Muscle Synergies in Parkinson’s Disease
Abstract
1. Introduction
2. Muscle Synergies: Theoretical Background
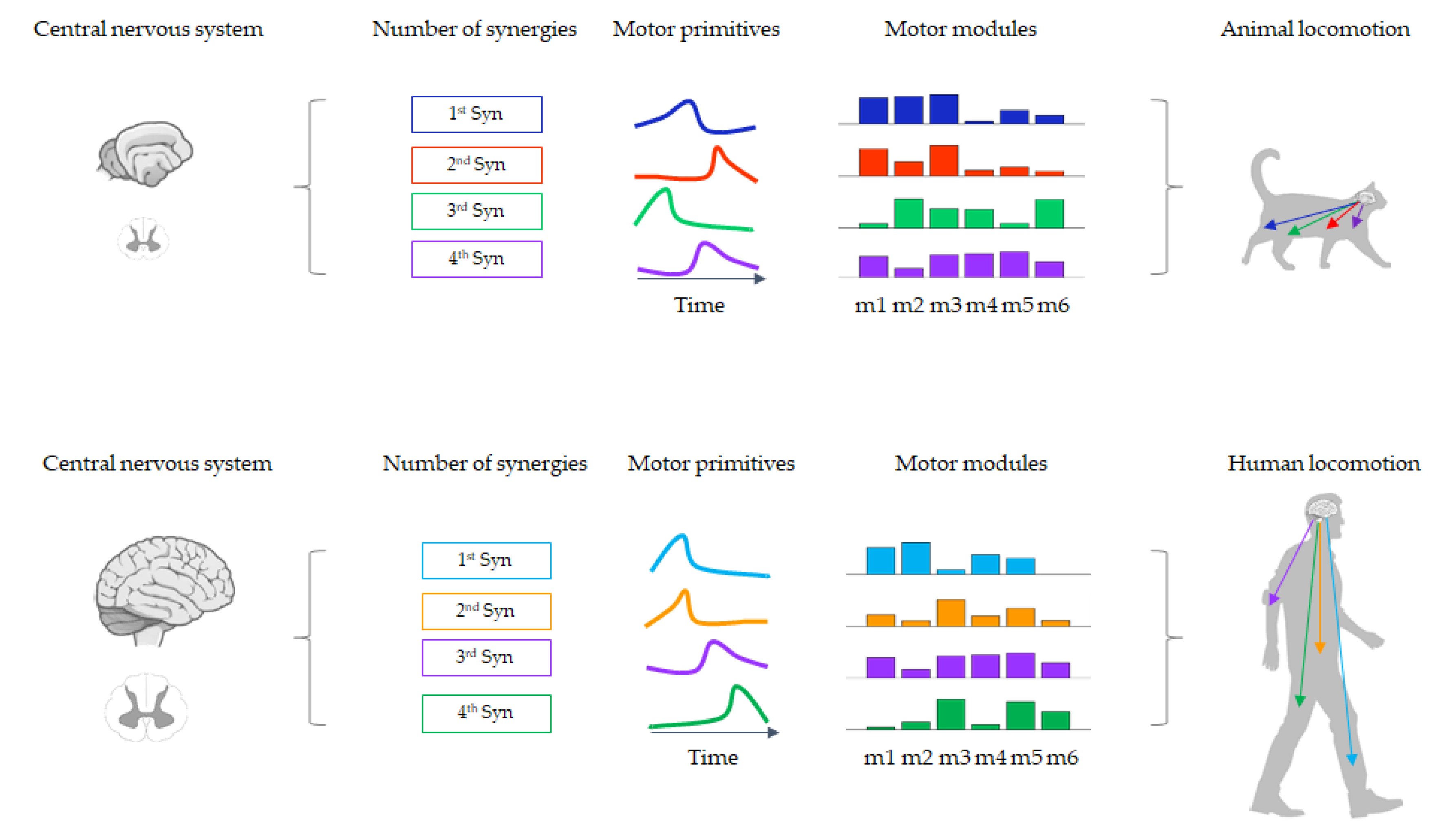
2.1. The Modularity of Movement and Muscle Synergies
2.2. Methods for Muscle Synergy Extraction
3. Literature Search Strategies and Criteria
4. Muscle Synergies in Parkinson’s Disease
4.1. Balance
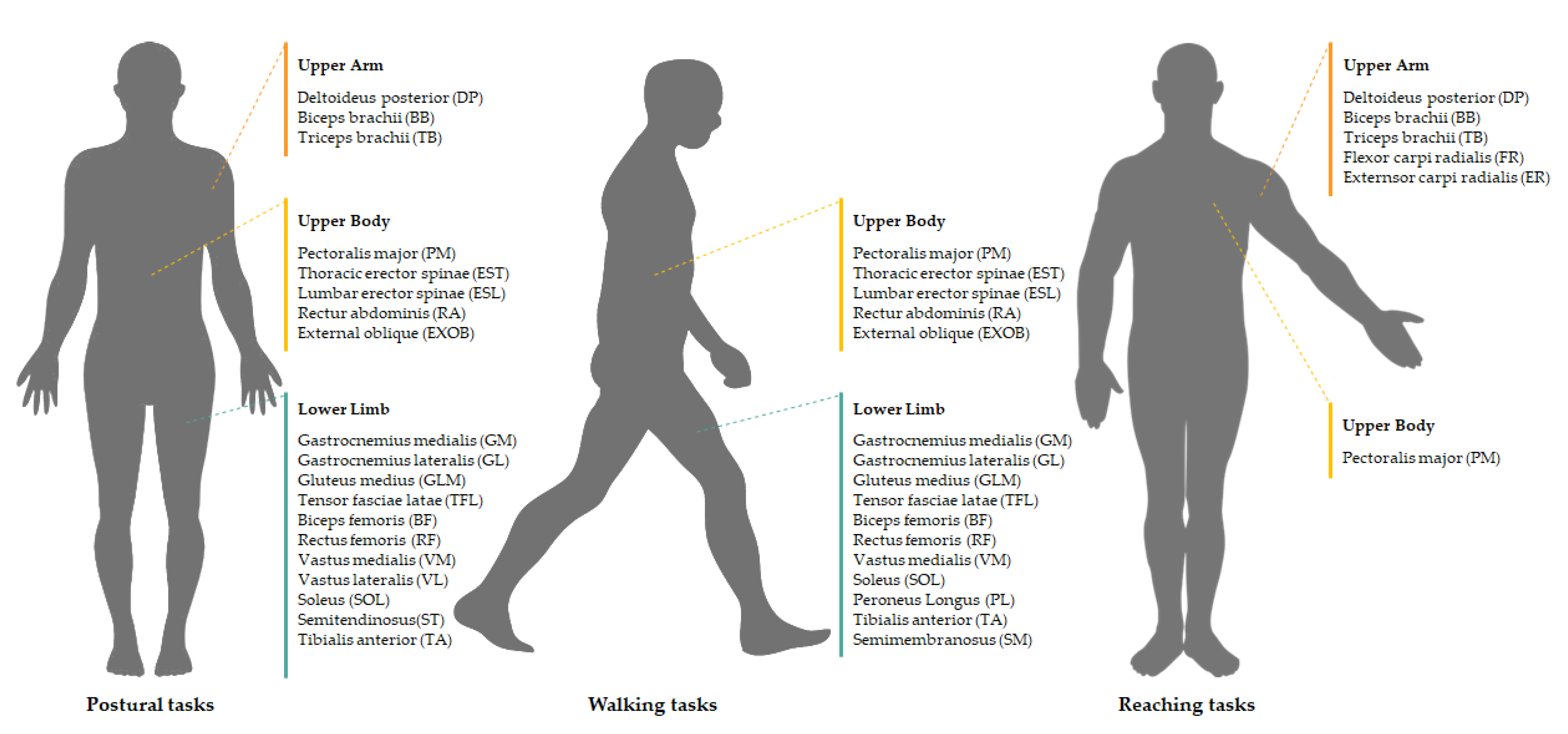
4.2. Locomotion
4.3. Upper Limb Movements
5. Discussion
6. Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| DBS | Deep Brain Stimulation |
| ELM | Extreme Learning Machine |
| EMG | Electromyography |
| H&Y | Hoehn and Yahr |
| HS | Healthy Subjects |
| ICA | Independent Component Analysis |
| M1 | Primary Motor Cortex |
| NMF | Non-Negative Matrix Factorization |
| PCA | Principal Component Analysis |
| PD | Parkinson’s Disease |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VAF | Variability accounted for |
References
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Suppa, A.; Bologna, M.; Conte, A.; Berardelli, A.; Fabbrini, G. The effect of L-dopa in Parkinson’s disease as revealed by neurophysiological studies of motor and sensory functions. Expert Rev. Neurother. 2017, 17, 181–192. [Google Scholar] [CrossRef]
- Okun, M.S. Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2012, 367, 1529–1538. [Google Scholar] [CrossRef]
- Debû, B.; Godeiro, C.D.O.; Lino, J.C.; Moro, E. Managing Gait, Balance, and Posture in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 23. [Google Scholar] [CrossRef]
- Suppa, A.; Kita, A.; Leodori, G.; Zampogna, A.; Nicolini, E.; Lorenzi, P.; Rao, R.; Irrera, F. l-DOPA and Freezing of Gait in Parkinson’s Disease: Objective Assessment through a Wearable Wireless System. Front. Neurol. 2017, 8, 406. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Crouse, J.J.; Phillips, J.R.; Jahanshahi, M.; Moustafa, A.A. Postural instability and falls in Parkinson’s disease. Rev. Neurosci. 2016, 27, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Bharti, K.; Suppa, A.; Tommasin, S.; Zampogna, A.; Pietracupa, S.; Berardelli, A.; Pantano, P. Neuroimaging advances in Parkinson’s disease with freezing of gait: A systematic review. NeuroImage. Clin. 2019, 24, 102059. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Mussa-Ivaldi, F.A.; Giszter, S. Computations underlying the execution of movement: A biological perspective. Science 1991, 253, 287–291. [Google Scholar] [CrossRef]
- Tresch, M.C.; Saltiel, P.; Bizzi, E. The construction of movement by the spinal cord. Nat. Neurosci. 1999, 2, 162–167. [Google Scholar] [CrossRef]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Lemay, M.A.; Galagan, J.E.; Hogan, N.; Bizzi, E. Modulation and vectorial summation of the spinalized frog’s hindlimb end-point force produced by intraspinal electrical stimulation of the cord. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 12–23. [Google Scholar] [CrossRef]
- Ting, L.H.; Macpherson, J.M. A Limited Set of Muscle Synergies for Force Control During a Postural Task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef]
- Krouchev, N.; Kalaska, J.F.; Drew, T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J. Neurophysiol. 2006, 96, 1991–2010. [Google Scholar] [CrossRef]
- D’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Akay, T.; Mayer, W.P.; Wells, T.L.; Schroll, A.; Arampatzis, A. Modular organization of murine locomotor pattern in the presence and absence of sensory feedback from muscle spindles. J. Physiol. 2019, 597, 3147–3165. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef] [PubMed]
- D’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef] [PubMed]
- Bernshteĭn, N.A. The Coordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- Greger, R.; Windhorst, U. Comprehensive Human Physiology: From Cellular Mechanisms to Integration; Springer: Berlin, Germany, 1996; ISBN 978-3-642-64619-5. [Google Scholar]
- Lacquaniti, F. Central representations of human limb movement as revealed by studies of drawing and handwriting. Trends Neurosci. 1989, 12, 287–291. [Google Scholar] [CrossRef]
- Tresch, M.C.; Bizzi, E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp. Brain Res. 1999, 129, 401–416. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K.; D’Avella, A.; Saltiel, P.; Tresch, M. Combining modules for movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef]
- D’Avella, A. Modularity for Motor Control and Motor Learning. In Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 2016; Volume 629, pp. 3–19. ISBN 978-0-387-77063-5. [Google Scholar]
- Lee, W.A. Neuromotor synergies as a basis for coordinated intentional action. J. Mot. Behav. 1984, 16, 135–170. [Google Scholar] [CrossRef]
- D’Avella, A. Muscle Synergies. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin, Germany, 2009; pp. 2509–2512. ISBN 978-3-540-29678-2. [Google Scholar]
- D’Avella, A.; Tresch, M.C. Modularity in the motor system: Decomposition of muscle patterns as combinations of time-varying synergies. In Advances in neural information processing systems; Curran Associates: Red Hook, NY, USA, 2002. [Google Scholar]
- Bizzi, E.; Cheung, V.C.K. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013, 7, 1–6. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Avella, A.; Tresch, M.C.; Bizzi, E. Central and Sensory Contributions to the Activation and Organization of Muscle Synergies during Natural Motor Behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef]
- Hart, C.B.; Giszter, S.F. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J. Neurosci. 2004, 24, 5269–5282. [Google Scholar] [CrossRef] [PubMed]
- Torres-oviedo, G.; Ting, L.H. Muscle Synergies Characterizing Human Postural Responses. J. Neurophysiol. 2007, 98, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, S.; Krouchev, N.; Drew, T. Sequential activation of motor cortical neurons contributes to intralimb coordination during reaching in the cat by modulating muscle synergies. J. Neurophysiol. 2011, 105, 388–409. [Google Scholar] [CrossRef] [PubMed]
- Overduin, S.A.; D’Avella, A.; Roh, J.; Bizzi, E. Modulation of muscle synergy recruitment in primate grasping. J. Neurosci. 2008, 28, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Monaco, V.; Ghionzoli, A.; Micera, S. Age-related modifications of muscle synergies and spinal cord activity during locomotion. J. Neurophysiol. 2010, 104, 2092–2102. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef]
- Muceli, S.; Boye, A.T.; D’Avella, A.; Farina, D. Identifying representative synergy matrices for describing muscular activation patterns during multidirectional reaching in the horizontal plane. J. Neurophysiol. 2010, 103, 1532–1542. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging human locomotion: Stability and modular organisation in unsteady conditions. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor patterns in human walking and running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef]
- Chvatal, S.A.; Ting, L.H. Common muscle synergies for balance and walking. Front. Comput. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef]
- Alessandro, C.; Delis, I.; Nori, F.; Panzeri, S.; Berret, B. Muscle synergies in neuroscience and robotics: From input-space to task-space perspectives. Front. Comput. Neurosci. 2013, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Kutch, J.J.; Valero-Cuevas, F.J. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput. Biol. 2012, 8, e1002434. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, P.; Wyler-Duda, K.; D’Avella, A.; Tresch, M.C.; Bizzi, E. Muscle synergies encoded within the spinal cord: Evidence from focal intraspinal NMDA iontophoresis in the frog. J. Neurophysiol. 2001, 85, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Lemay, M.A.; Grill, W.M. Modularity of motor output evoked by intraspinal microstimulation in cats. J. Neurophysiol. 2004, 91, 502–514. [Google Scholar] [CrossRef]
- Guertin, P.A. Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. Front. Neurol. 2012, 3, 183. [Google Scholar] [CrossRef]
- Roh, J.; Cheung, V.C.K.; Bizzi, E. Modules in the brain stem and spinal cord underlying motor behaviors. J. Neurophysiol. 2011, 106, 1363–1378. [Google Scholar] [CrossRef]
- Overduin, S.A.; D’Avella, A.; Carmena, J.M.; Bizzi, E. Microstimulation activates a handful of muscle synergies. Neuron 2012, 76, 1071–1077. [Google Scholar] [CrossRef]
- Holdefer, R.N.; Miller, L.E. Primary motor cortical neurons encode functional muscle synergies. Exp. Brain Res. 2002, 146, 233–243. [Google Scholar] [CrossRef]
- Rathelot, J.-A.; Strick, P.L. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl. Acad. Sci. USA 2009, 106, 918–923. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef]
- Zariffa, J.; Steeves, J.; Pai, D.K. Changes in hand muscle synergies in subjects with spinal cord injury: Characterization and functional implications. J. Spinal Cord Med. 2012, 35, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Sui, Y.; Sayenko, D.; Burdick, J.W. Motor Control After Human SCI Through Activation of Muscle Synergies Under Spinal Cord Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1331–1340. [Google Scholar] [CrossRef]
- Santuz, A.; Brüll, L.; Ekizos, A.; Schroll, A.; Eckardt, N.; Kibele, A.; Schwenk, M.; Arampatzis, A. Neuromotor Dynamics of Human Locomotion in Challenging Settings. Iscience 2020, 23, 100796. [Google Scholar] [CrossRef] [PubMed]
- Tresch, M.C.; Cheung, V.C.K.; D’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.H.; Chiel, H.J.; Trumbower, R.D.; Allen, J.L.; McKay, J.L.; Hackney, M.E.; Kesar, T.M. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron 2015, 86, 38–54. [Google Scholar] [CrossRef]
- Bruton, M.; O’Dwyer, N. Synergies in coordination: A comprehensive overview of neural, computational, and behavioral approaches. J. Neurophysiol. 2018, 120, 2761–2774. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. On the Methodological Implications of Extracting Muscle Synergies from Human Locomotion. Int. J. Neural Syst. 2017, 27, 1750007. [Google Scholar] [CrossRef]
- Devarajan, K.; Cheung, V.C.K. On nonnegative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput. 2014, 26, 1128–1168. [Google Scholar] [CrossRef]
- Soomro, M.H.; Conforto, S.; Giunta, G.; Ranaldi, S.; De Marchis, C. Comparison of Initialization Techniques for the Accurate Extraction of Muscle Synergies from Myoelectric Signals via Nonnegative Matrix Factorization. Appl. Bionics Biomech. 2018, 2018, 3629347. [Google Scholar] [CrossRef]
- Shourijeh, M.S.; Flaxman, T.E.; Benoit, D.L. An approach for improving repeatability and reliability of non-negative matrix factorization for muscle synergy analysis. J. Electromyogr. Kinesiol. 2016, 26, 36–43. [Google Scholar] [CrossRef]
- Kolda, T.G.; Bader, B.W. Tensor decompositions and applications. SIAM Rev. 2009, 51, 455–500. [Google Scholar] [CrossRef]
- Takiyama, K.; Yokoyama, H.; Kaneko, N.; Nakazawa, K. Speed-dependent and mode-dependent modulations of spatiotemporal modules in human locomotion extracted via tensor decomposition. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Antuvan, C.W.; Bisio, F.; Marini, F.; Yen, S.-C.; Cambria, E.; Masia, L. Role of Muscle Synergies in Real-Time Classification of Upper Limb Motions using Extreme Learning Machines. J. Neuroeng. Rehabil. 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.E.; Iqbal, K.; White, G.; Hutchinson, T.E. A systematic review on muscle synergies: From building blocks of motor behavior to a neurorehabilitation tool. Appl. Bionics Biomech. 2018, 2018, 3615368. [Google Scholar] [CrossRef] [PubMed]
- Ebied, A.; Kinney-Lang, E.; Spyrou, L.; Escudero, J. Evaluation of matrix factorisation approaches for muscle synergy extraction. Med Eng. Phys. 2018, 57, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Impaired synergic control of posture in Parkinson’s patients without postural instability. Gait Posture 2016, 44, 209–215. [Google Scholar] [CrossRef]
- Kieliba, P.; Tropea, P.; Pirondini, E.; Coscia, M.; Micera, S.; Artoni, F. How are Muscle Synergies Affected by Electromyography Pre-Processing? IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 882–893. [Google Scholar] [CrossRef]
- Ramos, F.M.; D’Avella, A.; Hayashibe, M. Identification of time-varying and time-scalable synergies from continuous electromyographic patterns. IEEE Robot. Autom. Lett. 2019, 4, 3053–3058. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef]
- Oliveira, A.S.C.; Gizzi, L.; Farina, D.; Kersting, U.G. Motor modules of human locomotion: Influence of EMG averaging, concatenation, and number of step cycles. Front. Hum. Neurosci. 2014, 8, 335. [Google Scholar] [CrossRef]
- Endres, D.M.; Chiovetto, E.; Giese, M.A. Model selection for the extraction of movement primitives. Front. Comput. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.K.; Devarajan, K.; Severini, G.; Turolla, A.; Bonato, P. Decomposing time series data by a non-negative matrix factorization algorithm with temporally constrained coefficients. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3496–3499. [Google Scholar]
- Shuman, B.R.; Schwartz, M.H.; Steele, K.M. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Front. Comput. Neurosci. 2017, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bulea, T.C.; Damiano, D.L. Novel Methods to Enhance Precision and Reliability in Muscle Synergy Identification during Walking. Front. Hum. Neurosci. 2016, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Palermo, E.; Del Prete, Z.; Rossi, S. On the Reliability and Repeatability of Surface Electromyography Factorization by Muscle Synergies in Daily Life Activities. Appl. Bionics Biomech. 2018, 2018, 5852307. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.H.; Kim, T.H.; Wang, F.; Vyas, S.; Ryu, S.I.; Shenoy, K.V.; Schnitzer, M.; Kolda, T.G.; Ganguli, S. Unsupervised Discovery of Demixed, Low-Dimensional Neural Dynamics across Multiple Timescales through Tensor Component Analysis. Neuron 2018, 98, 1099–1115. [Google Scholar] [CrossRef]
- Ting, L.H.; Chvatal, S.A. Decomposing Muscle Activity in Motor Tasks—Methods and Interpretation. In Motor Control: Theories, Experiments, and Applications; Danion, F., Latash, M.L., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 102–138. [Google Scholar]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients with Parkinson’s disease. J. Electromyogr. Kinesiol. 2017, 33, 20–26. [Google Scholar] [CrossRef]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Dewald, J.P.; Pope, P.S.; Given, J.D.; Buchanan, T.S.; Rymer, W.Z. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 1995, 118, 495–510. [Google Scholar] [CrossRef]
- Latash, M.L. Towards physics of neural processes and behavior. Neurosci. Biobehav. Rev. 2016, 69, 136–146. [Google Scholar] [CrossRef]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef]
- Roemmich, R.T.; Fregly, B.J.; Hass, C.J. Neuromuscular complexity during gait is not responsive to medication in persons with Parkinson’s disease. Ann. Biomed. Eng. 2014, 42, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Motor equivalence and structure of variance: Multi - muscle postural synergies in Parkinson’ s disease. Exp. Brain Res. 2017, 235, 2243–2258. [Google Scholar] [CrossRef] [PubMed]
- Falaki, A.; Jo, H.J.; Lewis, M.M.; O’Connell, B.; De Jesus, S.; McInerney, J.; Huang, X.; Latash, M.L. Systemic effects of deep brain stimulation on synergic control in Parkinson’s disease. Clin. Neurophysiol. 2018, 129, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Mileti, I.; Zampogna, A.; Taborri, J.; Martelli, F.; Rossi, S.; Del Prete, Z.; Paoloni, M.; Suppa, A.; Palermo, E. Parkinson’s disease and Levodopa effects on muscle synergies in postural perturbation. In Proceedings of the Medical Measurements and Applications, Istanbul, Turkey, 26–28 June 2019. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, S.; Hao, M.; Xiao, Q.; Lan, N. The impact of evoked cutaneous afferents on voluntary reaching movement in patients with Parkinson’s disease. J. Neural Eng. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McKay, J.L.; Sawers, A.; Hackney, M.E.; Ting, L.H. Increased neuromuscular consistency in gait and balance after partnered, dance-based rehabilitation in Parkinson’s disease. J. Neurophysiol. 2017, 118, 363–373. [Google Scholar] [CrossRef]
- Hu, Z.X.; Xu, S.Q.; Hao, M.Z.; Xiao, Q.; Lan, N. Muscle synergy changes with cutaneous stimulation during resting tremor and reaching task in Parkinson’s disease. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering, San Francisco, CA, USA, 20–23 March 2019. [Google Scholar] [CrossRef]
- Shuman, B.; Goudriaan, M.; Bar-On, L.; Schwartz, M.H.; Desloovere, K.; Steele, K.M. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait Posture 2016, 45, 127–132. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front. Hum. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef]
- Steele, K.M.; Rozumalski, A.; Schwartz, M.H. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 2015, 57, 1176–1182. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Masiello, D.; Rossi, S. Factorization of EMG via muscle synergies in walking task: Evaluation of intra-subject and inter-subject variability. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017; pp. 1–6. [Google Scholar]
- Scalona, E.; Taborri, J.; Del Prete, Z.; Palermo, E.; Rossi, S. EMG factorization during walking: Does digital filtering influence the accuracy in the evaluation of the muscle synergy number? In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Hug, F.; Turpin, N.A.; Guével, A.; Dorel, S. Is interindividual variability of EMG patterns in trained cyclists related to different muscle synergies? J. Appl. Physiol. 2010, 108, 1727–1736. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Kunimasa, Y.; Kijima, K.; Ishikawa, M.; Arampatzis, A. Lower complexity of motor primitives ensures robust control of high-speed human locomotion. bioRxiv 2020. [Google Scholar] [CrossRef]
- Santuz, A.; Akay, T. Fractal analysis of muscle activity patterns during locomotion: Pitfalls and how to avoid them Running head: Fractal analysis of locomotor primitives. bioRxiv 2020. [Google Scholar] [CrossRef]
- Taborri, J.; Mileti, I.; Del Prete, Z.; Rossi, S.; Palermo, E. Yaw Postural Perturbation Through Robotic Platform: Aging Effects on Muscle Synergies. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, the Netherlands, 26–29 August 2018; pp. 916–921. [Google Scholar]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; d’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor patterns in cerebellar ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Israeli-Korn, S.D.; Barliya, A.; Paquette, C.; Franzén, E.; Inzelberg, R.; Horak, F.B.; Flash, T. Intersegmental coordination patterns are differently affected in Parkinson’s disease and cerebellar ataxia. J. Neurophysiol. 2019, 121, 672–689. [Google Scholar] [CrossRef]
- Vinueza Veloz, M.F.; Zhou, K.; Bosman, L.W.J.; Potters, J.W.; Negrello, M.; Seepers, R.M.; Strydis, C.; Koekkoek, S.K.E.; De Zeeuw, C.I. Cerebellar control of gait and interlimb coordination. Brain Struct. Funct. 2015, 220, 3513–3536. [Google Scholar] [CrossRef]
- Bharti, K.; Suppa, A.; Pietracupa, S.; Upadhyay, N.; Giannì, C.; Leodori, G.; Di Biasio, F.; Modugno, N.; Petsas, N.; Grillea, G.; et al. Abnormal Cerebellar Connectivity Patterns in Patients With Parkinson’s Disease and Freezing of Gait. Cerebellum 2019, 18, 298–308. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Thaut, M.H.; McIntosh, G.C.; Rice, R.R.; Miller, R.A.; Rathbun, J.; Brault, J.M. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 1996, 11, 193–200. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Lowenthal, J.; Herman, T.; Gruendlinger, L.; Peretz, C.; Giladi, N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur. J. Neurosci. 2007, 26, 2369–2375. [Google Scholar] [CrossRef]
- Delval, A.; Moreau, C.; Bleuse, S.; Tard, C.; Ryckewaert, G.; Devos, D.; Defebvre, L. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin. Neurophysiol. 2014, 125, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, T.; Fukuyama, H.; Katsumi, Y.; Honda, M.; Shibasaki, H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann. Neurol. 1999, 45, 329–336. [Google Scholar] [CrossRef]
- Erra, C.; Mileti, I.; Germanotta, M.; Petracca, M.; Imbimbo, I.; De Biase, A.; Rossi, S.; Ricciardi, D.; Pacilli, A.; Di Sipio, E.; et al. Immediate effects of rhythmic auditory stimulation on gait kinematics in Parkinson’s disease ON/OFF medication. Clin. Neurophysiol. 2019, 130, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications; Routledge: New York, NY, USA, 2008; ISBN 9780203958827. [Google Scholar]

| Sex (F/M) | Age (years) | Body Weight (kg) | Height (m) | Disease Duration (years) | Onset Side (L/R/B) | Clinical Phenotype (TD/PIGD) | H&Y | UPDRS-III | BBS | MMSE | LEDD (mg) | DBS (years) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ON | OFF | |||||||||||||
| [87] | - | 67 ± 8 | 80 ± 14 | 1.7 ± 0.1 | - | - | - | - | - | - | - | - | - | |
| [88] | 2 F | 66 ± 7 | 77 ± 9 | 1.7 ± 0.1 | 4 ± 2 | - | - | 37 ± 7 | 41 ± 10 | - | - | - | - | |
| 7 M | ||||||||||||||
| [71] | 6 F4 M | 69 ± 6 | - | - | 3.5 ± 1.9 | 3 L | - | 14 ± 10 | - | - | - | 412 ± 191 | - | |
| 6 R | ||||||||||||||
| 2 B | ||||||||||||||
| [83] | 4 F 6 M | 69 ± 6 | 80 ± 15 | 1.7 ± 0.1 | 6 ± 4 | 3 L 5 R 2 B | II-III | 18 ± 10 | 27 ± 11 | - | - | 578 ± 144 | - | |
| [90] | 10 M | 61 ± 10 | - | - | 11 ± 5 | 3 L 6 R 1 B | - | 27 ± 12 * 37 ± 22 ** | - | - | - | 715 ± 444 | 1.57 ± 1.2 | |
| [91] | 10 M | 61 ± 4 | - | - | - | - | I-II | 21 ± 8 | 32 ± 11 | 53 ± 5 | 29 ± 2 | - | - | |
| [92] | 2 F | 64 ± 10 | - | - | 7 ± 4 | - | 10 TD | II-III | 19 ± 4 | - | - | 30 ± 1 | 423 ± 213 | - |
| 8 M | ||||||||||||||
| [93] | 1 F 5 M | 64 ± 17 | 72 ± 13 | 1.8 ± 0.1 | 7 ± 5 | - | 1 TD 4 PIGD 1 Undet. | I-III | 30 ± 5 | - | - | - | - | - |
| [Ref] | Subjects | State of Therapy | Recorded Muscles | Experimental Task | Synergy Extraction | Main Findings | Conclusions | |
|---|---|---|---|---|---|---|---|---|
| [87] | 15 PD and 14 HS | ON | Eight leg muscles bilaterally: SOL, GM, TA, VM, RF, SM, BF, GLM | 10 minutes walking on a treadmill | NMF and %VAF | 95% of PD require four or fewer muscle synergies, compared to 57% HS. Similar muscle weights but shifted muscle activation profile in PD. Association between walking speed and total %VAF in PD | Altered timing of modular activation may be responsible for abnormal motor control during gait in PD, rather than different muscle weighting vectors | |
| [88] | Nine PD | ON and OFF | Eight leg muscles bilaterally: SOL, GM, TA, VM, RF, SM, BF, GLM | Overground walking and walking on a treadmill | NMF and %VAF | No differences between ON and OFF therapy for total %VAF, NoS and the muscle weighting vector. Negative correlation between total %VAF and walking speed, but no correlations with other spatiotemporal gait parameters | Dopaminergic therapy does not influence the number, structure or timing of muscle synergies | |
| [71] | 11 PD 11 HS | ON | 13 leg and trunk muscle of the right side: TA, SOL, GM, GL, BF, ST, RF, VL, VM, TFL, ESL, EST, RA | Quiet standing, voluntary sway, releasing a load and fast body motion | PCA analysis with Varimax rotation and factor extraction | Four muscle synergies identified using PCA with rotation. Muscle synergies account for a lower amount of variance in PD (71.5±1.74%) than HS (78.3±1.74%). Muscle synergies are predictors of centre of pressure changes in all subjects | Organization of muscles into muscle synergies is less consistent in PD compared with HS | |
| [83] | 10 PD | ON and OFF | 13 leg and trunk muscles of the right side: TA, SOL, GM, GL, BF, ST, RF, VL, VM, TFL, ESL, EST, RA | Quiet standing, voluntary sway, releasing a load and fast body motion | PCA analysis with Varimax rotation and factor extraction | Four muscle synergies identified using PCA with rotation. Muscle synergies account for a larger amount of variance in PD during ON (74.7±2.4%) than OFF (68.6±2.2%) therapy. Muscle synergies are predictors of centre of pressure changes | In PD, dopaminergic therapy makes the organization of muscles into muscle synergies more consistent during postural tasks | |
| [90] | 10 PD | ON with DBS-OFF or DBS-ON | Three leg and trunk muscles of the right side: TA, SOL, GM, GL, BF, ST, RF, VL, VM, TFL, ESL, EST, RA | Quiet standing, voluntary sway, releasing a load | PCA analysis with Varimax rotation and factor extraction | In postural tasks, four muscle synergies were identified using PCA with rotation. Muscle synergies account for similar amounts of variance in DBS-OFF (75.3±2.9%) and DBS-ON (75.1±2.9%). Muscle synergies are predictors of centre of pressure changes regardless of DBS status | DBS does not influence the organization of muscles into muscle synergies | |
| [91] | 10 PD and 10 HS | ON and OFF | six upper body muscles bilaterally: PM, DP, BB, TB, EXOB, ESL | Standing while balancing external yaw perturbation | NMF and %VAF | Higher values of total %VAF in PD than HS for NoS less than 4. Similar total %VAF during OFF and ON therapy. NoS positively correlate with MMSE scores and negatively with sub-item 3.14 of UPDRS-III (“body bradykinesia”) | PD use a lower number of muscle synergies to maintain balance. l-dopa does not influence muscle synergies during yaw postural perturbations. | |
| [93] | 6 PD | ON | 13 lower back and right leg muscle: RA, EXOB, EST, GLM, TFL, BF, VM, GM, GL, SOL, PL | Overground walking trial and standing while balancing a ramp-and-hold external perturbation before and after a rehabilitation program (three weeks of daily adapted tango classes) | NMF and %VAF | No differences in NoS after rehabilitation training. Rehabilitation improves motor module distinctness (i.e., well-defined biomechanical output between modules), consistency (reduced variability within motor modules) and generalizability (increased sharing of motor modules across gait and balance tasks) | Within- and between-module parameters (e.g., consistency, distinctness and generalizability) reflect motor performance in PD better than NoS | |
| [92] | 10 PD and 8 HS | ON | Six right arm and upper body muscles: PM, DP, BB, TB, FR, ER | Resting tremor and reaching task with and without transcutaneous electrical stimulation of the radial nerve | NMF and %VAF | Three muscle synergies were found both in resting tremor and in reaching tasks. Cutaneous stimulation does not alter synergy vectors, but differently change the time profile of muscle synergies during resting tremor and reaching tasks | The different effects of cutaneous electrical stimulation on vector patterns and the time profile of muscle synergies may imply different spinal pathways for these signals | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle Synergies in Parkinson’s Disease. Sensors 2020, 20, 3209. https://doi.org/10.3390/s20113209
Mileti I, Zampogna A, Santuz A, Asci F, Del Prete Z, Arampatzis A, Palermo E, Suppa A. Muscle Synergies in Parkinson’s Disease. Sensors. 2020; 20(11):3209. https://doi.org/10.3390/s20113209
Chicago/Turabian StyleMileti, Ilaria, Alessandro Zampogna, Alessandro Santuz, Francesco Asci, Zaccaria Del Prete, Adamantios Arampatzis, Eduardo Palermo, and Antonio Suppa. 2020. "Muscle Synergies in Parkinson’s Disease" Sensors 20, no. 11: 3209. https://doi.org/10.3390/s20113209
APA StyleMileti, I., Zampogna, A., Santuz, A., Asci, F., Del Prete, Z., Arampatzis, A., Palermo, E., & Suppa, A. (2020). Muscle Synergies in Parkinson’s Disease. Sensors, 20(11), 3209. https://doi.org/10.3390/s20113209











