In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Preparation of MEA Integrated with Optical Fiber
2.2. ChR2 Transduction and Optrode Implantation
2.3. Electrophysiological Recording and Optical Stimulation
2.4. Histology
2.5. Statistics
- (1)
- Spike firing rate analysis was defined as the mean number of spikes per second each bin. Here we took the average of multiple recording channels.
- (2)
- Interspike interval (ISI) analysis was defined as the number of spikes each bin of the discharge interval (from 0–100 ms).
- (3)
- Auto-correlation analysis was defined as the number of neuron firing time differences (from −50 to 50 ms) in each time window.
- (4)
- LFP power was defined as the integral of the mean squared LFP. Here we took the average of multiple recording channels.
3. Results
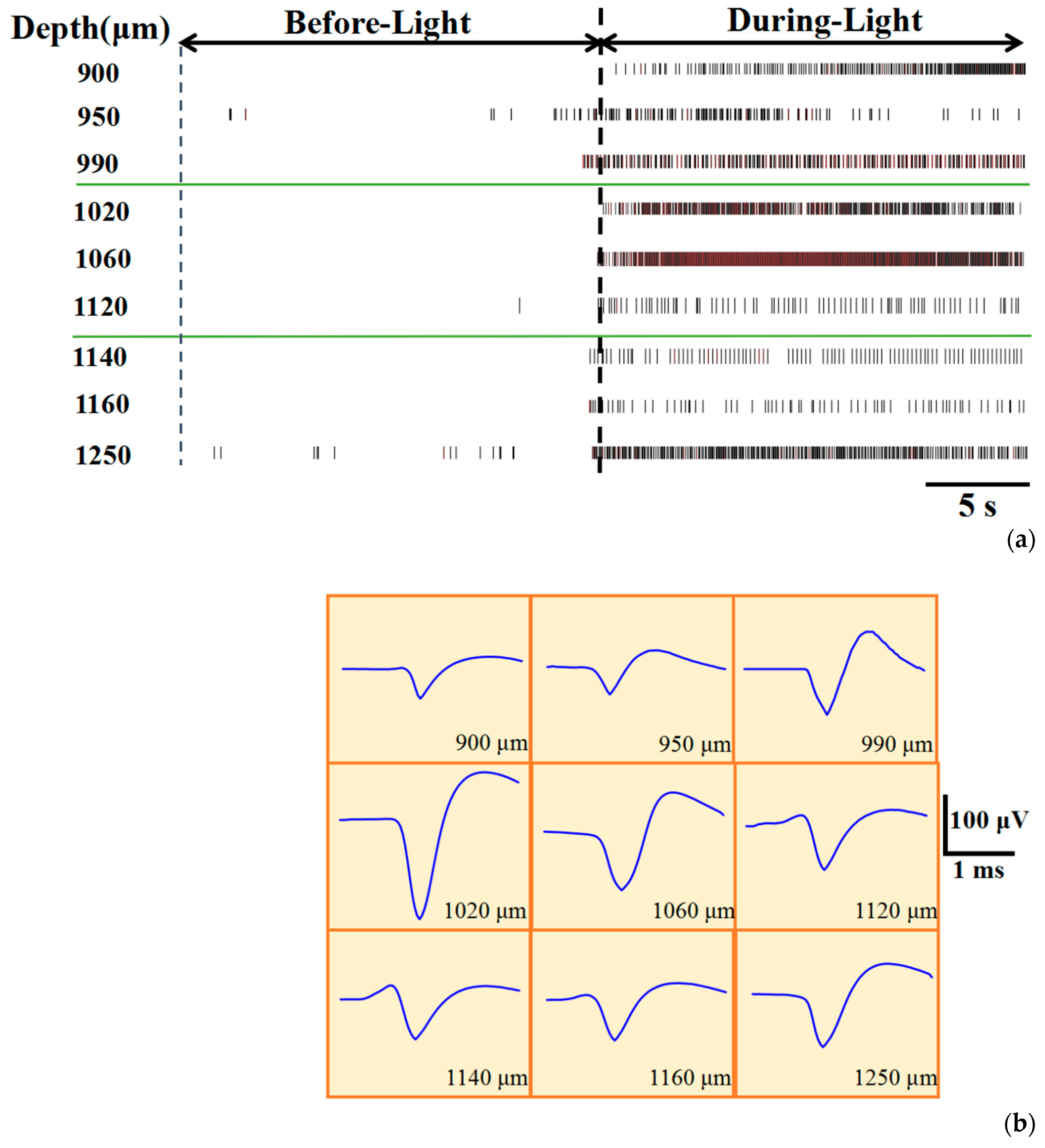
3.1. Optical Stimulation Enhances Neuronal Activity
3.2. Optical Stimulation Activates a Possible Local Inhibitory Circuit
3.3. Effects of Different Optical Stimulation Patterns on Optogenetic Neuromodulation
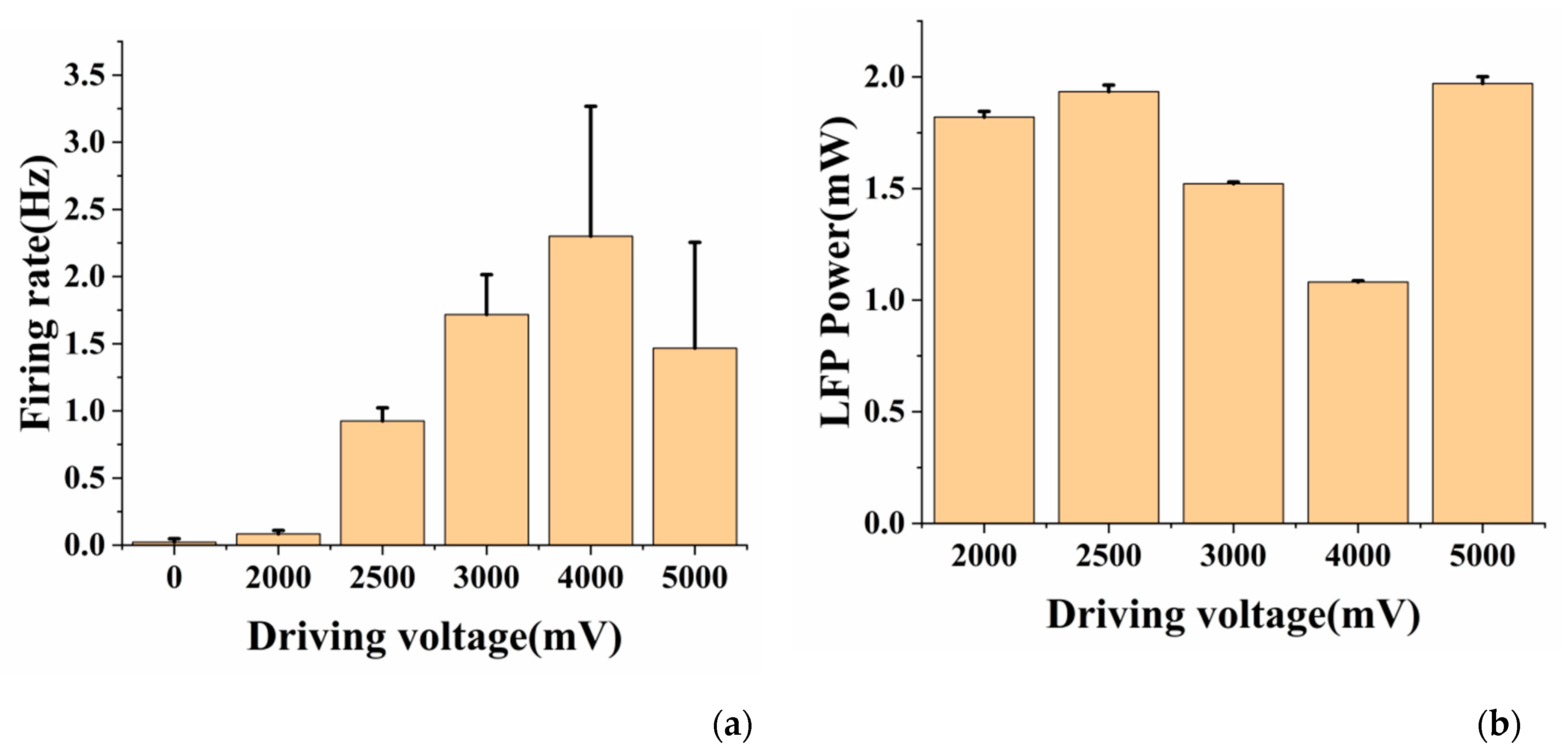
3.4. Effects of Different Light Powers on Optogenetic Neuromodulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, T.J.; Feng, J.; Luo, J.; Yang, P.; Samineni, V.K.; Gereau, R.W.t.; Kelley, N.; Hu, H.; Spencer, N.J. Optogenetic induction of colonic motility in mice. Gastroenterology 2018, 155, 514–528 e516. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Deisseroth, K. Recent advances in optogenetics and pharmacogenetics. Brain. Res. 2013, 1511, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.Y.; Boyden, E.S. Optogenetics and Translational Medicine. Sci. Transl. Med. 2013, 5, 177ps5. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in neural systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef]
- Zhang, F.; Vierock, J.; Yizhar, O.; Fenno, L.E.; Tsunoda, S.; Kianianmomeni, A.; Prigge, M.; Berndt, A.; Cushman, J.; Polle, J.; et al. The Microbial opsin family of optogenetic tools. Cell 2011, 147, 1446–1457. [Google Scholar] [CrossRef]
- Bernstein, J.G.; Boyden, E.S. Optogenetic tools for analyzing the neural circuits of behavior. Trends. Cogn. Sci. 2011, 15, 592–600. [Google Scholar] [CrossRef]
- Buzsaki, G.; Stark, E.; Berenyi, A.; Khodagholy, D.; Kipke, D.R.; Yoon, E.; Wise, K.D. Tools for probing local circuits: High-density silicon probes combined with optogenetics. Neuron 2015, 86, 92–105. [Google Scholar] [CrossRef]
- Goncalves, S.B.; Ribeiro, J.F.; Silva, A.F.; Costa, R.M.; Correia, J.H. Design and manufacturing challenges of optogenetic neural interfaces: A review. J. Neural Eng. 2017, 14, 041001. [Google Scholar] [CrossRef]
- Zhao, H. Recent progress of development of optogenetic implantable neural probes. Int. J. Mol. Sci. 2017, 18, 1751. [Google Scholar] [CrossRef]
- Royer, S.; Zemelman, B.V.; Barbic, M.; Losonczy, A.; Buzsaki, G.; Magee, J.C. Multi-array silicon probes with integrated optical fibers: Light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 2010, 31, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Stark, E.; Koos, T.; Buzsaki, G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J. Neurophysiol. 2012, 108, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Shin, H.-J.; Cho, I.-J.; Yoon, E.-S.; Suh, J.-K.F.; Im, M.; Yoon, E.; Kim, Y.-J.; Kim, J.; IEEE. The First Neural Probe Integrated with Light Source (Blue Laser Diode) for Optical Stimulation and Electrical Recording. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2961–2964. [Google Scholar]
- Wang, J.; Wagner, F.; Borton, D.A.; Zhang, J.; Ozden, I.; Burwell, R.D.; Nurmikko, A.V.; van Wagenen, R.; Diester, I.; Deisseroth, K. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J. Neural Eng. 2012, 9, 016001. [Google Scholar] [CrossRef] [PubMed]
- Schwaerzle, M.; Seidl, K.; Schwarz, U.T.; Paul, O.; Ruther, P. Ultracompact optrode with integrated laser diode chips and su-8 waveguides for optogenetic applications. In Proceedings of the 26th IEEE International Conference on Micro Electro Mechanical Systems, Taipei, China, 20–24 January 2013; pp. 1029–1032. [Google Scholar]
- Son, Y.; Lee, H.J.; Kim, J.; Shin, H.; Choi, N.; Lee, C.J.; Yoon, E.S.; Yoon, E.; Wise, K.D.; Kim, T.G.; et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Sci. Rep. 2015, 5, 15466. [Google Scholar] [CrossRef]
- Huang, W.C.; Chi, H.S.; Lee, Y.C.; Lo, Y.C.; Liu, T.C.; Chiang, M.Y.; Chen, H.Y.; Li, S.J.; Chen, Y.Y.; Chen, S.Y. Gene-embedded nanostructural biotic-abiotic optoelectrode arrays applied for synchronous brain optogenetics and neural signal recording. ACS Appl. Mater. Interfaces 2019, 11, 11270–11282. [Google Scholar] [CrossRef]
- Cao, H.; Gu, L.; Mohanty, S.K.; Chiao, J.C. An integrated mu LED optrode for optogenetic stimulation and electrical recording. IEEE Trans. Biomed. Eng. 2013, 60, 225–229. [Google Scholar] [CrossRef]
- Wu, F.; Stark, E.; Ku, P.C.; Wise, K.D.; Buzsaki, G.; Yoon, E. Monolithically integrated muLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 2015, 88, 1136–1148. [Google Scholar] [CrossRef]
- Scharf, R.; Tsunematsu, T.; McAlinden, N.; Dawson, M.D.; Sakata, S.; Mathieson, K. Depth-specific optogenetic control in vivo with a scalable, high-density muLED neural probe. Sci. Rep. 2016, 6, 28381. [Google Scholar] [CrossRef]
- Reddy, J.W.; Kimukin, I.; Stewart, L.T.; Ahmed, Z.; Barth, A.L.; Towe, E.; Chamanzar, M. High density, double-sided, flexible optoelectronic neural probes with Embedded muLEDs. Front. Neurosci. 2019, 13, 745. [Google Scholar] [CrossRef]
- Fan, X.; Song, Y.; Ma, Y.; Zhang, S.; Xiao, G.; Yang, L.; Xu, H.; Zhang, D.; Cai, X. In situ real-time monitoring of glutamate and electrophysiology from cortex to hippocampus in mice based on a microelectrode array. Sensors 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Tsien, J.Z. Large-scale neural ensembles in mice: Methods for recording and data analysis. In Electrophysiological Recording Techniques; Vertes, R.P., Stackman, R.W., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 54, p. 103126. [Google Scholar]
- Aravanis, A.M.; Wang, L.-P.; Zhang, F.; Meltzer, L.A.; Mogri, M.Z.; Schneider, M.B.; Deisseroth, K. An optical neural interface:in vivocontrol of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007, 4, S143–S156. [Google Scholar] [CrossRef] [PubMed]
- Binding, J.; Ben Arous, J.; Leger, J.F.; Gigan, S.; Boccara, C.; Bourdieu, L. Brain refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy. Opt. Express. 2011, 19, 4833–4847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; González-Vila, Á.; Liu, F.; Liu, Y.; Li, K.; Guo, T. Twist sensor based on surface plasmon resonance excitation using two spectral combs in one tilted fiber Bragg grating. J. Opt. Soc. Am. B 2019, 36, 1176–1182. [Google Scholar] [CrossRef]
- Su, H.; Iordachita, I.I.; Tokuda, J.; Hata, N.; Liu, X.; Seifabadi, R.; Xu, S.; Wood, B.; Fischer, G.S. Fiber optic force sensors for mri-guided interventions and rehabilitation: A Review. IEEE Sens. J. 2017, 17, 1952–1963. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, S.; Song, Y.; Zhang, Y.; Li, Z.; Gao, F.; Xie, J.; Sha, L.; Xu, Q.; Shen, Y.; et al. In situ detection of neurotransmitters and epileptiform electrophysiology activity in awake mice brains using a nanocomposites modified microelectrode array. Sens. Actuators B Chem. 2019, 288, 601–610. [Google Scholar] [CrossRef]
- Noh, K.N.; Park, S.I.; Qazi, R.; Zou, Z.; Mickle, A.D.; Grajales-Reyes, J.G.; Jang, K.I.; Gereau, R.W.t.; Xiao, J.; Rogers, J.A.; et al. Miniaturized, battery-free optofluidic systems with potential for wireless pharmacology and optogenetics. Small 2018, 14, 1702479. [Google Scholar] [CrossRef]
- Park, S.I.; Shin, G.; McCall, J.G.; Al-Hasani, R.; Norris, A.; Xia, L.; Brenner, D.S.; Noh, K.N.; Bang, S.Y.; Bhatti, D.L.; et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, E8169–E8177. [Google Scholar] [CrossRef]
- Kim, T.I.; McCall, J.G.; Jung, Y.H.; Huang, X.; Siuda, E.R.; Li, Y.; Song, J.; Song, Y.M.; Pao, H.A.; Kim, R.H.; et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 2013, 340, 211–216. [Google Scholar] [CrossRef]
- Matarese, B.F.E.; Feyen, P.L.C.; de Mello, J.C.; Benfenati, F. Sub-millisecond Control of Neuronal Firing by Organic Light-Emitting Diodes. Front. Bioeng. Biotechnol. 2019, 7, 278. [Google Scholar] [CrossRef]
- McCall, J.G.; Kim, T.I.; Shin, G.; Huang, X.; Jung, Y.H.; Al-Hasani, R.; Omenetto, F.G.; Bruchas, M.R.; Rogers, J.A. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 2013, 8, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Schwaerzle, M.; Pothof, F.; Paul, O.; Ruther, P. High-Resolution Neural Depth Probe with Integrated 460 Nm Light Emitting Diode For Optogenetic Applications. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems, Anchorage, AK, USA, 21–25 January 2015; pp. 1774–1777. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Song, Y.; Xu, S.; Dai, Y.; Wang, Y.; Lu, B.; Xie, J.; Wang, H.; Cai, X. In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber. Sensors 2020, 20, 4526. https://doi.org/10.3390/s20164526
Fan P, Song Y, Xu S, Dai Y, Wang Y, Lu B, Xie J, Wang H, Cai X. In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber. Sensors. 2020; 20(16):4526. https://doi.org/10.3390/s20164526
Chicago/Turabian StyleFan, Penghui, Yilin Song, Shengwei Xu, Yuchuan Dai, Yiding Wang, Botao Lu, Jingyu Xie, Hao Wang, and Xinxia Cai. 2020. "In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber" Sensors 20, no. 16: 4526. https://doi.org/10.3390/s20164526
APA StyleFan, P., Song, Y., Xu, S., Dai, Y., Wang, Y., Lu, B., Xie, J., Wang, H., & Cai, X. (2020). In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber. Sensors, 20(16), 4526. https://doi.org/10.3390/s20164526




