.B.; Güngör, A.; Ilbey, S.; Guven, H.E. Trajectory Analysis for Field Free Line Magnetic Particle Imaging. Med. Phys. 2019, 46, 1592–1607. [Google Scholar] [CrossRef]
- Carson, J.R. A generalization of the reciprocal theorem. Bell Syst. Tech. J. 1924, 3, 393–399. [Google Scholar] [CrossRef]
- Rau, U. Reciprocity relation between photovoltaic quantum efficiency and electroluminescent emission of solar cells. Phys. Rev. B Condens. Matter 2007, 76, 85303. [Google Scholar] [CrossRef]
- Bevir, M.K. The theory of induced voltage electromagnetic flowmeters. J. Fluid Mech. 2006, 43, 577–590. [Google Scholar] [CrossRef]
- Jozwik, K.; Obidowski, D. Numerical simulations of the blood flow through vertebral arteries. J. Biomech. 2010, 43, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Viedma, A.; Jiménez-Ortiz, C.; Marco, V. Extended Willis circle model to explain clinical observations in periorbital arterial flow. J. Biomech. 1997, 30, 265. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-Ameer, S.S.; Al-Magaishi, S.H. Electrical properties with relaxation through human blood. Biomicrofluidics 2010, 4, 34101. [Google Scholar] [CrossRef]
- Knopp, T.; Erbe, M.; Sattel, T.F.; Biederer, S.; Buzug, T.M. Generation of a static magnetic field-free line using two Maxwell coil pairs. Appl. Phys. Lett. 2010, 97, 092505. [Google Scholar] [CrossRef]
- Knopp, T.; Erbe, M.; Sattel, T.F.; Biederer, S.; Buzug, T.M. A Fourier slice theorem for magnetic particle imaging using a field-free line. Inverse. Probl. 2011, 27, 095004. [Google Scholar] [CrossRef]
- Ertan, K.; Soheil, T.; Atalar, E. Driving mutually coupled gradient array coils in magnetic resonance imaging. Magn. Reson. Med. 2019, 82, 1187–1198. [Google Scholar] [CrossRef]
- Szwast, M.; Piatkiewicz, W. Hall Effect in Electrolyte Flow Measurements: Introduction to Blood Flow Measurements. Artif. Organs 2012, 36, 551–555. [Google Scholar] [CrossRef]
- Kanai, H.; Yamano, E.; Nakayama, K.; Kawamura, N.; Furuhata, H. Transcutaneous Blood Flow Measurement by Electromagnetic Induction. IEEE Trans. Biomed. Eng. 2007, BME-21, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Guldvog, I.; Kjaernes, M.; Thoresen, M.; Walloe, L. Blood flow in arteries determined transcutaneously by an ultrasonic doppler velocitymeter as compared to electromagnetic measurements on the exposed vesels. Acta Physiol. 2010, 109, 211–216. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, G.; Yang, D.; Xu, B. Application of Linear Gradient Magnetic Field in Arterial Profile Scanning Imaging. Sensors 2020, 20, 4547. https://doi.org/10.3390/s20164547
Liu Y, Liu G, Yang D, Xu B. Application of Linear Gradient Magnetic Field in Arterial Profile Scanning Imaging. Sensors. 2020; 20(16):4547. https://doi.org/10.3390/s20164547
Chicago/Turabian StyleLiu, Yanjun, Guoqiang Liu, Dan Yang, and Bin Xu. 2020. "Application of Linear Gradient Magnetic Field in Arterial Profile Scanning Imaging" Sensors 20, no. 16: 4547. https://doi.org/10.3390/s20164547
APA StyleLiu, Y., Liu, G., Yang, D., & Xu, B. (2020). Application of Linear Gradient Magnetic Field in Arterial Profile Scanning Imaging. Sensors, 20(16), 4547. https://doi.org/10.3390/s20164547