Multiangle Long-Axis Lateral Illumination Photoacoustic Imaging Using Linear Array Transducer
Abstract
1. Introduction
2. Materials and Methods
2.1. Monte Carlo Simulation of Illumination Schemes
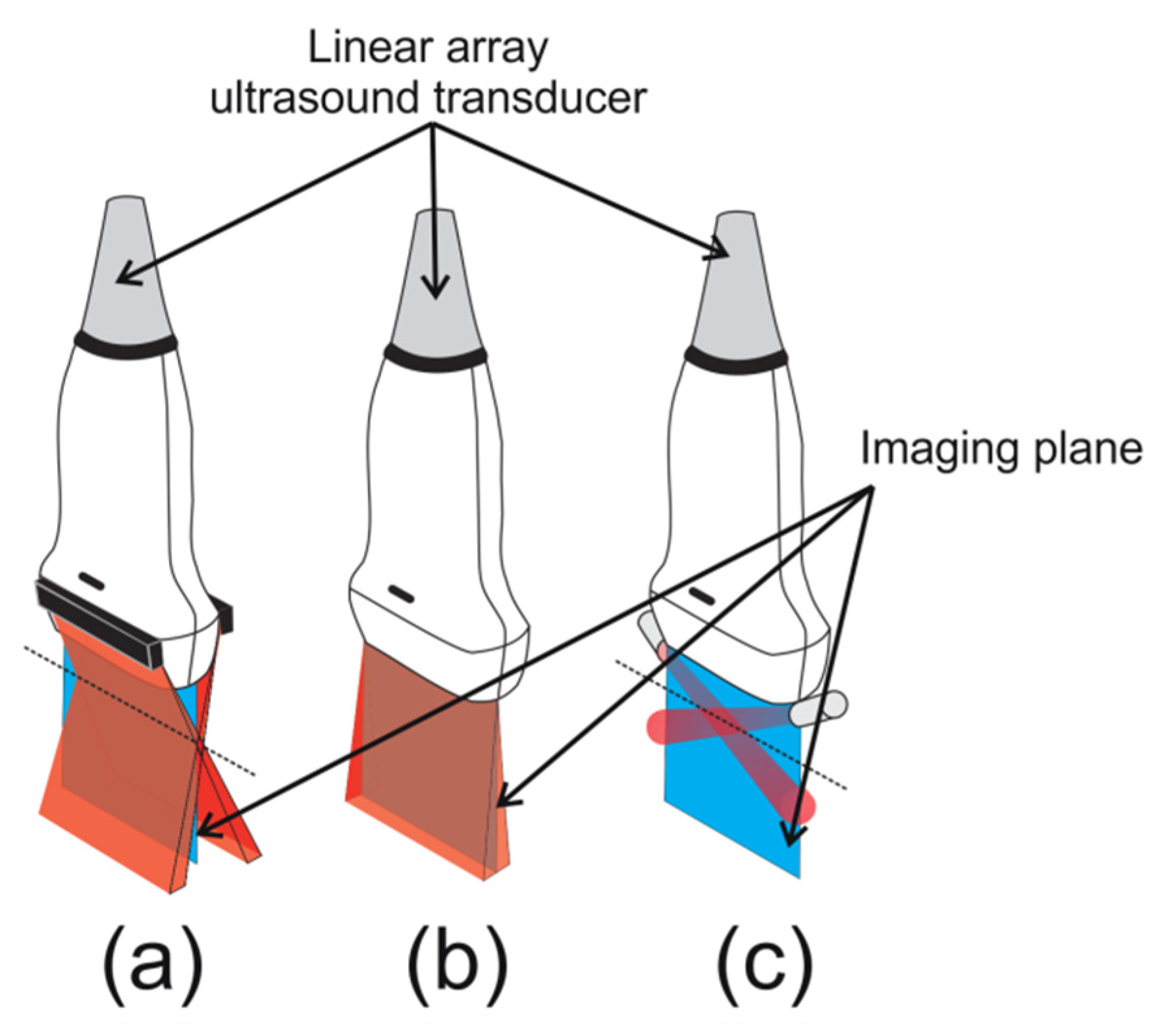
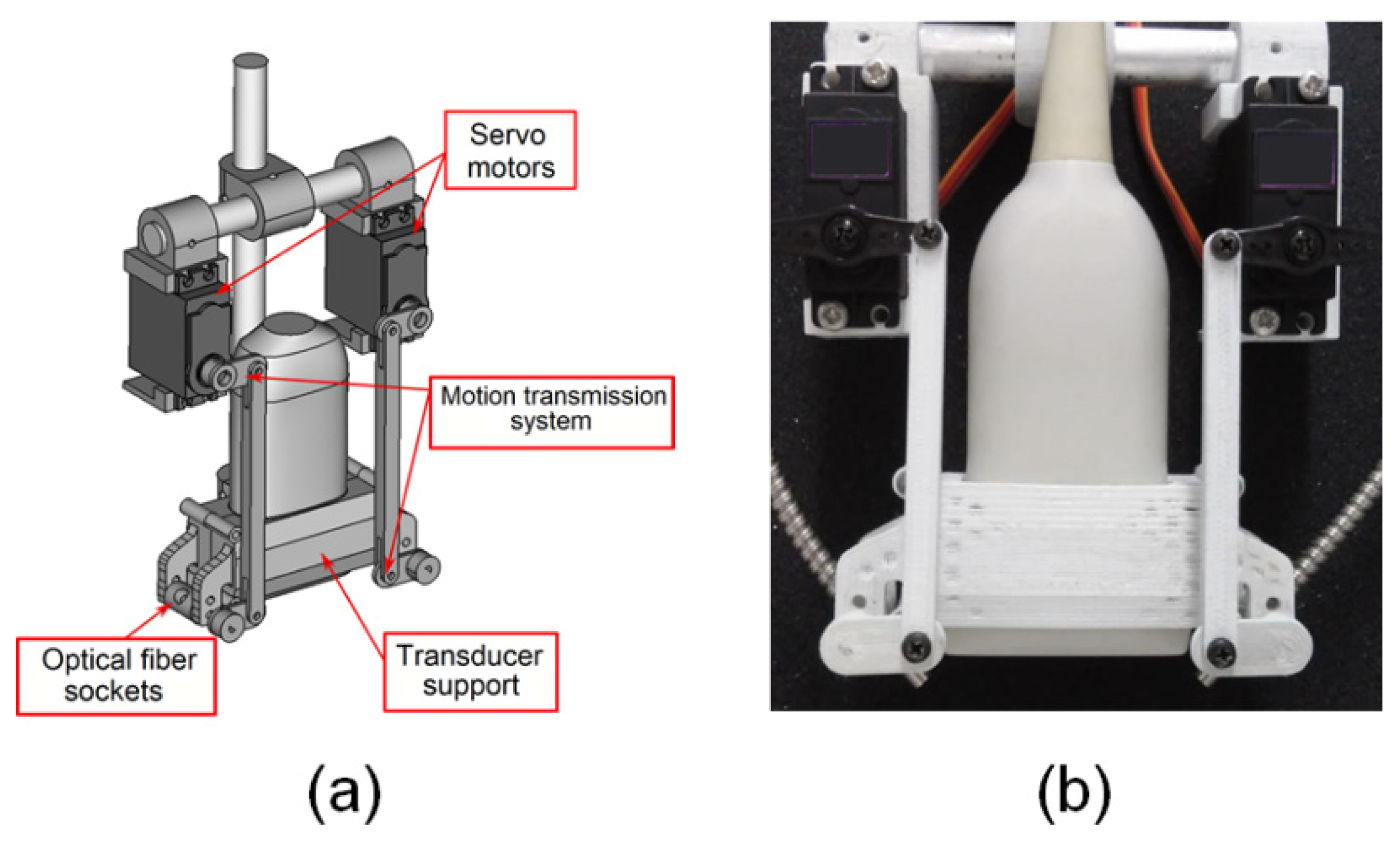
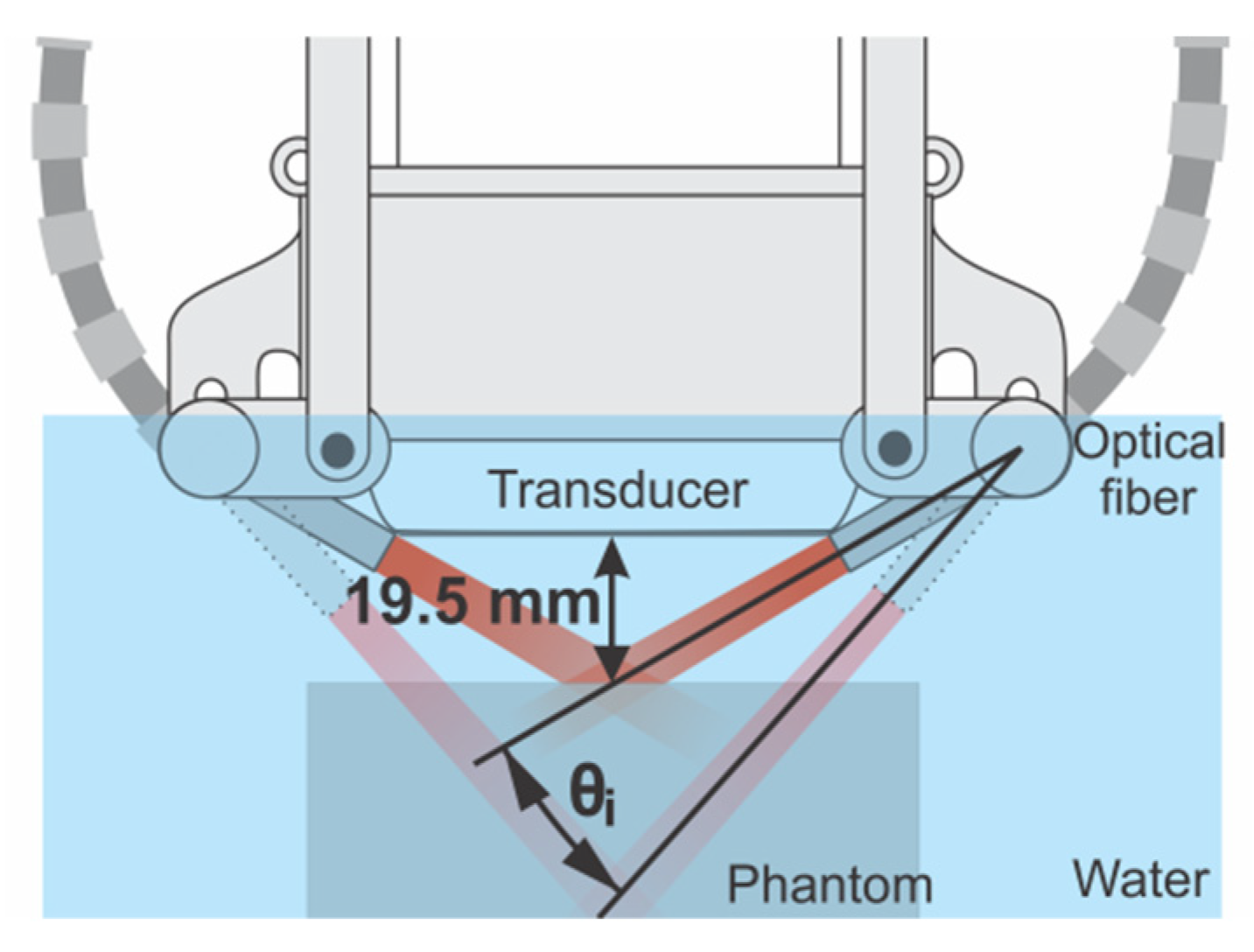
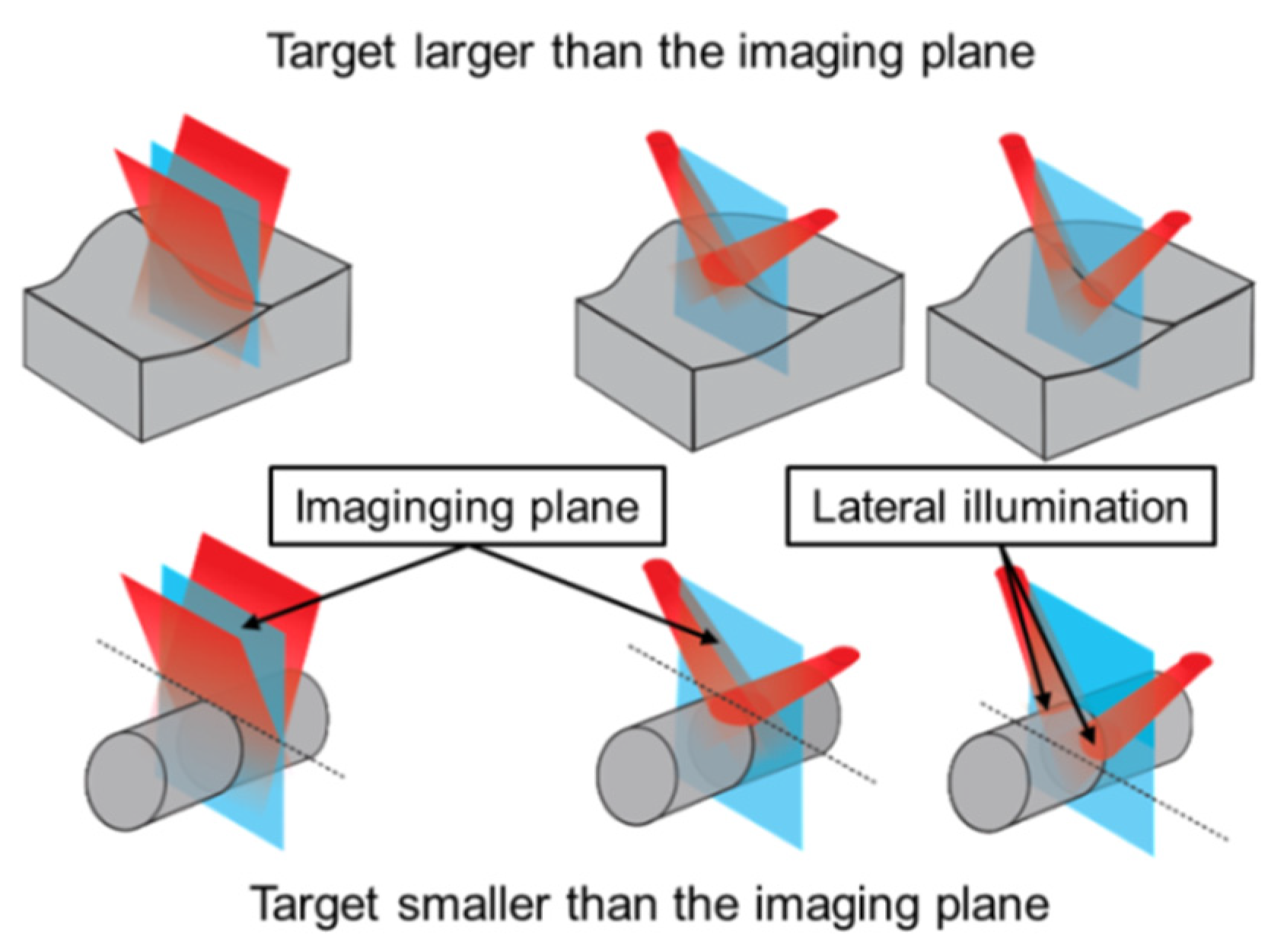
2.2. Device for Multiangle Long-Axis Lateral Illumination
2.3. Coherently Summing the PA Subframes
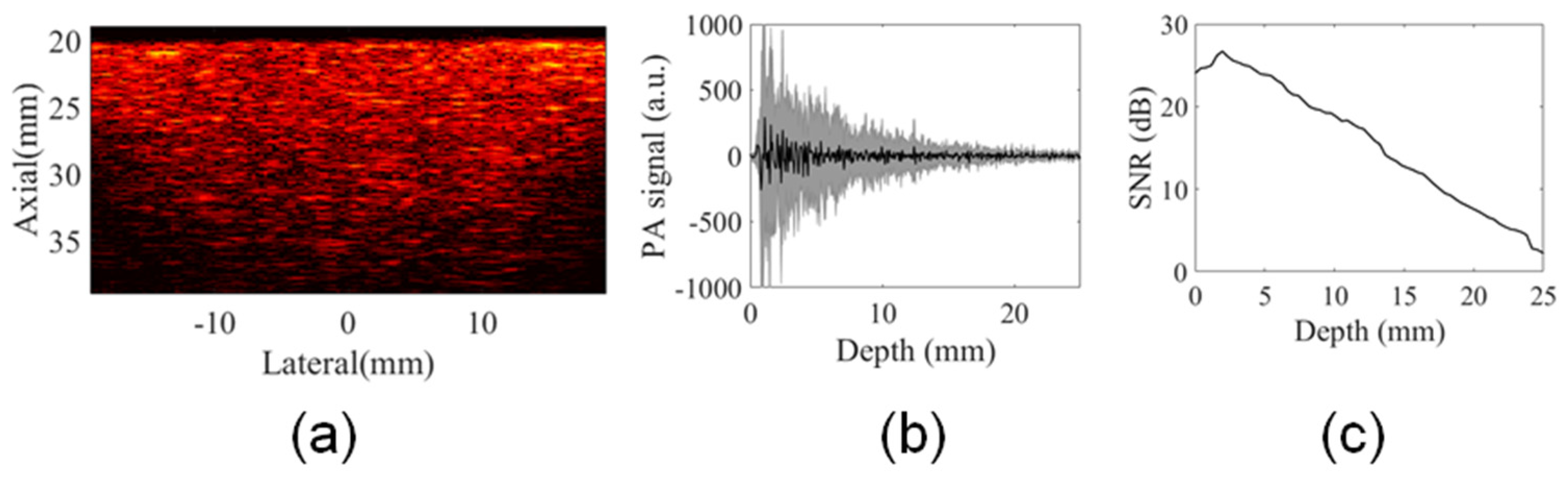
2.4. Phantom Experiments: Evaluation of Multiangle Long-Axis Lateral Illumination PAI
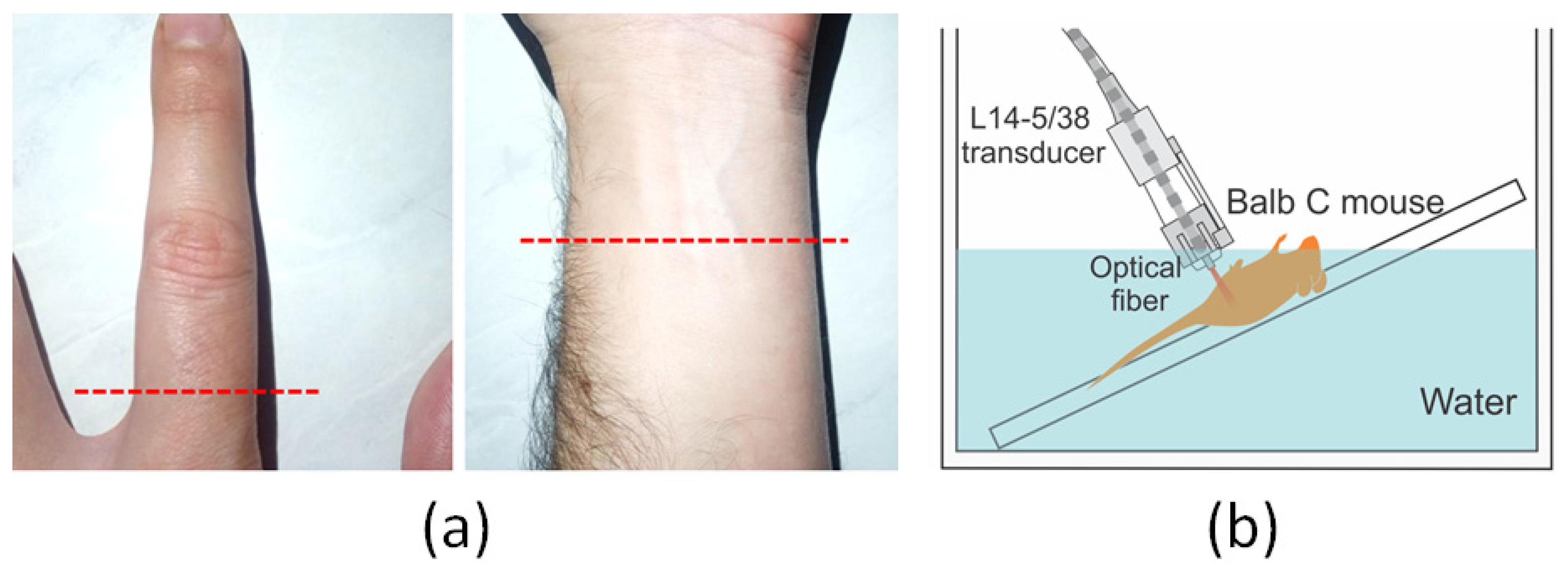
2.5. In Vivo Experiments: Human (Finger and Forearm) and Animal (Balb/C Mouse)
3. Results and Discussion
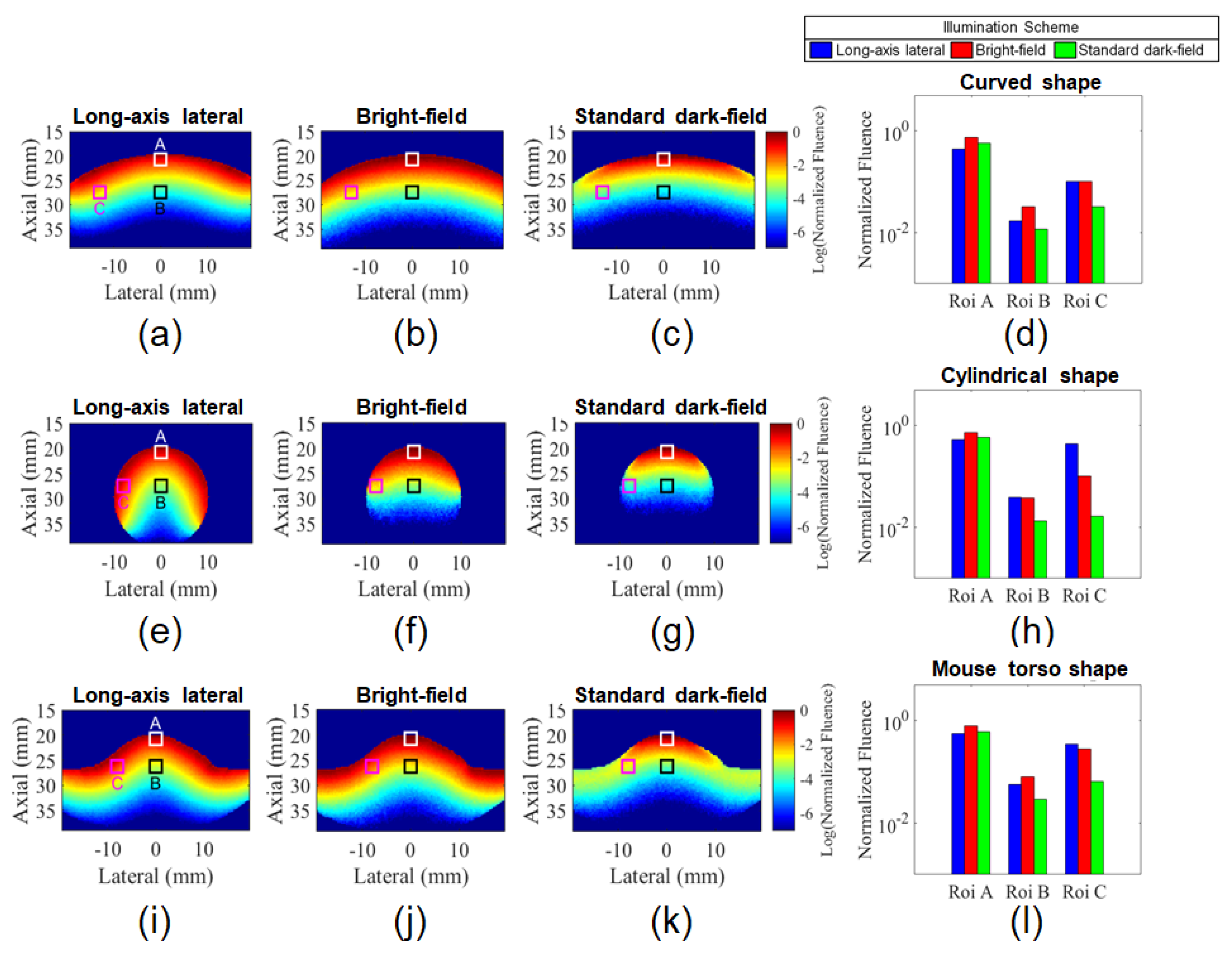
3.1. Comparison of Illumination Schemes for Different Target Shapes Using Monte Carlo simulation
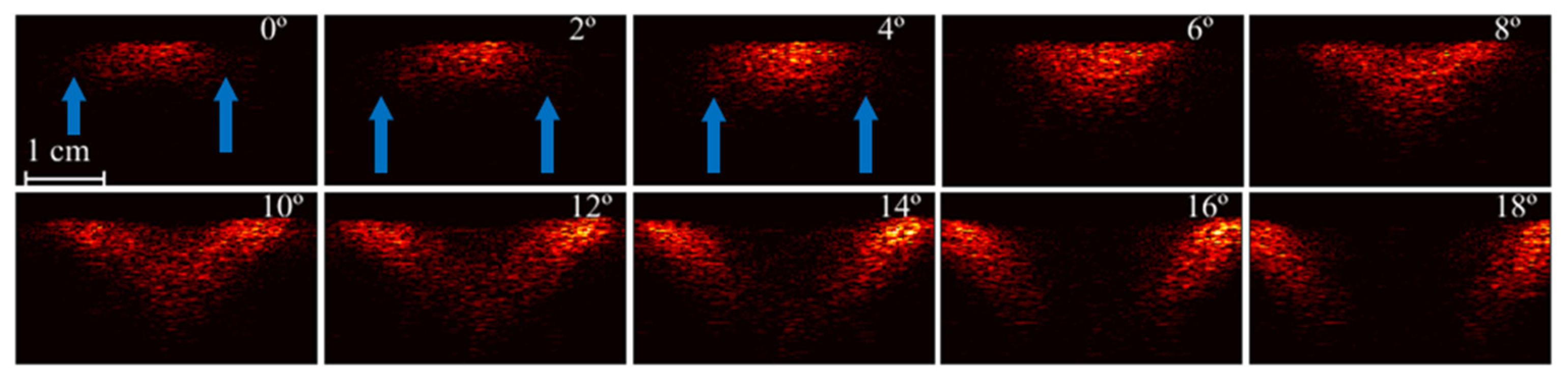
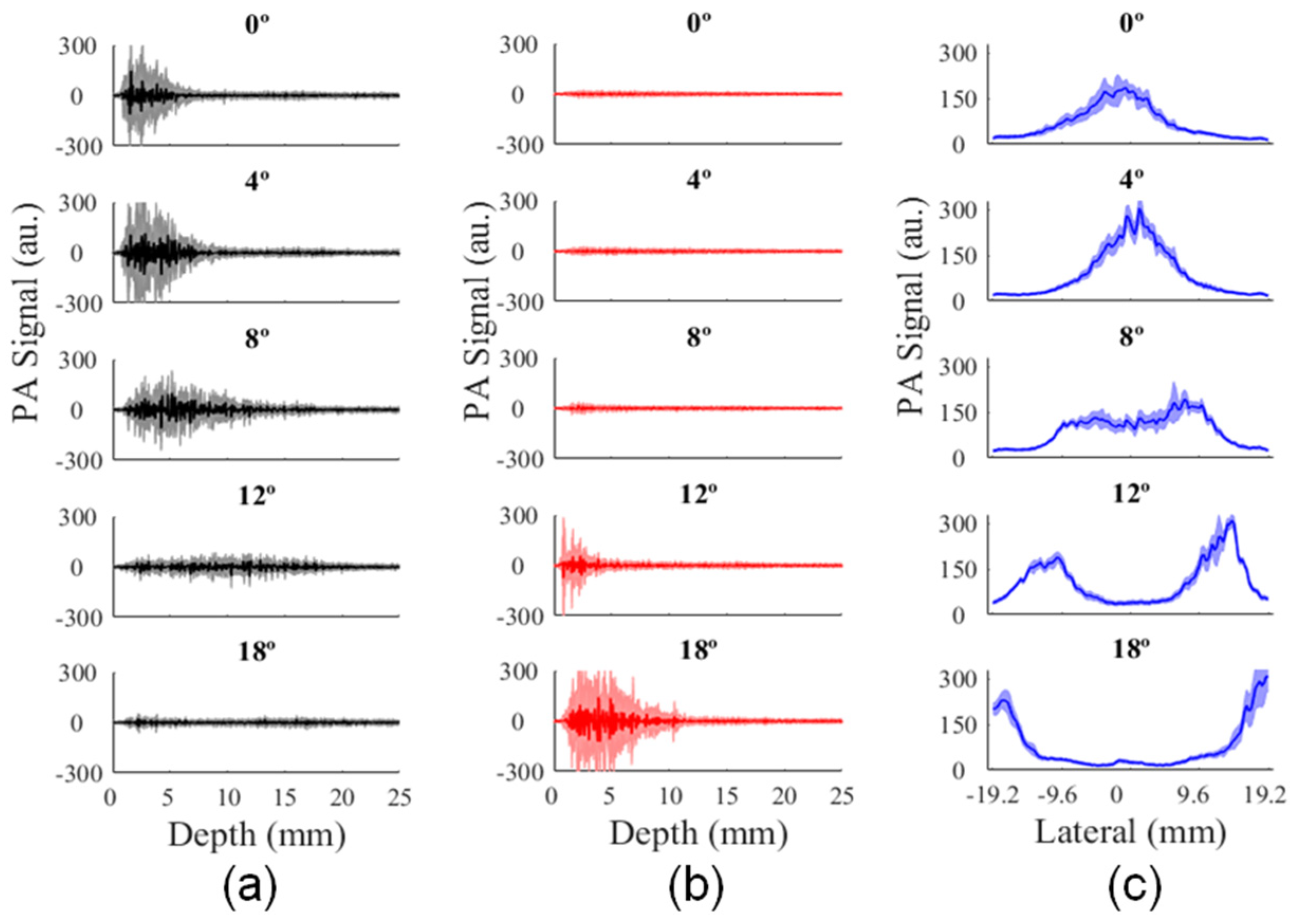
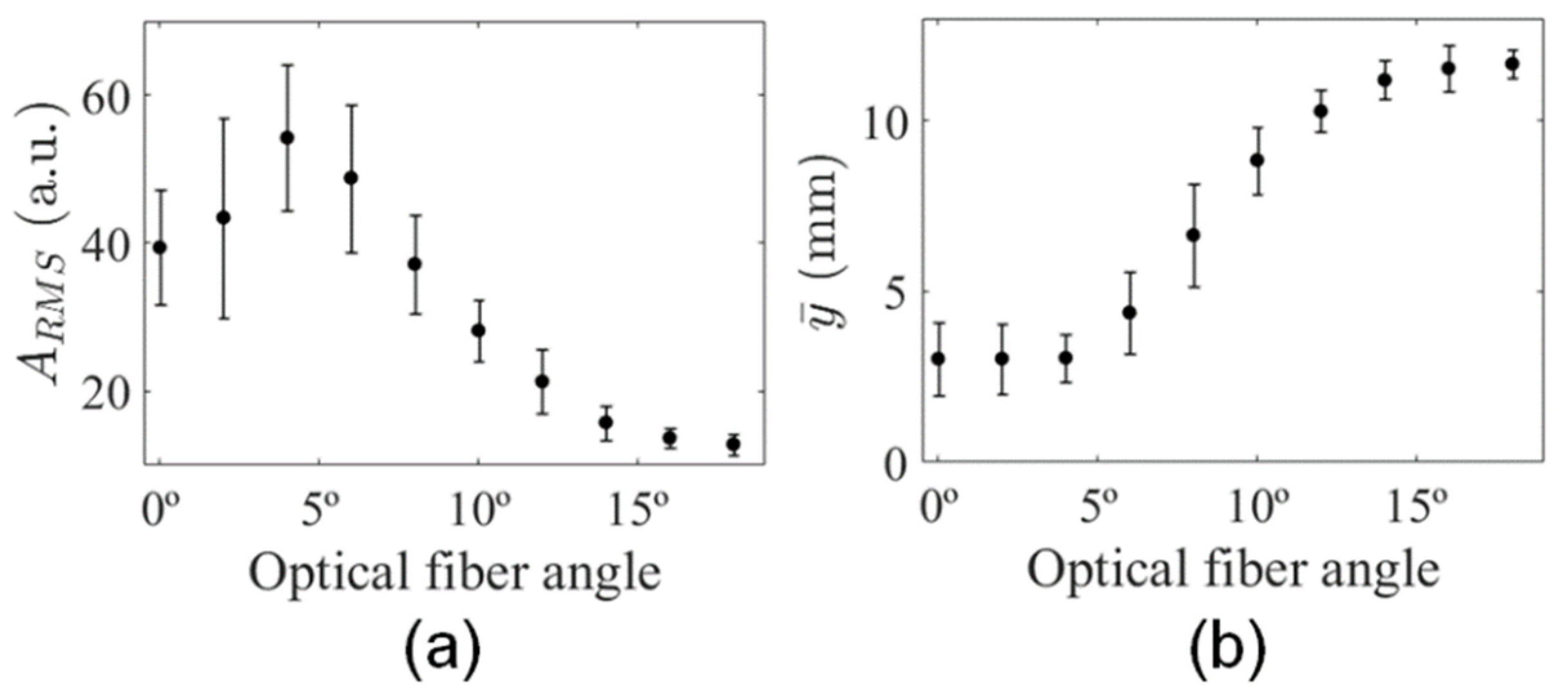
3.2. Analysis of Illumination Angles Contribution to the PA Image of the Phantom
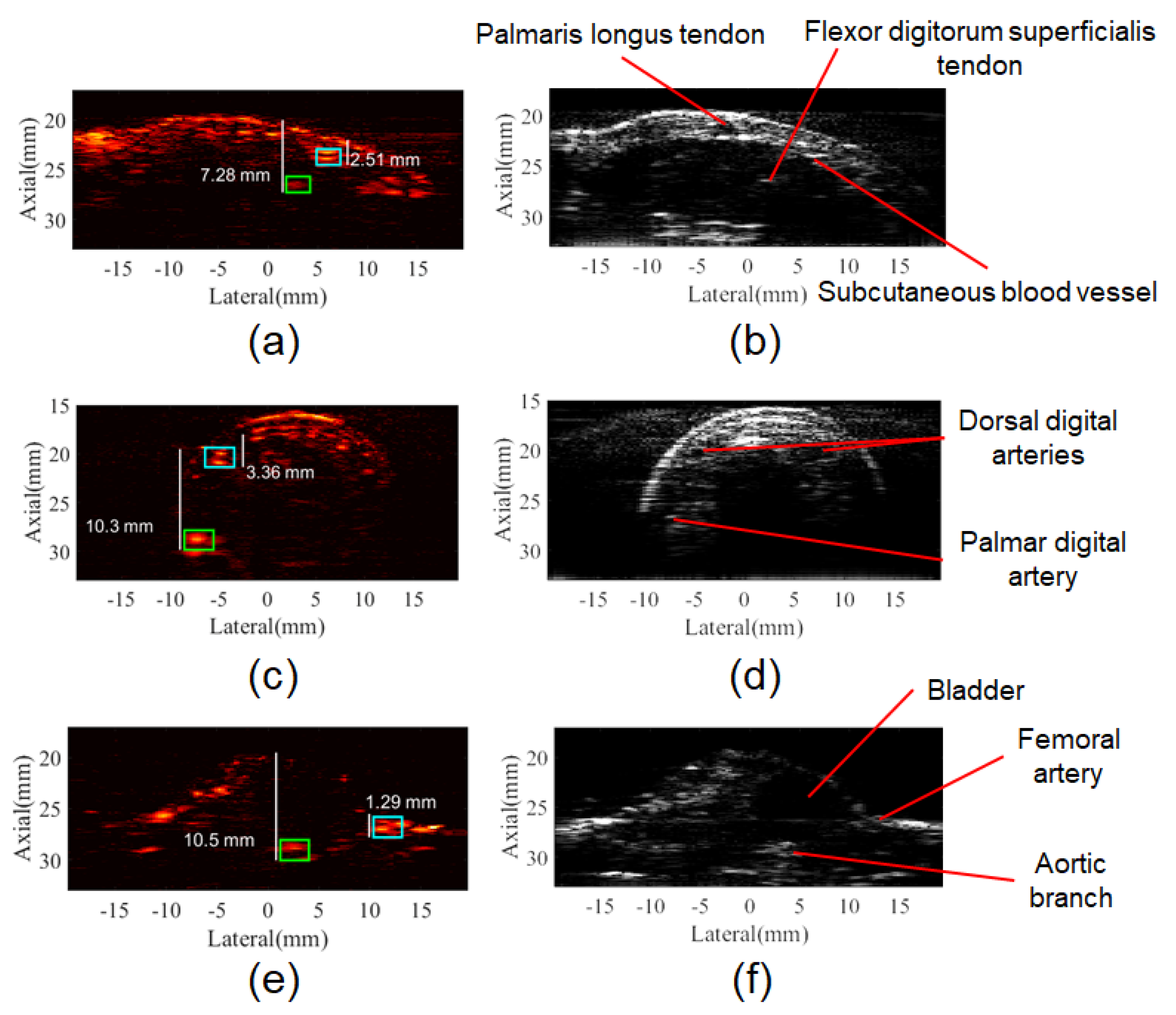
3.3. In Vivo PA Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Generation of ultrasonic waves from a layered photoacoustic source. Nature 1992, 359, 710–713. [Google Scholar] [CrossRef]
- Kruger, R.A. Photoacoustic ultrasound. Med. Phys. 1994, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Xu, M.; Yokoo, S.; Fry, E.S.; Wang, L.V. Photoacoustic tomography of biological tissues with high cross-section resolution: Reconstruction and experiment. Med. Phys. 2002, 29, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science (80-) 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Larina, I.V.; Larin, K.V.; Esenaliev, R.O. Real-time optoacoustic monitoring of temperature in tissues. J. Phys. D Appl. Phys. 2005, 38, 2633–2639. [Google Scholar] [CrossRef]
- Shah, J.; Park, S.; Aglyamov, S.R.; Larson, T.; Ma, L.; Sokolov, K.V.; Johnston, K.P.; Milner, T.E.; Emelianov, S.Y. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008, 13, 034024. [Google Scholar] [CrossRef]
- Yao, J.; Ke, H.; Tai, S.; Zhou, Y.; Wang, L.V. Absolute photoacoustic thermometry in deep tissue. Opt. Lett. 2013, 38, 5228–5231. [Google Scholar] [CrossRef]
- Ke, H.; Tai, S.; Wang, L.V. Photoacoustic thermography of tissue. J. Biomed. Opt. 2014, 19, 026003. [Google Scholar] [CrossRef]
- Razansky, D.; Vinegoni, C.; Ntziachristos, V. Multispectral photoacoustic imaging of fluorochromes in small animals. Opt. Lett. 2007, 32, 2891. [Google Scholar] [CrossRef]
- Laufer, J.; Zhang, E.; Beard, P. Evaluation of absorbing chromophores used in tissue phantoms for quantitative photoacoustic spectroscopy and imaging. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 600–607. [Google Scholar] [CrossRef]
- Laufer, J.G. Pulsed near-infrared photoacoustic spectroscopy of blood. Proc. SPIE 2004, 5320, 57–68. [Google Scholar] [CrossRef]
- Laufer, J.; Elwell, C.; Delpy, D.; Beard, P. In vitro measurements of absolute blood oxygen saturation using pulsed near-infrared photoacoustic spectroscopy: Accuracy and resolution. Phys. Med. Biol. 2005, 50, 4409–4428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Appl. Phys. Lett. 2007, 90, 5–7. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, S.; Xing, D. In vivo detection of hemoglobin oxygen saturation and carboxyhemoglobin saturation with multiwavelength photoacoustic microscopy. Opt. Lett. 2012, 37, 3414. [Google Scholar] [CrossRef]
- Shao, Q.; Ashkenazi, S. Photoacoustic lifetime imaging for direct in vivo tissue oxygen monitoring. J. Biomed. Opt. 2015, 20, 036004. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, 1–31. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Clingman, B.; Zalev, J.; Stavros, A.T.; Yang, W.T.; Parikh, J.R. Clinical optoacoustic imaging combined with ultrasound for coregistered functional and anatomical mapping of breast tumors. Photoacoustics 2018, 12, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xu, G.; Cao, M.; Marquardt, A.; Francis, S.; Gandikota, G.; Wang, X. A Functional Study of Human Inflammatory Arthritis Using Photoacoustic Imaging. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Tian, C.; Xu, G.; Sarazin, J.; Schiopu, E.; Gandikota, G.; Wang, X. Photoacoustic tomography for human musculoskeletal imaging and inflammatory arthritis detection. Photoacoustics 2018, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, J.J.; Jaeger, M.; Lemor, R.; Weber, P.; Frenz, M. Combined ultrasound and optoacoustic system for real-time high-contrast vascular imaging in vivo. IEEE Trans. Med. Imaging 2005, 24, 436–440. [Google Scholar] [CrossRef]
- Daoudi, K.; Van Den Berg, P.J.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365. [Google Scholar] [CrossRef]
- Hai, P.; Zhou, Y.; Zhang, R.; Ma, J.; Li, Y.; Wang, L.V. Label-free high-throughput detection and quantification of circulating melanoma tumor cell clusters. J. Biomed. Opt. 2016, 22, 1-041004–1-041005-6. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, J.; Wang, L.V. Cuffing-based photoacoustic flowmetry in humans in the optical diffusive regime. J. Biophotonics 2016, 9, 208–212. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, M.; Jiang, Y.; Li, C. Optical fluence compensation for handheld photoacoustic probe: An in vivo human study case. J. Innov. Opt. Health Sci. 2017, 10, 1740002-1–1740002-18. [Google Scholar] [CrossRef]
- Montilla, L.G.; Olafsson, R.; Bauer, D.R.; Witte, R.S. Real-time photoacoustic and ultrasound imaging: A simple solution for clinical ultrasound systems with linear arrays. Phys. Med. Biol. 2013, 58. [Google Scholar] [CrossRef]
- Wang, G.W.G.; Zhao, H.Z.H.; Ren, Q.R.Q.; Li, C.L.C. Simulation of light delivery for photoacoustic breast imaging using the handheld probe. Chin. Opt. Lett. 2014, 12, 051703–051706. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jeong, E.J.; Song, H.W.; Ahn, C.G.; Noh, H.W.; Sim, J.Y.; Song, D.H.; Jeon, M.Y.; Lee, S.; Kim, H.; et al. Photoacoustic imaging probe for detecting lymph nodes and spreading of cancer at various depths. J. Biomed. Opt. 2017, 22, 091513. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cong, B.; Gong, X.; Song, L.; Liu, C. Compact and low-cost handheld quasibright-field linear-array probe design in photoacoustic computed tomography. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Held, G.; Preisser, S.; Akarçay, H.G.; Peeters, S.; Frenz, M.; Jaeger, M. Effect of irradiation distance on image contrast in epi-optoacoustic imaging of human volunteers. Biomed. Opt. Express 2014, 5, 3765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frenz, M.; Jaeger, M. Optimization of tissue irradiation in optoacoustic imaging using a linear transducer: Theory and experiments. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2008: The Ninth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-optics, San Jose, CA, USA, 19–24 January 2008. [Google Scholar]
- Preisser, S.; Held, G.; Akarçay, H.G.; Jaeger, M.; Frenz, M. Study of clutter origin in in-vivo epi-optoacoustic imaging of human forearms. J. Opt. 2016, 18, 094003. [Google Scholar] [CrossRef]
- Haisch, C.; Eilert-Zell, K.; Vogel, M.M.; Menzenbach, P.; Niessner, R. Combined optoacoustic/ultrasound system for tomographic absorption measurements: Possibilities and limitations. Anal. Bioanal. Chem. 2010, 397, 1503–1510. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Periyasamy, V.; Wen, K.K.; Pramanik, M. Optimizing light delivery through fiber bundle in photoacoustic imaging with clinical ultrasound system: Monte Carlo simulation and experimental validation. J. Biomed. Opt. 2016, 22, 041008. [Google Scholar] [CrossRef]
- Sangha, G.S.; Hale, N.J.; Goergen, C.J. Adjustable photoacoustic tomography probe improves light delivery and image quality. Photoacoustics 2018, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sowers, T.; Yoon, H.; Emelianov, S. Investigation of light delivery geometries for photoacoustic applications using Monte Carlo simulations with multiple wavelengths, tissue types, and species characteristics. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Ha, S.; Kim, K. A new design of light illumination scheme for deep tissue photoacoustic imaging. Opt. Express 2012, 20, 22649. [Google Scholar] [CrossRef]
- Yu, J.; Schuman, J.S.; Lee, J.K.; Lee, S.G.; Chang, J.H.; Kim, K. A Light Illumination Enhancement Device for Photoacoustic Imaging: In Vivo Animal Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1205–1211. [Google Scholar] [CrossRef]
- Xia, W.; Kuniyil Ajith Singh, M.; Maneas, E.; Sato, N.; Shigeta, Y.; Agano, T.; Ourselin, S.; JWest, S.; EDesjardins, A. Handheld real-time LED-based photoacoustic and ultrasound imaging system for accurate visualization of clinical metal needles and superficial vasculature to guide minimally invasive procedures. Sensors 2018, 18, 1394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors 2020, 20, 2484. [Google Scholar] [CrossRef]
- Kuriakose, M.; Nguyen, C.D.; Singh, M.K.A.; Mallidi, S. Optimizing irradiation geometry in LED-based photoacoustic imaging with 3d printed flexible and modular light delivery system. Sensors 2020, 20, 3789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Adelegan, O.J.; Yamaner, F.Y.; Oralkan, O. Backward-Mode Photoacoustic Imaging Using Illumination Through a CMUT with Improved Transparency. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Davies, A.; Dewhurst, R.J.; Payne, P.A. Photo-acoustic probe for intra-arterial imaging and therapy. Electron. Lett. 1993, 29, 1632–1633. [Google Scholar] [CrossRef]
- Laufer, J.; Zhang, E.; Raivich, G.; Beard, P. Three-dimensional noninvasive imaging of the vasculature in the mouse brain using a high resolution photoacoustic scanner. Appl. Opt. 2009, 48, 299–306. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Gong, X.; Zheng, R.; Bai, Y.; Xing, M.; Du, X.; Liu, X.; Zeng, J.; Lin, R.; et al. Linear array-based real-time photoacoustic imaging system with a compact coaxial excitation handheld probe for noninvasive sentinel lymph node mapping. Biomed. Opt. Express 2018, 9, 1408–1422. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, R.S.A.; Zhang, H.; Nyayapathi, N.; Oh, K.W.; Xia, J. Optimizing the light delivery of linear-array-based photoacoustic systems by double acoustic reflectors. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Ida, T.; Iwazaki, H.; Kawaguchi, Y.; Kawauchi, S.; Ohkura, T.; Iwaya, K.; Tsuda, H.; Saitoh, D.; Sato, S.; Iwai, T. Burn depth assessments by photoacoustic imaging and laser Doppler imaging. Wound Repair Regen. 2016, 24, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.W.; Nguyen, T.M.; Xia, J.; Arnal, B.; Wong, E.Y.; Pelivanov, I.M.; O’Donnell, M. Real-time integrated photoacoustic and ultrasound (PAUS) imaging system to guide interventional procedures: Ex vivo study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Boas, D.A. Monte Carlo Simulation of Photon Migration in 3D Turbid Media Accelerated by Graphics Processing Units. Opt. Express 2009, 17, 20178. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Chamberlain, S.R.; Falcone, D.R.; Bruce, R.; Heinmiller, A.; Xia, J.; Seshadri, M. Performance Characteristics of Photoacoustic Imaging Probes with Varying Frequencies and Light-delivery Schemes. Ultrason. Imaging 2019, 41, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Pavan, T.Z.; Madsen, E.L.; Frank, G.R.; Carneiro, A.A.O.; Hall, T.J. Nonlinear elastic behavior of phantom materials for elastography. Phys. Med. Biol. 2010, 55, 2679. [Google Scholar] [CrossRef]
- Uliana, J.H.; Sampaio, D.R.T.; Carneiro, A.A.O.; Pavan, T.Z. Photoacoustic-based thermal image formation and optimization using an evolutionary genetic algorithm. Res. Biomed. Eng. 2018, 34, 147–156. [Google Scholar] [CrossRef]
- Naser, M.A.; Sampaio, D.R.; Muñoz, N.M.; Wood, C.A.; Mitcham, T.M.; Stefan, W.; Sokolov, K.V.; Pavan, T.Z.; Avritscher, R.; Bouchard, R.R. Improved Photoacoustic-Based Oxygen Saturation Estimation With SNR-Regularized Local Fluence Correction. IEEE Trans. Med. Imaging 2019, 38, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, X.; Jin, H.; Zhang, R.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Handheld Photoacoustic Imager for Theranostics in 3D. IEEE Trans. Med. Imaging 2019, 38, 2037–2046. [Google Scholar] [CrossRef]
- Singh, C.P.; Bindra, K.S.; Bhalerao, G.M.; Oak, S.M. Investigation of optical limiting in iron oxide nanoparticles. Opt. Express 2008, 16, 8440. [Google Scholar] [CrossRef]
- Jacques, S.L.; Prahl, S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water. Available online: https://omlc.org/spectra/hemoglobin/index.html (accessed on 16 July 2020).
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M.; Boone, J.M. The Essential Physics of Medical Imaging, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Alles, E.J.; Jaeger, M.; Bamber, J.C. Photoacoustic clutter reduction using short-lag spatial coherence weighted imaging. IEEE Int. Ultrason. Symp. IUS 2014, 41–44. [Google Scholar] [CrossRef]
- Netter, F.H. Atlas of Human Anatomy, 6th ed.; Sauders Elsevier: Philadelphia, PA, USA, 2014; Volume 1. [Google Scholar]
- Lee, H.D.; Shin, J.G.; Hyun, H.; Yu, B.A.; Eom, T.J. Label-free photoacoustic microscopy for in-vivo tendon imaging using a fiber-based pulse laser. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Deana, A.M.; Yoshimura, T.M.; da Silva, D.F.; França, C.M.; Hamblin, M.R.; Ribeiro, M.S. The optical properties of mouse skin in the visible and near infrared spectral regions. J. Photochem. Photobiol. B Biol. 2016, 160, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J. The Anatomy of the Laboratory Mouse; Academic Press: London, UK; New York, NY, USA, 1965. [Google Scholar]
- Choi, W.; Park, E.Y.; Jeon, S.; Kim, C. Clinical photoacoustic imaging platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Phantom | Finger and Forearm | Balb/C Mouse |
|---|---|---|---|
| Angle step | 2° | 4° | 4° |
| Elevation step | 2 mm | 2 mm | 2 mm |
| Image axial | 45 mm | 35 mm | 35 mm |
| Image lateral | 38 mm | 38 mm | 38 mm |
| Number of angle steps (n) | 9 | 4 | 4 |
| Number of elevation steps | 19 | 9 | 5 |
| Number of frames per angle () | 2 | 2 | 2 |
| Wavelength | 720 nm | 800 nm | 800 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uliana, J.H.; Sampaio, D.R.T.; Fernandes, G.S.P.; Brassesco, M.S.; Nogueira-Barbosa, M.H.; Carneiro, A.A.O.; Pavan, T.Z. Multiangle Long-Axis Lateral Illumination Photoacoustic Imaging Using Linear Array Transducer. Sensors 2020, 20, 4052. https://doi.org/10.3390/s20144052
Uliana JH, Sampaio DRT, Fernandes GSP, Brassesco MS, Nogueira-Barbosa MH, Carneiro AAO, Pavan TZ. Multiangle Long-Axis Lateral Illumination Photoacoustic Imaging Using Linear Array Transducer. Sensors. 2020; 20(14):4052. https://doi.org/10.3390/s20144052
Chicago/Turabian StyleUliana, João H., Diego R. T. Sampaio, Guilherme S. P. Fernandes, María S. Brassesco, Marcello H. Nogueira-Barbosa, Antonio A. O. Carneiro, and Theo Z. Pavan. 2020. "Multiangle Long-Axis Lateral Illumination Photoacoustic Imaging Using Linear Array Transducer" Sensors 20, no. 14: 4052. https://doi.org/10.3390/s20144052
APA StyleUliana, J. H., Sampaio, D. R. T., Fernandes, G. S. P., Brassesco, M. S., Nogueira-Barbosa, M. H., Carneiro, A. A. O., & Pavan, T. Z. (2020). Multiangle Long-Axis Lateral Illumination Photoacoustic Imaging Using Linear Array Transducer. Sensors, 20(14), 4052. https://doi.org/10.3390/s20144052






