1. Introduction
The current energy transition from fossil fuels to renewable sources is a major worldwide challenge. One of the potential methods to facilitate this transition is the use of natural gas as a replacement of oil-based products in heating and transportation. This may facilitate a possible future transition to biogas and other renewable sources. Since natural gas is not produced at the locations of consumption, it is often compressed and cooled to Liquified Natural Gas (LNG) and transported by ships all over the world. LNG plays an increasingly important role as a transport fuel in gas and dual-fuel engines. However, it is well known that the composition of LNG depends heavily on the source and upgrading process. This composition has a major influence on the energy content and combustion properties of the fuel. Monitoring gas compositions and the associated Methane Number (MN) is critical for the optimal functioning of these engines when using LNG from different sources [
1,
2,
3]. The methane number is a measure for the LNG quality or combustion performance of the gas.
Several sensor solutions have been proposed for the assessment of the composition and quality of natural gas, of which the majority are based on large laboratory equipment, such as Raman spectroscopy [
4,
5,
6], gas chromatography [
7], and infrared spectroscopy [
8,
9]. These laboratory analytical techniques generally perform very well with respect to selectivity and sensitivity but are too costly for ubiquitous gas quality monitoring for LNG engines. As a lower cost and smaller alternative, some new developments have been presented in the field of miniaturized micro electromechanical system (MEMS)-based natural gas sensors [
10,
11,
12], which are based on the optical interaction of infrared light and chemical species present in the gas. One of the major challenges in the infrared-based detection of the components in (liquified) natural gas is the high chemical similarity of the hydrocarbon species. For this reason, an alternative gas sensor was developed with capacitive transducer technology, which enables the differentiation of various hydrocarbons in a natural gas mixture based on the combination of chemical interaction, critical temperature, boiling point, and size. This technology was presented in a previous paper for its relevance for the assessment of the composition of LNG, and was based on the absorption of gas molecules in porous absorbing coatings applied to a capacitive sensing platform [
13,
14,
15]. In this paper, more detailed results were presented and a correlation was made with the Methane Number. A series of 16 reference gas mixtures (having high concentrations of n- and iso-butane, and n- and iso-pentane) were assessed with the new capacitive sensor array (CSA). The results, i.e., selectivity and sensitivity, were compared to a MEMS benchmark technology based on tunable filter infrared spectroscopy (TFIR) [
16,
17].
The previous study [
15] showed that the selectivity of the sensor array was very good for higher hydrocarbons larger than propane but showed some significant cross-sensitivity for ethane and propane. In the present study, we modified some of the coatings used to reduce the cross-sensitivity and optimized the data processing in order to improve the selectivity of the lower hydrocarbons. In addition to the data from the coated chips, we also included a few combinations of coated chips (e.g., product or ratio of two chips). In this way, the temperature influence could be reduced and the responses to some gasses could be amplified. In this paper, we also calculated the MN of the various gas mixtures and assessed the optimal way of calculating the MN. We used the NPL (National Physics Laboratory, UK) algorithm [
18] as a benchmark to calculate the MN from the composition.
2. Materials and Methods
2.1. Capacitive Sensor Array—Technology
The development and construction of the capacitive sensor array has already been discussed in detail in the previous paper [
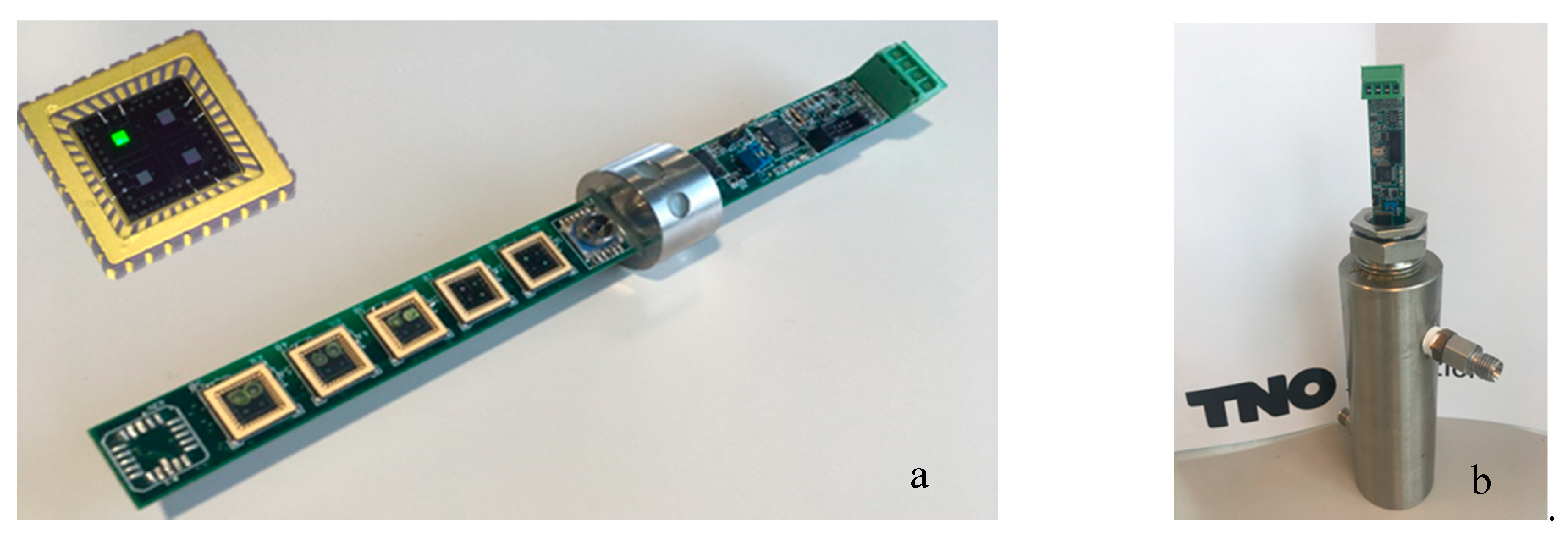
15]. In short, a series of capacitive sensor chips, consisting of aluminum interdigitated electrodes on a silicon substrate, was coated with the responsive coatings. The sensing chips were bonded to a Printed Circuit Board (PCB). The coatings absorbed the gasses in the LNG gas mixtures, which resulted in a change in the dielectric constant of the coating. This change was measured by the interdigitated electrode using AD7746 Capacitance-to-Digital converter chips (Analog Devices, Norwood, MA, USA), connected to the PCB. A microprocessor was applied to the PCB to read the digital signals from the AD chips to preprocess the temperature, pressure, and sensor chip data into a readable table and transmit the data via a USB connection to an external PC or laptop. The sensor is shown in
Figure 1.
In the previous study [
15], a series of eight coatings was synthesized, which gave a reasonable sensitivity and selectivity of the measured gasses over a large concentration range. We concluded that the cross-sensitivity of ethane and propane was high, and tried to mitigate this by the implementation of several alternative coatings. The list of coatings used is shown in
Table 1.
Three coating formulations listed in
Table 1 have been changed with respect to the previous study: Two MOF coatings and one Z26 coating, having no polymeric binder, and HAD, which is a soluble fluoropolymer Poly(tetrafluoroethylene-co- 2,2,4-trifluoro-5-trifluoromethoxy-1,3-dioxole) (Hyflon AD 40 HS, Merck, Darmstadt, Germany).
In addition to the values of the capacitances, a temperature and pressure reading was included in the data file. The temperature chip had a resolution of 0.1 °C, and the pressure sensor of 1 mbar. The microprocessor can send the data to the external computer with a frequency of ca 0.1 Hz.
2.2. Capacitive Sensor Array—Exposure Experiments
The sensor array that was manufactured, having ten coated electrodes, one temperature sensor, and one pressure sensor was exposed to various gas mixtures containing methane, ethane, propane, n-butane, iso-butane, n-pentane, iso-pentane, and nitrogen. A series of 20 different gas mixtures was used to calibrate the sensor array. The final validation was done with a series of 16 gas mixtures that were prepared at the Technical University of Braunschweig (TUBS) and calibrated at Physikalisch-Technische Bundesanstalt (PTB) [
19]. This series was used for the comparison of the capacitive sensor and the Tunable Filter Infrared sensor. The pressure was set at 1.2 bar(a), and the temperature was not regulated, but only changed a few degrees during the experiment (~26 °C to 27 °C) as a result of fluctuating laboratory temperature and heating of the sensor electronics. The temperature difference between the calibration and validation experiments was within 1 °C. The composition of the gas mixtures was regulated by flow controllers (Bronkhorst High Tech, Ruurlo, Netherlands) and validated using a gas chromatograph (Compact GC4.0, Global Analyzer Solutions, Breda, Netherlands). The exposure time of the sensor to the gas mixtures was three hours for each mixture. This long period was chosen because some of the sensor chip respond relatively slow to changes in gas composition. The most important reason is the presence of contamination in the gas tubes in the gas mixing station. The calibration mixtures are compiled from the separate gasses, and when very low amounts of, e.g., butane or pentane are needed, the flow through the tubes is very low. This means that it can take a long time before all gasses are mixed and fed to the sensor in the required composition. In addition, when starting up the experiment or a flow in a tube, some water that has migrated into the system during hours of nonoperation needs to be removed first, before a reliable measurement can be done. The validation gas mixtures do not need to be compiled from the separate gasses, so exposure to the sensor can be shorter. Nevertheless, an exposure time of three hours was used as well for the validation.
The sensor array was first calibrated using a selection of the gas mixtures, and subsequently validated using the set of 16 mixtures that was supplied by TUBS/PTB [
19]. The process and results of both the calibration and validation tests of a first series of experiments was discussed in the previous paper [
15]. In this paper, we mainly focused only the results of the validation tests on the 16 standard gas mixtures from TUBS/PTB.
The 16 standard gas mixtures that were used for the comparison between the two sensing solutions are listed in
Table 2. The values in the last columns are the Methane Numbers calculated from the composition using the NPL algorithm (MN(NPL)) and determined by TUBS/PTB using the engines test (MN(TUBS)).
The data processing has also been explained previously using simple multivariate matrix calculations. First a calibration matrix was generated using the test results of the calibration experiments. Then, this calibration matrix was used to calculate the composition and methane numbers from the chip data. In this paper, the processing was slightly different from the previous paper [
15]. Instead of using only the ten sensor chips, in the current paper, we also used combinations of two chips: One additional signal for the product of two chips (Ch
n × Ch
m), and one additional signal for the ratio of two chips (Ch
n/Ch
m). The extra signal of the product of two chips amplifies some of the signals for specific gasses, while the extra signal of the ratio of two chips reduces some of the responses that may cause cross-sensitivity and also reduces temperature effects. Since the data processing was multivariate linear regression using only linear dependencies on chip responses, combinations of chip responses were not included. So, by adding these two combined chip responses, we obtained twelve unique chip signals for the calculation of eight gas concentrations (CH
4, C
2H
6, C
3H
8, n-C
4H
10, iso-C
4H
10, n-C
5H
12, iso-C
5H
12, N
2). These two additional signals (Ch
n × Ch
m) and (Ch
n/Ch
m) were added to the response matrix in Equation (1). The chip numbers
n and
m are different for each gas and are listed in
Table 3. This means that the calibration procedure for each gas is different (using different combinations of signals). For the validation test, the same combinations of signals must be used. The temperature and pressure were not incorporated in the calibration and validation, since they were kept constant during all tests at 26.4 ± 0.4 °C and 1.2 ± 0.01 bar(a), respectively. When temperature and pressure fluctuate more, these parameters must be also included in the chip responses of Equation (1):
The gasses are listed in
Table 3. This can be simplified to:
[
M] is the response matrix for all chips. For the calculation of the gas concentrations from the chip responses, the relation has to be rewritten into:
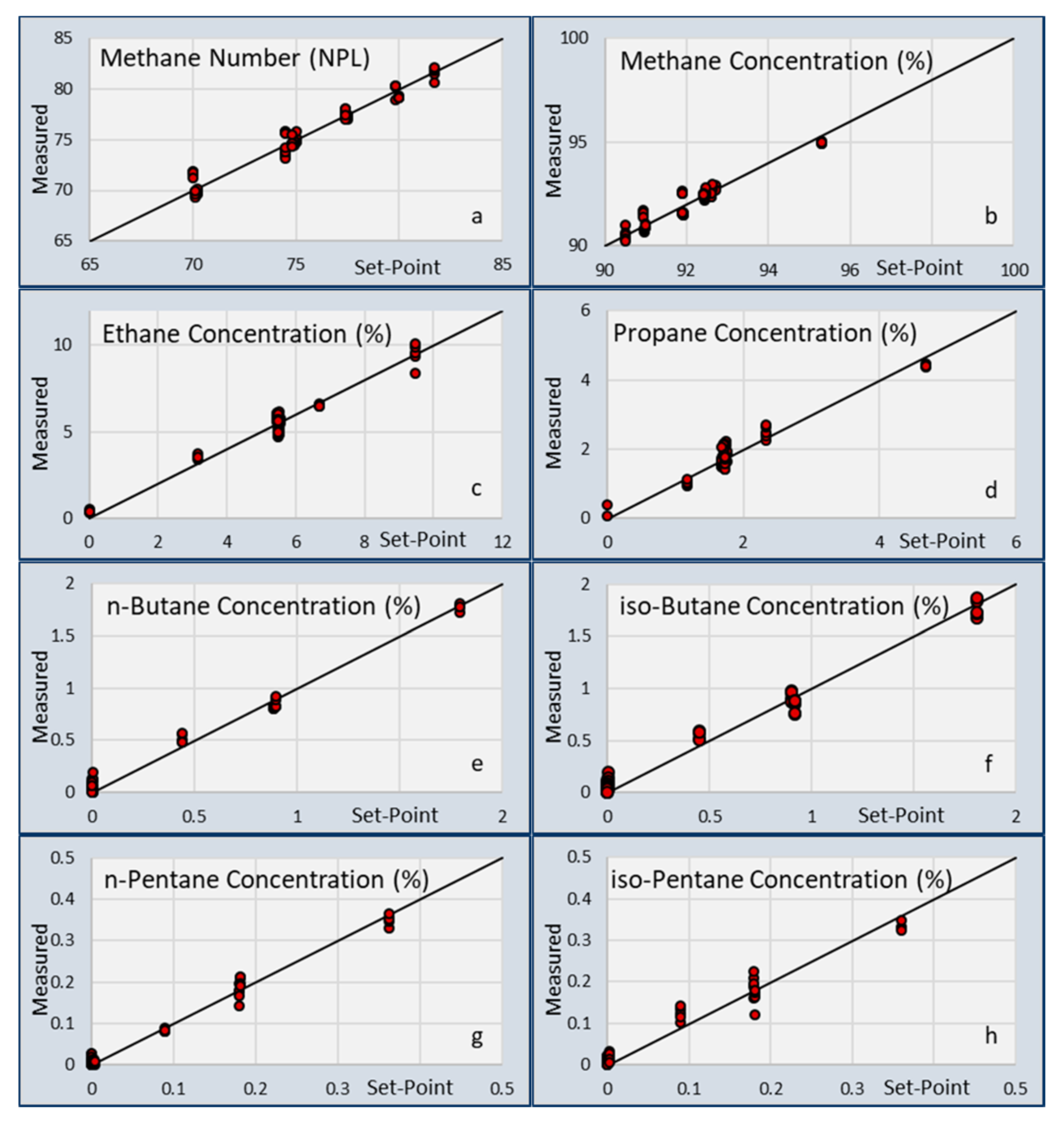
So, after obtaining the calibration matrix [M] from the calibration experiments, Equation (3) can be used to calculate the gas concentrations from the chip responses. The accuracy of this calculation was assessed by comparing the calculated gas concentration to the setpoint gas concentration and calculate the standard deviation of the difference.
2.3. Methane Number
The Methane Number of an LNG fuel is a measure for the combustion performance, just like the Octane Number for petrol engines. Unfortunately, there is no analytical relationship between the fuel composition and the MN. This parameter must be measured experimentally using a well-calibrated engine. In theory, a fuel with a MN of 100 has similar combustion properties as 100% methane. A fuel with a lower MN (e.g., 90) behaves like a mixture of methane and hydrogen (i.e., 90% CH4 and 10% H2). By assessing the knocking behavior of methane/hydrogen mixtures and comparing these with actual fuels, the experimental MN is obtained.
However, for sake of simplicity, to enable the calculation of the MN from the composition and not only rely on experimental engine tests, much research has been conducted to calculate or approach the MN using analytical models, such as Anstallt für Verbrennungsmotoren (AVL), Motorenwerke Mannheim (MWM), National Physical Laboratory (NPL), and Wärtsilä (WMN) [
20,
21]. Unfortunately, all of these MN calculators give different results. In the current paper, we used the NPL algorithm as a benchmark to calculate the MN of the calibration and validation gasses, and the values for the calculated MN from the composition that were generated by the sensors [
18].
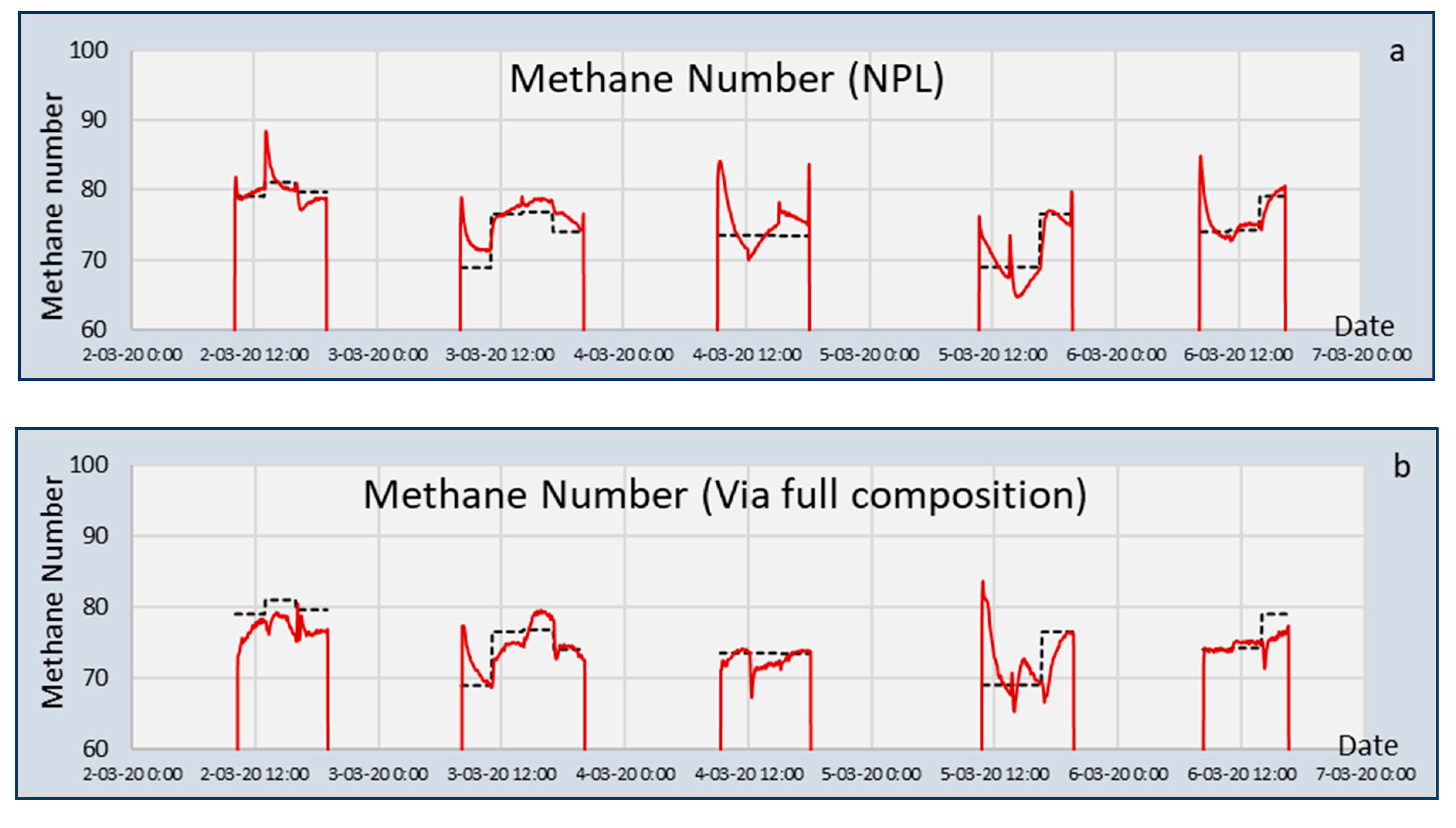
In addition to the calculation of the MN, the actual values of the sixteen validation gasses were determined by TUBS/PTB by engine tests [
19]. The values for the MN of these mixtures, as calculated using the NPL algorithm and as measured, are listed in
Table 2. The standard deviation of the difference between the two values was 1.18, with a very large contribution to this uncertainty of MIX3 containing just methane (94.6%), propane (4.9%), and nitrogen (0.5%).
2.4. Tunable Filter Infrared Sensor—Technology
In a TFIR detection method, the infrared (IR) light is passed through a gas mixture and analyzed using a MEMS-based IR filter and low-cost detector. This technology combines the small footprint and low-cost features from MEMS products with the chemical accuracy of IR analytical techniques. A great benefit of these IR-based methods with respect to other chemical sensors is the fact that the sensor is not in contact with the target gas, and can operate relative remotely from the cold fuel or hot exhaust gasses. Therefore, it is also typical to apply TFIR sensors as independent extractive gas analyzers in the same way as, e.g., analyzers based on Fourier transform infrared (FTIR) spectroscopy. However, being a simpler technique with no moving parts, TFIR is potentially more robust and economic solution than FTIR in applications where moderate sensitivity in sub-percentage levels of gas concentration is adequate.
The TFIR sensor applied in this work has been explained in more detail by the authors of [
15]. It was based on NIRONE NIR Spectral Sensor module at wavelength range 2.0–2.45 µm by Spectral Engines Ltd. The module was connected with NIR broadband light source and gas absorption cell using optical fibers. The optical path length of the sample cell was 400mm, the cell volume was approximately 200 mL, and the cell was temperature controlled. The gas sample cell output was open to ambient air and the actual measurements took place in ambient pressure. The Spectral Engines Sensor Control software was used for system control and collection of measured spectra.
2.5. Tunable Filter Infrared Sensor—Exposure Experiments
In all absorption spectrum measurements with the TFIR sensor sample, the cell temperature was set to a constant temperature of 45.0 °C. The sample gas was flowing continuously through the sample cell and the sample cell output was in ambient pressure. The typical gas flow rate was 1–2 L/min, and accordingly, gas exchange time for the gas cell and system response time was approximately 10 s. The background spectrum was measured using pure nitrogen when starting the measurements of the day, and the stability of the sensor was controlled by occasionally measuring nitrogen again. During a set measurement time of two seconds, a total of 100 spectra was collected internally by the control software and their average was calculated as an output and recorded. Furthermore, typically 10 or more consecutive series of spectra were recorded during a one-minute-maximum actual measurement time for each gas mixture in order to estimate standard deviation of the results. The average of these spectra was used as calculated measurement result for the measured gas mixture.
4. Discussion
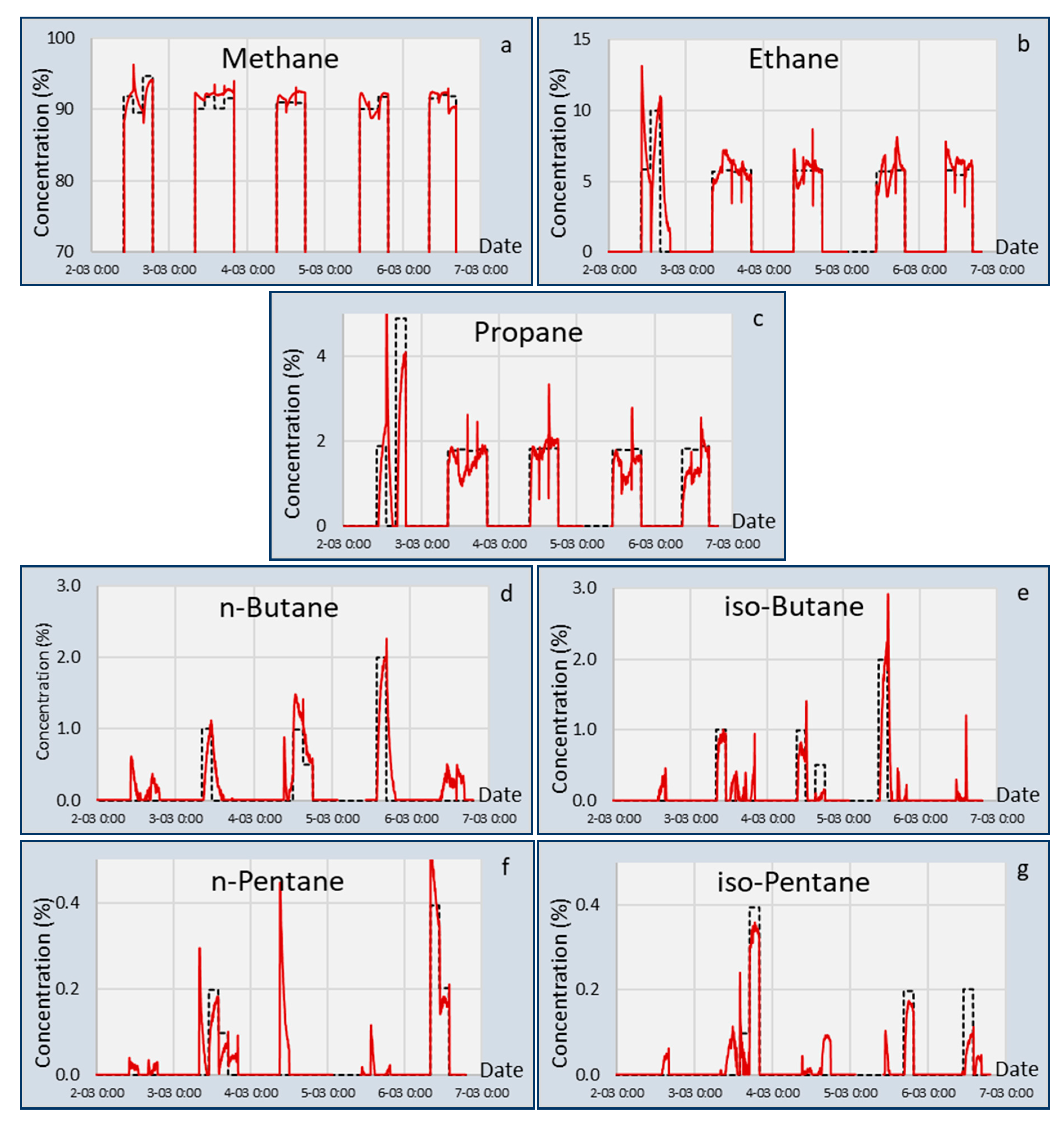
In
Section 3, the results of the LNG composition and Methane Number measurements are presented using the two sensors: The capacitive sensor array (CSA) and tunable filter infrared sensor (TFIR). The 95% confidence intervals of the experiments of
Figure 4,
Figure 5 and
Figure 7 are listed in
Table 5 for the measured gasses and Methane Number. It is apparent that both sensors showed different features, but both calculated the Methane Number with a good 95% CI below 0.5 units, although the stability of the TFIR signal was better than the CSA signal. For the individual gasses, it was observed that the CSA 95% CI for methane was better and for ethane was poorer than the TFIR values. Furthermore, those for propane and butane were larger and for pentane were smaller than FTIR. This is an indication that the TFIR is more accurate for ethane, but can detect the pentanes only with a large error. This is also reflected in
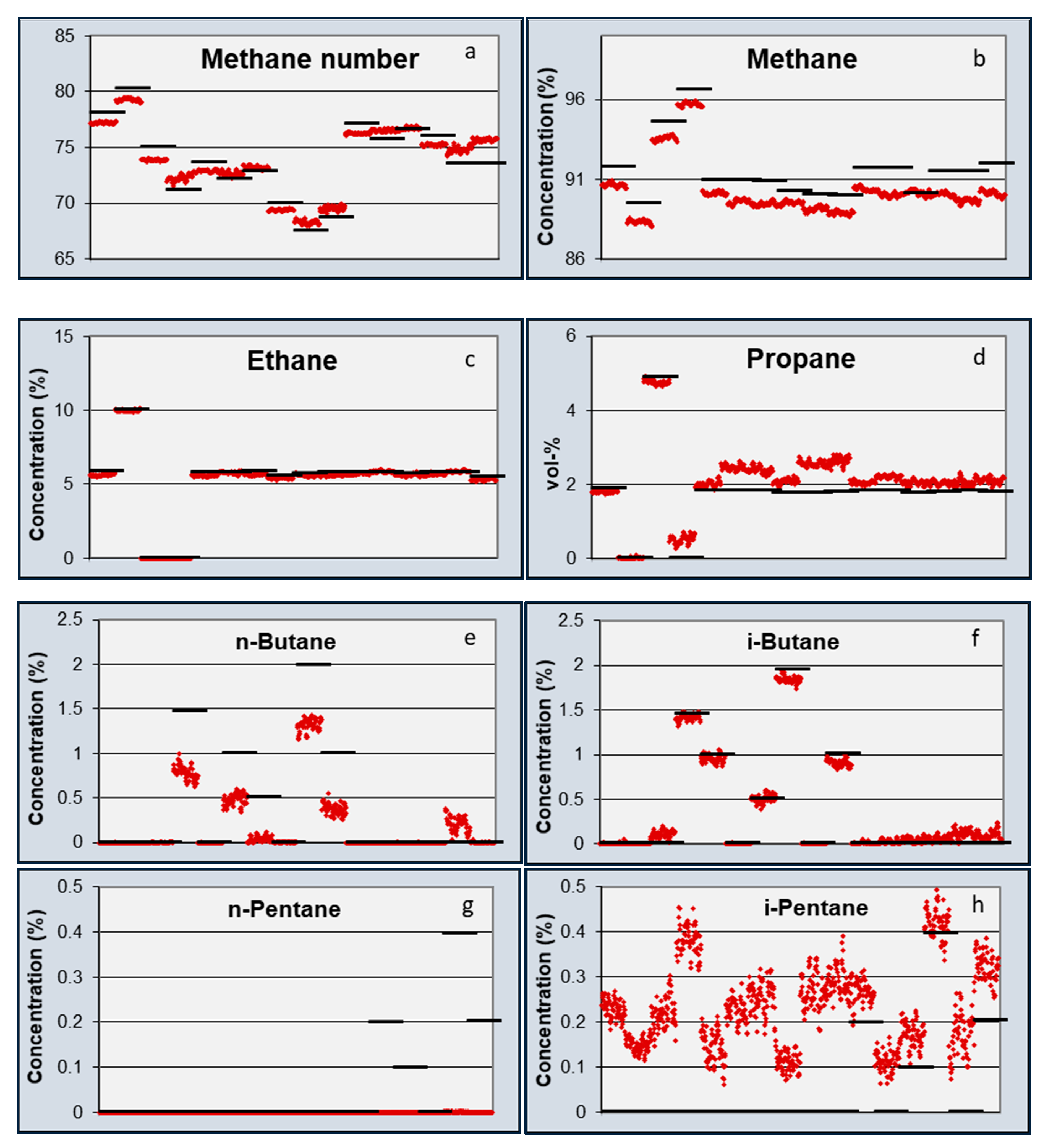
Table 6. From this table, it becomes clear that the TFIR sensor is more sensitive for the smaller hydrocarbons, and the CSA shows a higher accuracy for the larger hydrocarbons. Notably, ethane was still difficult to measure with the CSA, and gave a very accurate value using the TFIR. The reason for this difference may be found in the difference in sensing mechanism. The TFIR measured all gas components by the infrared assessment of the presence of chemical bonds in the mixtures. Every chemical bond has a proportional contribution to the total signal. So, low concentrations of components give a small contribution and generate a high uncertainty in the concentrations. The CSA is based on the absorption of gas components in responsive coatings. Since the absorption depends on the interaction between the gas and the coating chemistry, the absorption of one gas can be significantly higher than another gas. This is the case for the higher hydrocarbons that are preferably absorbed by most of the coatings. Another difference can be seen between the absorption of iso- and n-alkanes in the coatings. The signals of the n-alkanes were much higher than the iso-alkanes, which is represented in the higher accuracy of the n-alkanes in
Table 6.
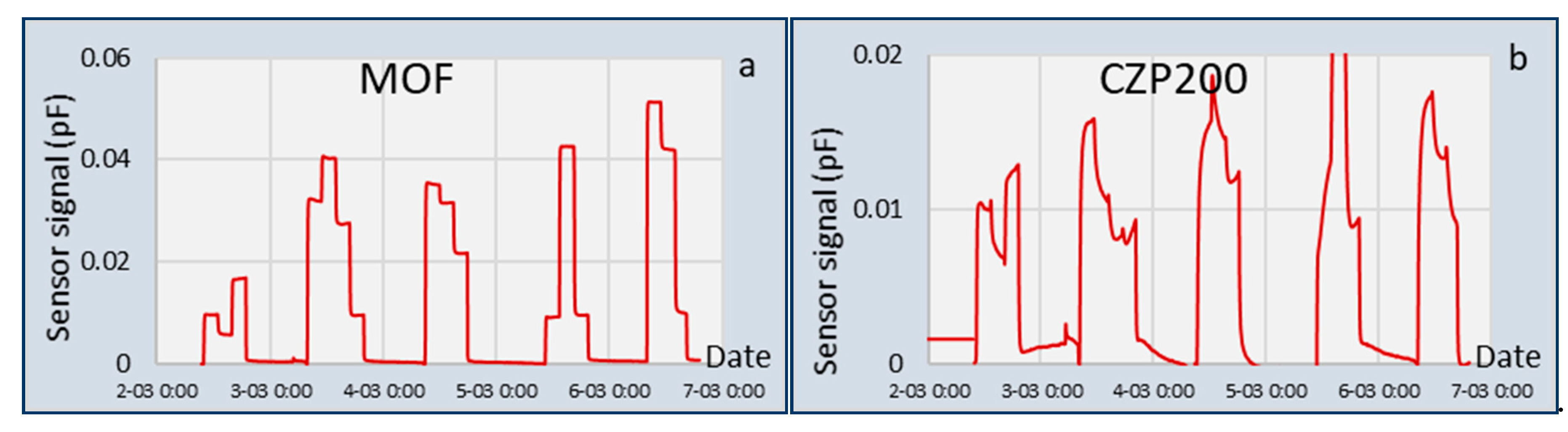
For the application of the two sensors for LNG composition measurements, some additional differences can be observed. The response times measured for the CSA were higher than for the TFIR. This was partly due to the experimental setup, in which the gas mixtures required some time to replace the previous mixture in the exposure chamber and remove all the water that was introduced by the switching of the gasses. However, the slower absorption rate of some gasses into the coatings also worsens the response times. When composition changes are small and slow, which typically is the case for an LNG burning engine using fuel from a large tank, it is expected that the CSA can follow these changes nicely, although the TFIR will be a faster sensor in all cases.
Second, once calibrated, the CSA can operate for a long period of time without additional calibration or baseline correction [
13]. The validation experiments of the CSA lasted for five days and started two weeks after the calibration. A nitrogen baseline was measured just before the first exposure to MIX1 and was used for the whole series of experiments. The TFIR used a new nitrogen baseline in the beginning of the measurement day. This is possible in laboratory conditions but will be a challenge with in-line field implementation. Accordingly, high stability of the system construction is a benefit.
Furthermore, pressure and temperature corrections need to be included for both sensors. In the current paper, pressure and temperature were kept relatively constant and TFIR gas sample cell was temperature controlled, which contributed to the very good results. In practice, it has been shown for the CSA that pressure and temperature compensation are possible, provided the calibration also includes these temperature and pressure ranges [
13]. The pressure compensation of TFIR sensor results is possible as well.
The Methane Numbers have been measured and calculated using several approaches. All of these values are listed in
Table 7. It is clear that every approach resulted in a different value. There were also some differences between the calculated values using the two algorithms and the experimental values obtained by TUBS/PTB. The standard deviation of the differences between the experiments and the algorithms was around 1 MN unit. This shows that every way of obtaining the Methane Number has its own uncertainties. The values for the spark-advanced service Methane Number (SA-SMN) were taken from [
19]. The 95% confidence intervals obtained for these experiments were in the range between 0.2 MN and 0.9 MN units, which is larger than the range of 0.25 MN to 0.5 MN units that was found for the CSA and TFIR sensors.
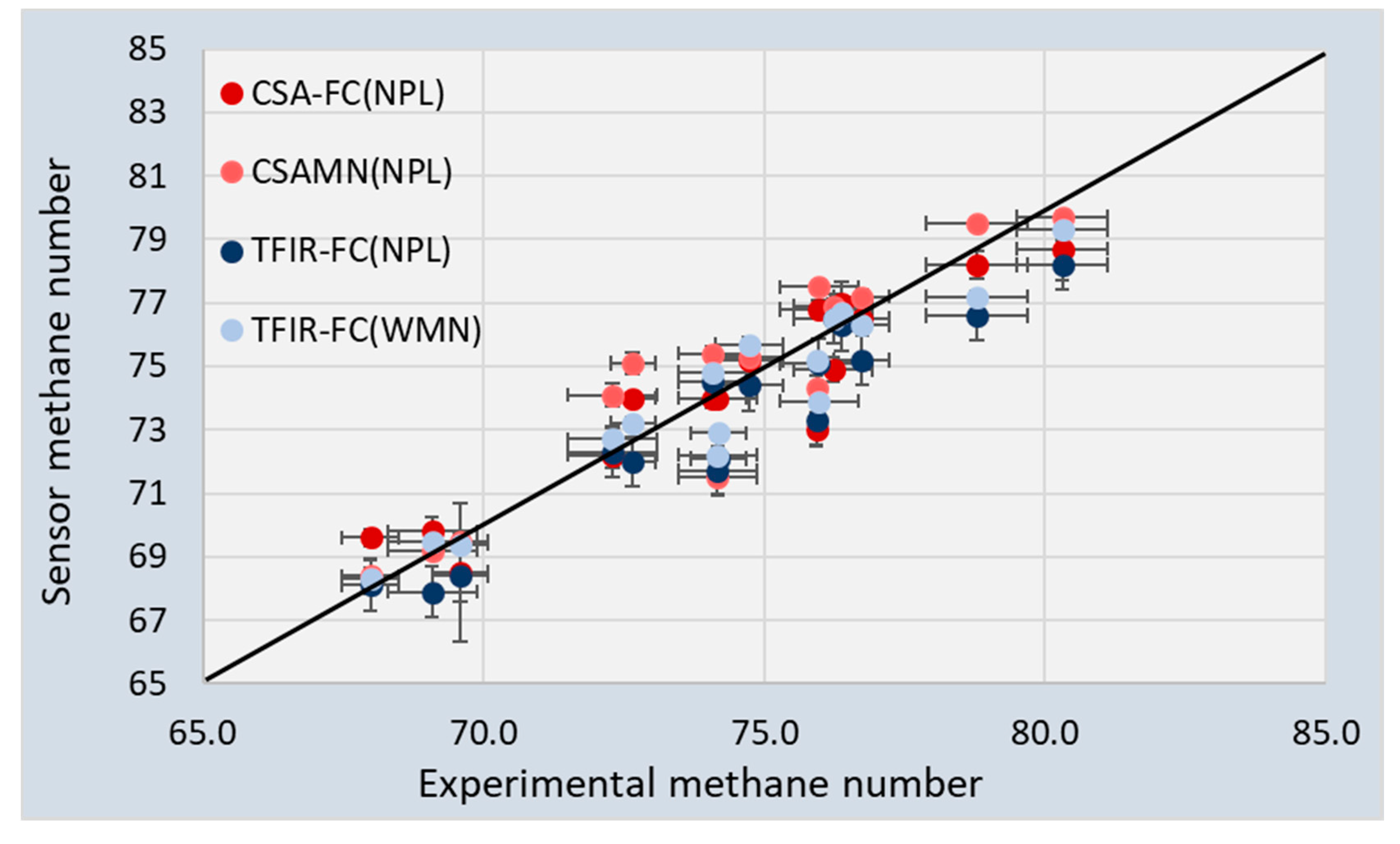
Figure 8 compares the sensor MNs with the experimental values (SA-SMN). The sensor data is not equally distributed above and below the black line. Furthermore, some of the sensor datapoints show a large deviation from the experimental value for all approaches. The 95% CI is shown in
Figure 8 for both the sensor data and experimental data.
It was found that the 95% CI for the Methane Numbers for both sensors was 0.25–0.50 vol%.
Figure 8 shows that the 95% CI for the experimental values was much larger: Between 0.2 and 0.9 vol% [
19].
An overview of the maximum and average deviation is listed in
Table 8. The table shows that the maximum differences between the calculated and the experimental Methane Numbers were similar for all approaches, at around 3 MN.
Figure 8 and
Table 8 show that the TFIR generally underestimated the MN with respect to the experimental values. On average, both CSA results had a standard deviation of 1.2–1.3. The TFIR results were slightly better when the WMN algorithm was used. A reason for this may be found in the slightly better correlation between WMN algorithm and SA-SMN. Furthermore, the results of the NPL and WMN algorithms using the GC data also showed a standard deviation of 1.1–1.2. This all indicates that the accuracy of both sensors is similar and can be compared very well with mathematical solutions. For the assessment of the Methane Number, the two sensors can be used equally well as a gas chromatograph.
The comparison of the presented sensors with alternative sensor solutions for the detection of the gas composition in a fuel or gas line is difficult, since there are not many examples published that enable the measurement of Methane Numbers. Many examples were presented in the literature for the measurement of methane concentrations (e.g., [
22,
23]). However, for the measurement and monitoring of the Methane Number or Wobbe Index, the detection of methane is not sufficient. A full composition of the fuel gasses is needed. Most full gas composition monitoring systems are based on GC or Raman, and they perform better than the capacitive sensor or the TFIR sensor with respect to selectivity (typical 0.1–0.3 vol-% accuracy), but this comes with a much higher price. Other composition monitoring solutions are comprised of a combination of various physical sensors (thermal conductivity, speed of sound, density, infrared sensor [
24,
25]) presented an accuracy of ~1% in the calculated concentrations or Wobbe Index. The accuracy in the results of such combined physical sensor approaches decreases upon the increase of the number of components in the gas. The authors of [
25] showed that the accuracy decreased from 1% for binary mixtures to 3% for ternary mixtures. The authors of [
24] presented an accuracy of 1% in the Wobbe Index, but no composition or Methane Number was measured or calculated. The FTIR solution by the authors of [
19] only calculated the Methane Number with a comparable accuracy as found in the present paper (~1.5 MN units) but required a large laboratory device.