Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate
Abstract
1. Introduction
2. Enzymatic Biosensors for Nitrate and Nitrite
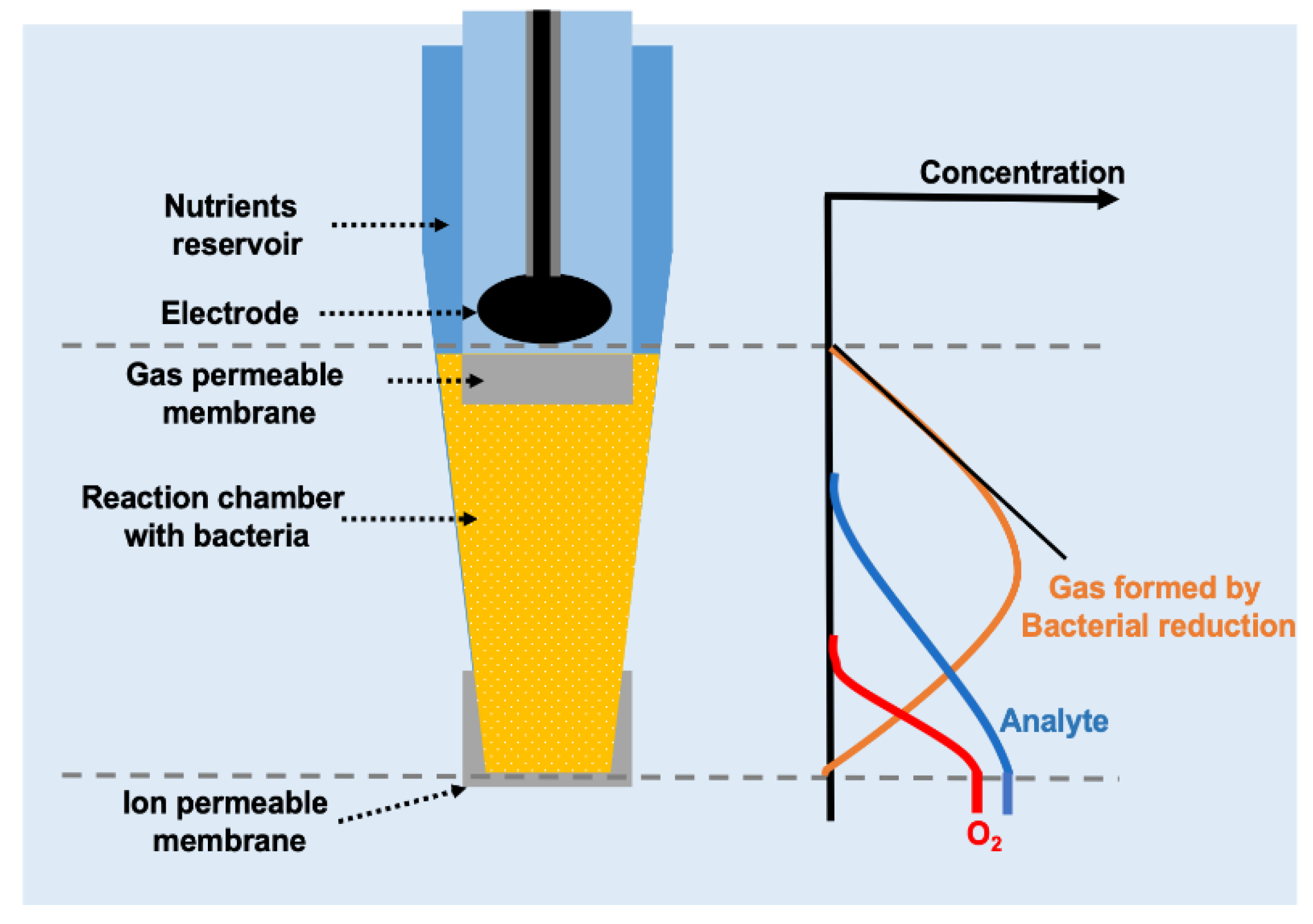
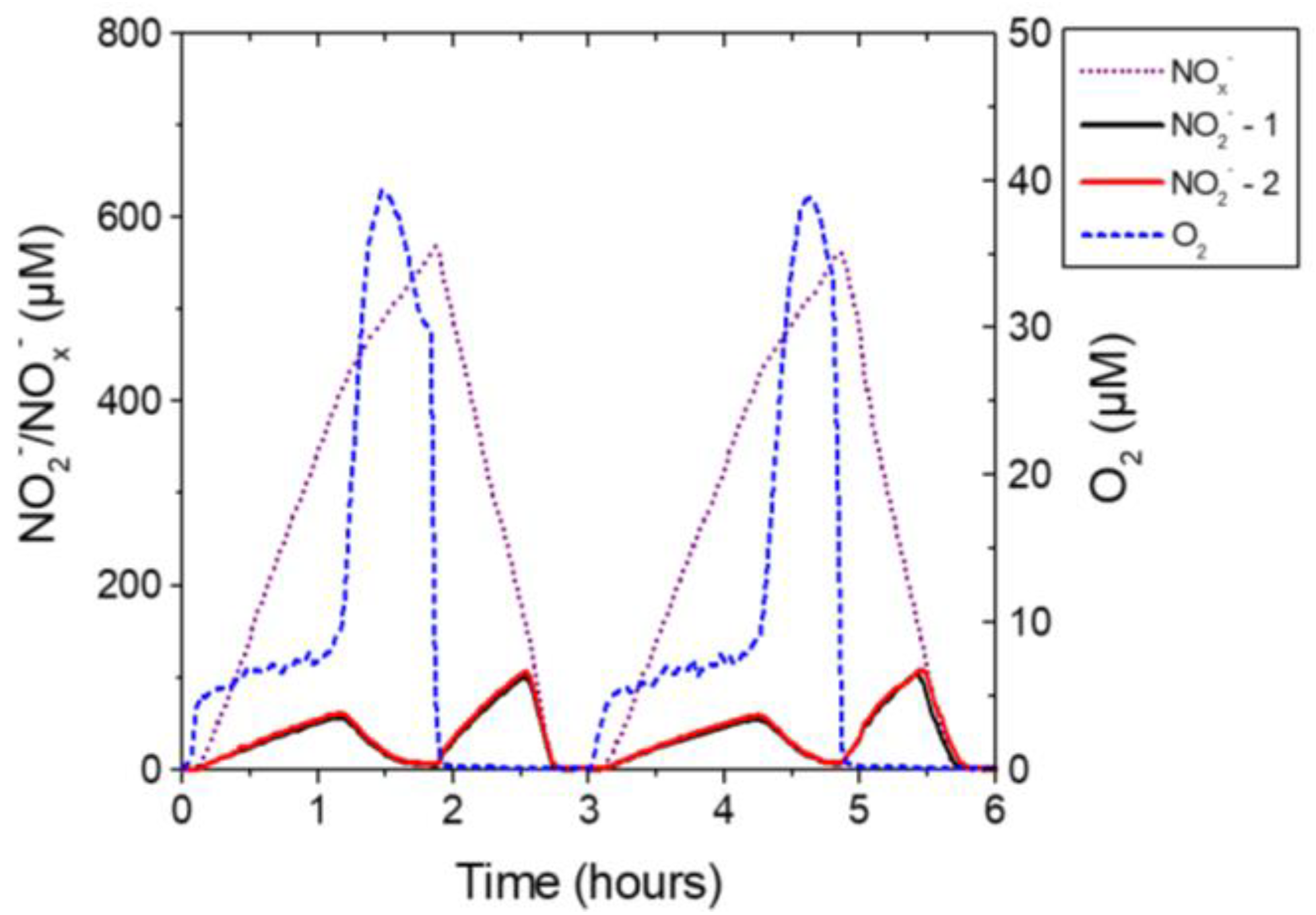
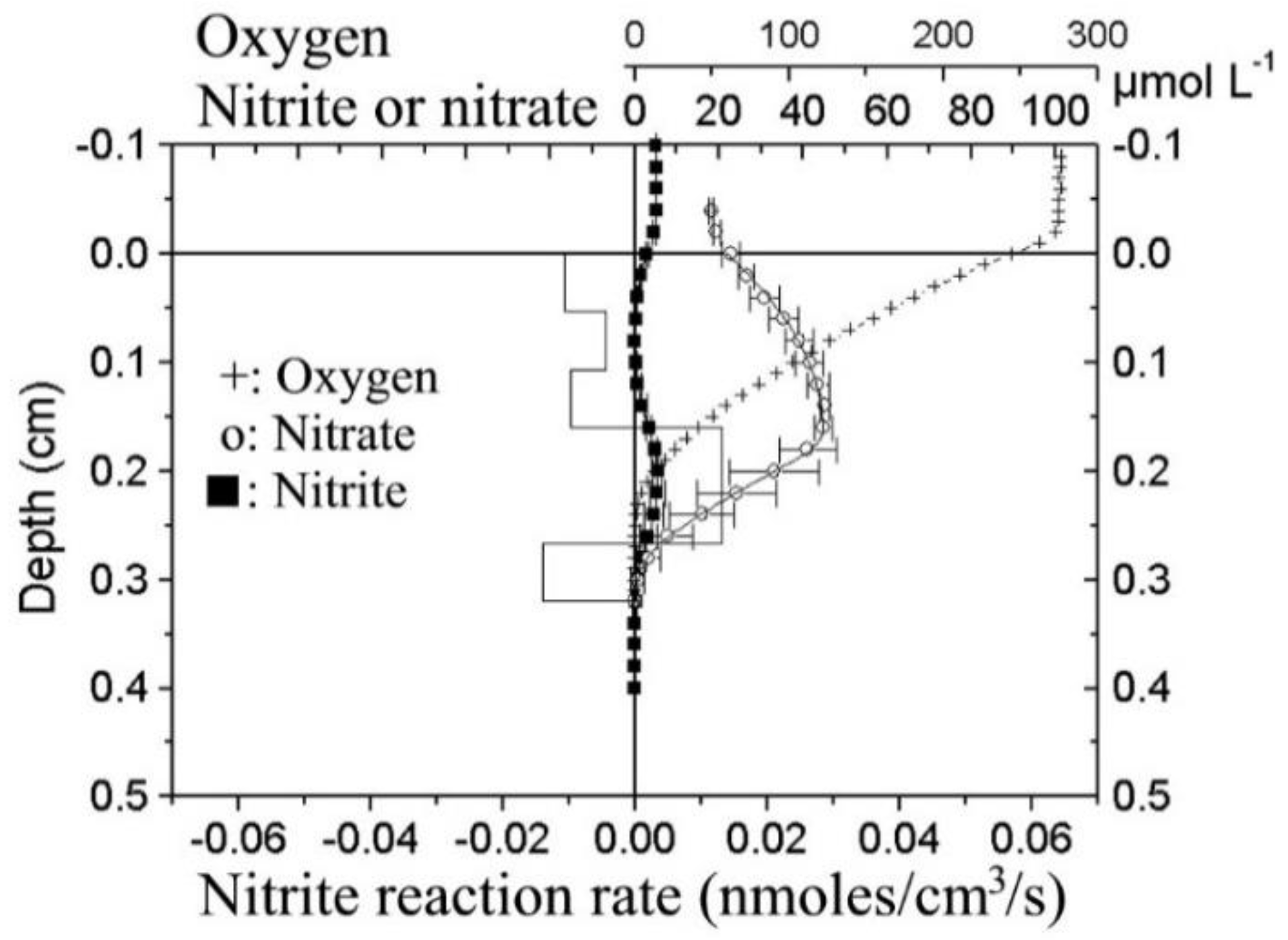
3. Bacteria-Based Biosensors for NO2− and NO3−
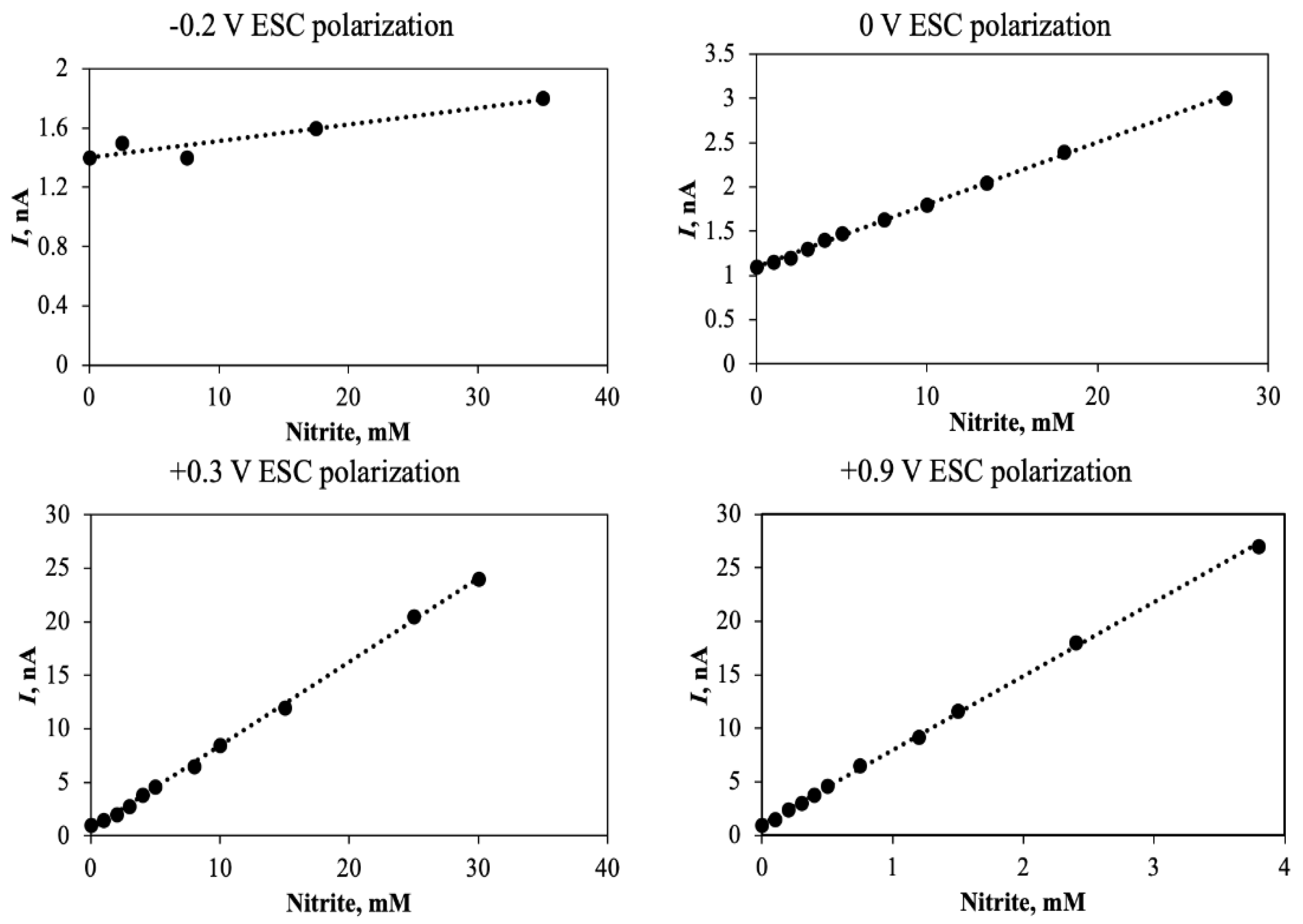
4. Scaling of Sensor Response by ESC
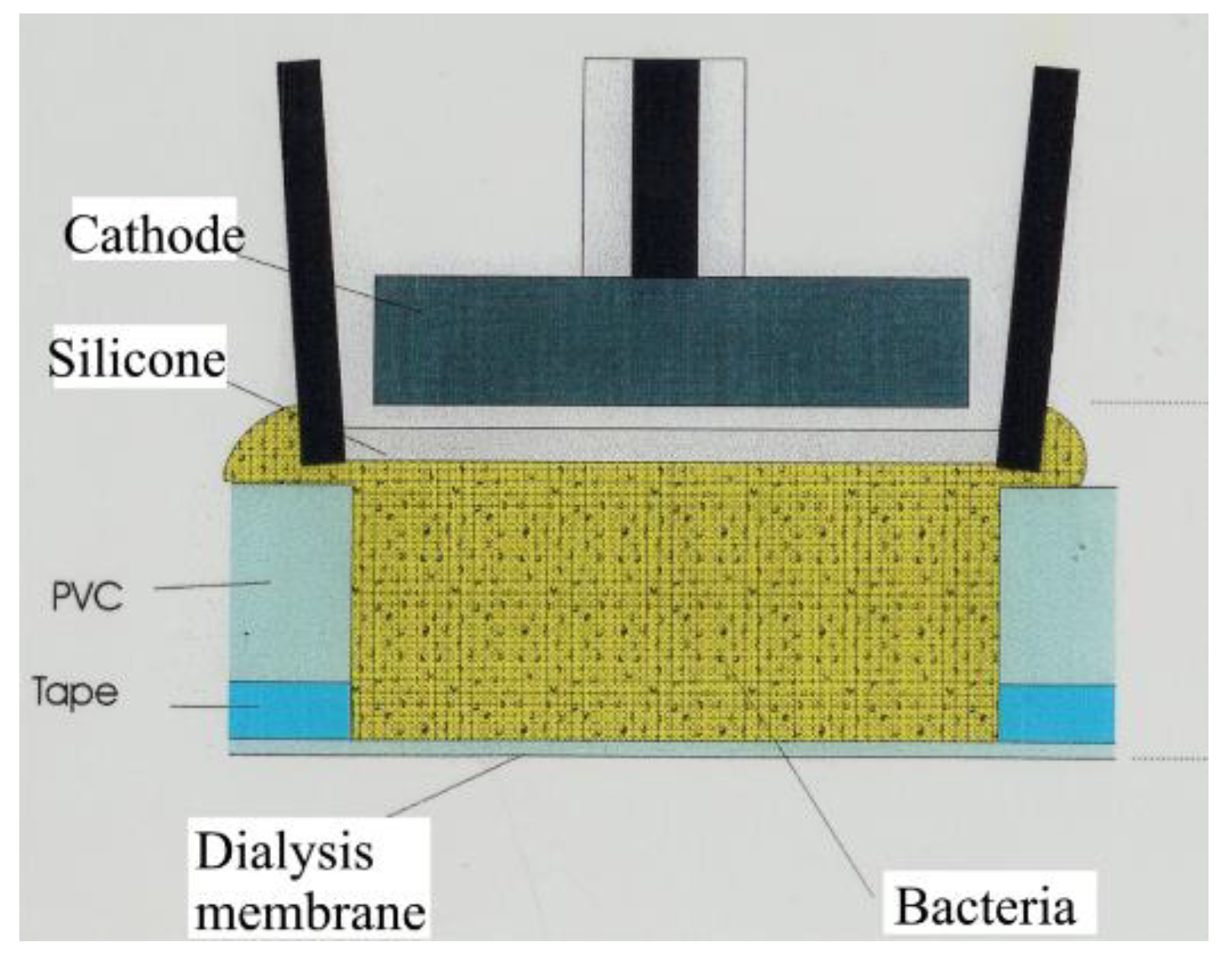
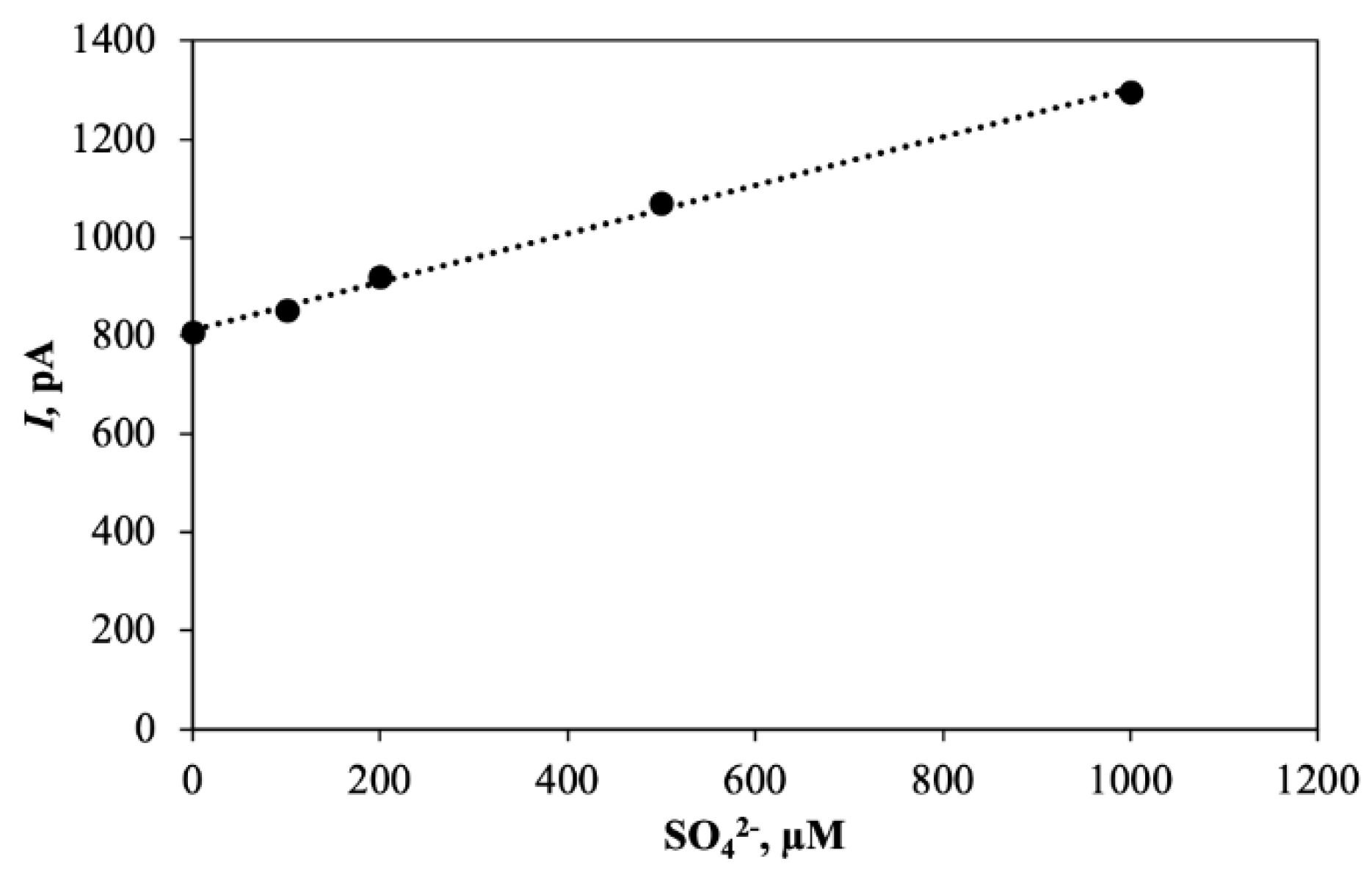
5. Biosensor for Sulfate
6. General Characteristics of NO3−, NO2−, and SO42− Sensors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.S.; Nuzzio, D.B.; Deering, T.W.; Taillefert, M.; Luther, G.W. Use of voltammetry to monitor O-2 using Au/Hg electrodes and to control physical sensors on an unattended observatory in the Delaware bay. Electroanalysis 2007, 19, 2110–2116. [Google Scholar] [CrossRef]
- Holmes, J.; Pathirathna, P.; Hashemi, P. Novel frontiers in voltammetric trace metal analysis: Towards real time, on-site, in situ measurements. Trend. Anal. Chem. 2019, 111, 206–219. [Google Scholar] [CrossRef]
- De Beer, D. Potentiometric microsensors for in situ measurements-in-aquatic environments. In Situ Monitoring of Aquatic Systems: Chemical Analysis and Speciation; John Wiley & Sons: New York, NY, USA, 2000; pp. 161–194. [Google Scholar]
- Chen, X.; Zhou, G.; Mao, S.; Chen, J. Rapid detection of nutrients with electronic sensors: A review. Environ. Sci. Nano. 2018, 5, 837–862. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, I.N.; Yoo, C.L.; Pyun, H.-J.; Cha, G.S.; Nam, H. Potentiometric evaluation of solvent polymeric carbonate-selective membranes based on molecular tweezer-type neutral carriers. Anal. Chem. 2000, 72, 4694–4699. [Google Scholar] [CrossRef]
- Suman, S.; Singh, R. Anion selective electrodes: A brief compilation. Microchem. J. 2019, 149, 1040–1045. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Mecharaj, M. Development of a whole cell biosensor for the detection of inorganic mercury. Environ. Technol. Innov. 2017, 8, 64–70. [Google Scholar] [CrossRef]
- Shin, H.J. Genetically engineered microbial biosensors for in situ monitoring of environmental pollution. Appl. Microbiol. 2011, 89, 867–877. [Google Scholar] [CrossRef]
- Xie, X.; Bakker, E. Non-Severinghaus potentiometric dissolved CO2 sensor with improved characteristics. Anal. Chem. 2013, 85, 1332–1336. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Garcia-Robledo, E.; Sveegaard, S.; Andersen, M.H.; Gothelf, K.V.; Larsen, L.H. Amperometic microsensor for measurement of gaseous and dissolved CO2. Sens. Actuators B Chem. 2019, 283, 349–354. [Google Scholar] [CrossRef]
- Jeroschewski, P.; Steuckart, C.; Kühl, M. An amperometric microsensor for the determination of H2S in aquatic environments. Anal. Chem. 1996, 68, 4351–4357. [Google Scholar] [CrossRef]
- Li, H.-L.; Chambers, J.; Hobbs, D. Electroreduction of nitrate ions in concentrated sodium hydroxide solutions at lead, zinc, nickel and phthalocyanine-modified electrodes. J. Appl. Electrochem. 1988, 18, 454–458. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Nei, L.; Davis, J.; Compton, R.G. Enhanced electrochemical detection of nitrite and nitrite at a Cu-30Ni alloy electrode. Anal. Lett. 2000, 33, 3127–3136. [Google Scholar] [CrossRef]
- Davis, J.; Compton, R.G. Sonoelectrochemically enhanced nitrite detection. Anal. Chim. Acta 2000, 404, 241–247. [Google Scholar] [CrossRef]
- Ohmori, T.; El-Deab, M.S.; Osawa, M. Electroreduction of nitrate ion to nitrite and ammonia on a gold electrode in acidic and basic sodium and cesium nitrate solutions. J. Electroanal. Chem. 1999, 470, 46–52. [Google Scholar] [CrossRef]
- Cattarin, S. Electrochemical reduction of nitrogen oxyanions in 1 M sodium hydroxide solutions at silver, copper and CuInSe2 electrodes. J. Appl. Electrochem. 1992, 22, 1077–1081. [Google Scholar] [CrossRef]
- Mori, V.; Bertotti, M. Amperometric detection with microelectrodes in FIA: Studies on the conversion of nitrate to nitrite in human saliva. Anal. Lett. 1999, 32, 25–37. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Kirstein, D.; Scheller, F.; Borcherding, H.; Ronnenberg, J.; Diekmann, S.; Steinrücke, P. Amperometric nitrate biosensors on the basis of Pseudomonas stutzeri nitrate reductase. J. Electroanal. Chem. 1999, 474, 43–51. [Google Scholar] [CrossRef]
- Qin, C.; Wang, W.; Chen, C.; Bu, L.; Wang, T.; Su, X.; Xie, Q.; Yao, S. Amperometric sensing of nitrite based on electroactive ferricyanide–poly (diallyldimethylammonium)–alginate composite film. Sens. Actuators B Chem. 2013, 181, 375–381. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.-P.; Gai, L.; Li, D.-J.; Li, Y.-B. An amperometric sensor based on ionic liquid and carbon nanotube modified composite electrode for the determination of nitrite in milk. Sens. Actuators B Chem. 2013, 181, 65–70. [Google Scholar] [CrossRef]
- Cosnier, S.; Innocent, C.; Jouanneau, Y. Amperometric detection of nitrate via a nitrate reductase immobilized and electrically wired at the electrode surface. Anal. Chem. 1994, 66, 3198–3201. [Google Scholar] [CrossRef]
- Moretto, L.M.; Ugo, P.; Zanata, M.; Guerriero, P.; Martin, C.R. Nitrate biosensor based on the ultrathin-film composite membrane concept. Anal. Chem. 1998, 70, 2163–2166. [Google Scholar] [CrossRef]
- Da Silva, S.; Shan, D.; Cosnier, S. Improvement of biosensor performances for nitrate determination using a new hydrophilic poly (pyrrole-viologen) film. Sens. Actuators B Chem. 2004, 103, 397–402. [Google Scholar] [CrossRef]
- Plumereé, N.; Henig, J.R.; Campbell, W.H. Enzyme-catalyzed O2 removal system for electrochemical analysis under ambient air: Application in an amperometric nitrate biosensor. Anal. Chem. 2012, 84, 2141–2146. [Google Scholar]
- Quan, D.; Shim, J.H.; Kim, J.D.; Park, H.S.; Cha, G.S.; Nam, H. Electrochemical determination of nitrate with nitrate reductase-immobilized electrodes under ambient air. Anal. Chem. 2005, 77, 4467–4473. [Google Scholar] [CrossRef]
- Papias, S.; Masson, M.; Pelletant, S.; Prost-Boucle, S.; Boutin, C. In situ continuous monitoring of nitrogen with ion-selective electrodes in a constructed wetland receiving treated wastewater: An operating protocol to obtain reliable data. Water Sci. Technol. 2018, 77, 1706–1713. [Google Scholar] [CrossRef]
- Ledford, S.H.; Toran, L. Downstream evolution of wastewater treatment plant nutrient signals using high-temporal monitoring. Hydrol. Process. 2019, 34, 852–864. [Google Scholar] [CrossRef]
- Murphy, M.J.; Siegel, L.M.; Tove, S.R.; Kamin, H. Siroheme: A new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. P. Natl. Acad. Sci. USA 1974, 71, 612–616. [Google Scholar] [CrossRef]
- Sasaki, S.; Karube, I.; Hirota, N.; Arikawa, Y.; Nishiyama, M.; Kukimoto, M.; Horinouchi, S.; Beppu, T. Application of nitrite reductase from Alcaligenes faecalis S-6 for nitrite measurement. Biosens. Bioelectron. 1998, 13, 1–5. [Google Scholar] [CrossRef]
- Quan, D.; Min, D.G.; Cha, G.S.; Nam, H. Electrochemical characterization of biosensor based on nitrite reductase and methyl viologen co-immobilized glassy carbon electrode. Bioelectrochemistry 2006, 69, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Strehlitz, B.; Gründig, B.; Schumacher, W.; Kroneck, P.M.; Vorlop, K.D.; Kotte, H. A nitrite sensor based on a highly sensitive nitrite reductase mediator-coupled amperometric detection. Anal. Chem. 1996, 68, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.M.; Gomes, S.P.; Araújo, A.N.; Conceição, M.; Montenegro, B.S.M.; Todorovic, S.; Viana, A.S.; Silva, R.J.C.; Moura, J.J.G.; Almeida, M.G. An efficient non-mediated amperometric biosensor for nitrite determination. Biosens. Bioelectrons. 2010, 25, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, K.; Lu, J.; Gao, Y.; Duan, C.; Deng, L.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of horseradish peroxidase immobilized on porous Co3O4 nanosheets and reduced graphene oxide composite modified electrode. Sens. Actuators B Chem. 2017, 238, 249–256. [Google Scholar] [CrossRef]
- Wei, W.; Jin, H.-H.; Zhao, G.-C. A reagentless nitrite biosensor based on direct electron transfer of hemoglobin on a room temperature ionic liquid/carbon nanotube-modified electrode. Microchim. Act. 2009, 164, 167–171. [Google Scholar] [CrossRef]
- Jiang, J.; Fan, W.; Du, X. Nitrite electrochemical biosensing based on coupled graphene and gold nanoparticles. Biosens. Bioelectrons. 2014, 51, 343–348. [Google Scholar] [CrossRef]
- Eguilaz, M.; Agüi, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. A biosensor based on cytochrome c immobilization on a poly-3-methylthiophene/multi-walled carbon nanotubes hybrid-modified electrode. Application to the electrochemical determination of nitrite. J. Electroanal. Chem. 2010, 644, 30–35. [Google Scholar] [CrossRef]
- Hong, J.; Dai, Z. Amperometric biosensor for hydrogen peroxide and nitrite based on hemoglobin immobilized on one-dimensional gold nanoparticle. Sens. Actuators B Chem. 2009, 140, 222–226. [Google Scholar] [CrossRef]
- Larsen, L.H.; Kjær, T.; Revsbech, N.P. A microscale NO3−biosensor for environmental applications. Anal. Chem. 1997, 69, 3527–3531. [Google Scholar] [CrossRef]
- Nielsen, M.; Larsen, L.H.; Jetten, M.S.M.; Revsbech, N.P. Bacterium-based NO2− biosensor for environmental applications. Appl. Environ. Microbiol. 2004, 70, 6551–6558. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Glud, R.N. Biosensor for laboratory and lander-based analysis of benthic nitrate plus nitrite distribution in marine environments. Limnol. Oceanogr.-Meth. 2009, 7, 761–770. [Google Scholar] [CrossRef]
- Juhler, S.; Revsbech, N.P.; Schramm, A.; Herrmann, M.; Ottosen, L.D.M.; Nielsen, L.P. Distribution and rate of microbial processes in an ammonia-loaded air filter biofilm. Appl. Environ. Microbiol. 2009, 75, 3705–3713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersen, K.; Kjær, T.; Revsbech, N.P. An oxygen insensitive microsensor for nitrous oxide. Sens. Actuators B Chem. 2001, 81, 42–48. [Google Scholar] [CrossRef]
- Nielsen, M. On-line determination of nitrite in wastewater treatment by use of a biosensor. Water Sci. Technol. 2002, 45, 69–76. [Google Scholar] [CrossRef]
- Sin, G.; Vanrolleghem, P.A. A nitrate biosensor based methodology for monitoring anoxic activated sludge activity. Water Sci. Technol. 2004, 50, 125–133. [Google Scholar] [CrossRef]
- Ivanikova, N.V.; McKay, R.M.L.; Bullerjahn, G.S. Construction and characterization of a cyanobacterial bioreporter capable of assessing nitrate assimilatory capacity in freshwaters. Limnol. Oceanogr.-Meth. 2005, 3, 86–93. [Google Scholar] [CrossRef][Green Version]
- Nielsen, M.; Gieseke, A.; De Beer, D.; Revsbech, N.P. Nitrate, nitrite, and nitrous oxide transformations in sediments along a salinity gradient in the Weser Estuary. Aquat. Microb. Ecol. 2009, 55, 39–52. [Google Scholar] [CrossRef]
- Kjær, T.; Larsen, L.H.; Revsbech, N.P. Sensitivity control of ion-selective biosensors by electrophoretically mediated analyte transport. Anal. Chim. Acta. 1999, 391, 57–63. [Google Scholar] [CrossRef]
- Marzocchi, U.; Revsbech, N.P. Electrophoretic sensitivity control applied on microscale NOx− biosensors with different membrane permeabilities. Sens. Actuators B Chem. 2014, 202, 307–313. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Abdolhosein Dehghan, M.; Bagherzadeh, M.; Kargar, H. Impedimetric and potentiometric investigation of a sulfate anion-selective elec-trode: Experiment and simulation. Anal. Chem. 2012, 84, 2614–2621. [Google Scholar] [CrossRef] [PubMed]

| Sensor Type. | Relevant Interference a | LOD, μM | Resp. 90% | Shelf Lifetime | Cont. Operat. b | Sea-Water | Response Scaling | Refs. |
|---|---|---|---|---|---|---|---|---|
| NO3− Potentiometric c | Cl− | 0.3 | 4 s | months | months | No | No | [4,5] |
| NO3−, chemical amperometric | O2 | 0.1 | NA | months | ≤ hours | No | No | [20] |
| NO3− Enzym. | O2 | 0.5 | 30 days | ≤ hours | No | No | [22,23,24,25,26,27] | |
| NO3− bacteria | N2O, NO2− | 0.1 | 30 | 30 days | 2 weeks | Yes | Yes | [41,42,43,44,45,46,47,49,50,51] |
| NO2− Potentiometric | Cl−, SCN− | 1 | 10 s | ? | ? | No d | No | [4,5] |
| NO2−, chemical reductive | O2, Br− | 5 | ? | 3 weeks | ≤ hours | No | No | [30] |
| NO2−, chemical oxidative | None? | 0.1 | 2 s | 100 days | ≤ hours | No | No | [21] |
| NO2−, enzymatic reductive | O2 | 0.07 | ? | 30 days | ≤ hours | No | No | [40] |
| NO2−, enzymatic oxidative | I−, Br− | 0.01 | 2 s | 30 days | ? | No | No | [38] |
| NO2−, bacteria | N2O | 0.1 | 30 | 30 days | 1 week | Yes | Yes | [42,46,49] |
| SO42−, Potentiometric | Cl− | 0.6 | 15 | ? | ? | No | No | [52] |
| SO42−, bacteria | H2S | 5 | 300 | ? | ? | Yes | Yes | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revsbech, N.P.; Nielsen, M.; Fapyane, D. Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors 2020, 20, 4326. https://doi.org/10.3390/s20154326
Revsbech NP, Nielsen M, Fapyane D. Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors. 2020; 20(15):4326. https://doi.org/10.3390/s20154326
Chicago/Turabian StyleRevsbech, Niels Peter, Michael Nielsen, and Deby Fapyane. 2020. "Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate" Sensors 20, no. 15: 4326. https://doi.org/10.3390/s20154326
APA StyleRevsbech, N. P., Nielsen, M., & Fapyane, D. (2020). Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors, 20(15), 4326. https://doi.org/10.3390/s20154326





