Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity
Abstract
1. Introduction
2. Sensors for Continuous Intravascular Monitoring
2.1. Ion Selective Electrodes
2.2. ISE Biocompatibility
2.3. Oxygen Electrodes
3. Blood as a Reactive Sample Matrix
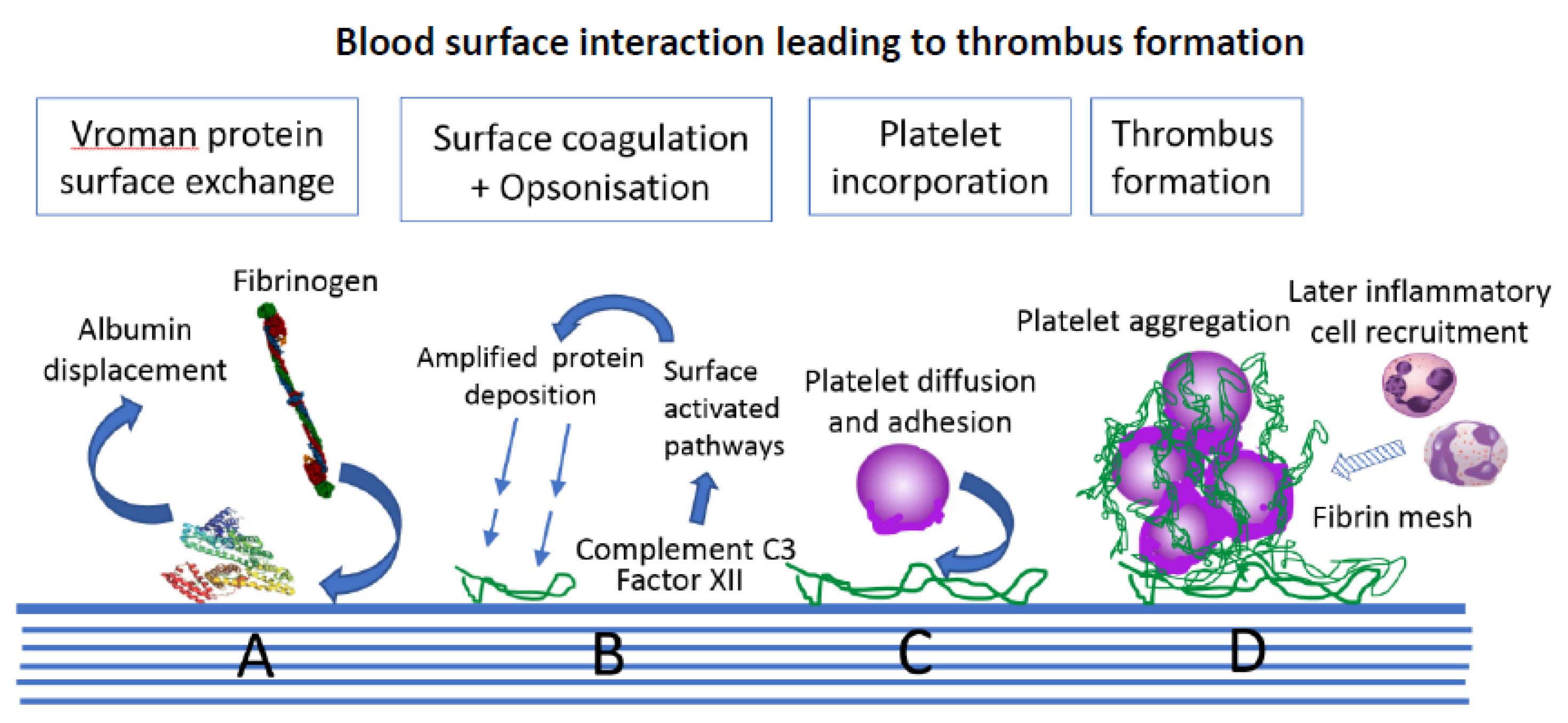
3.1. Protein Surface Interactions
3.2. Blood Biological Reactivity
4. Tissue Oxygen Electrodes
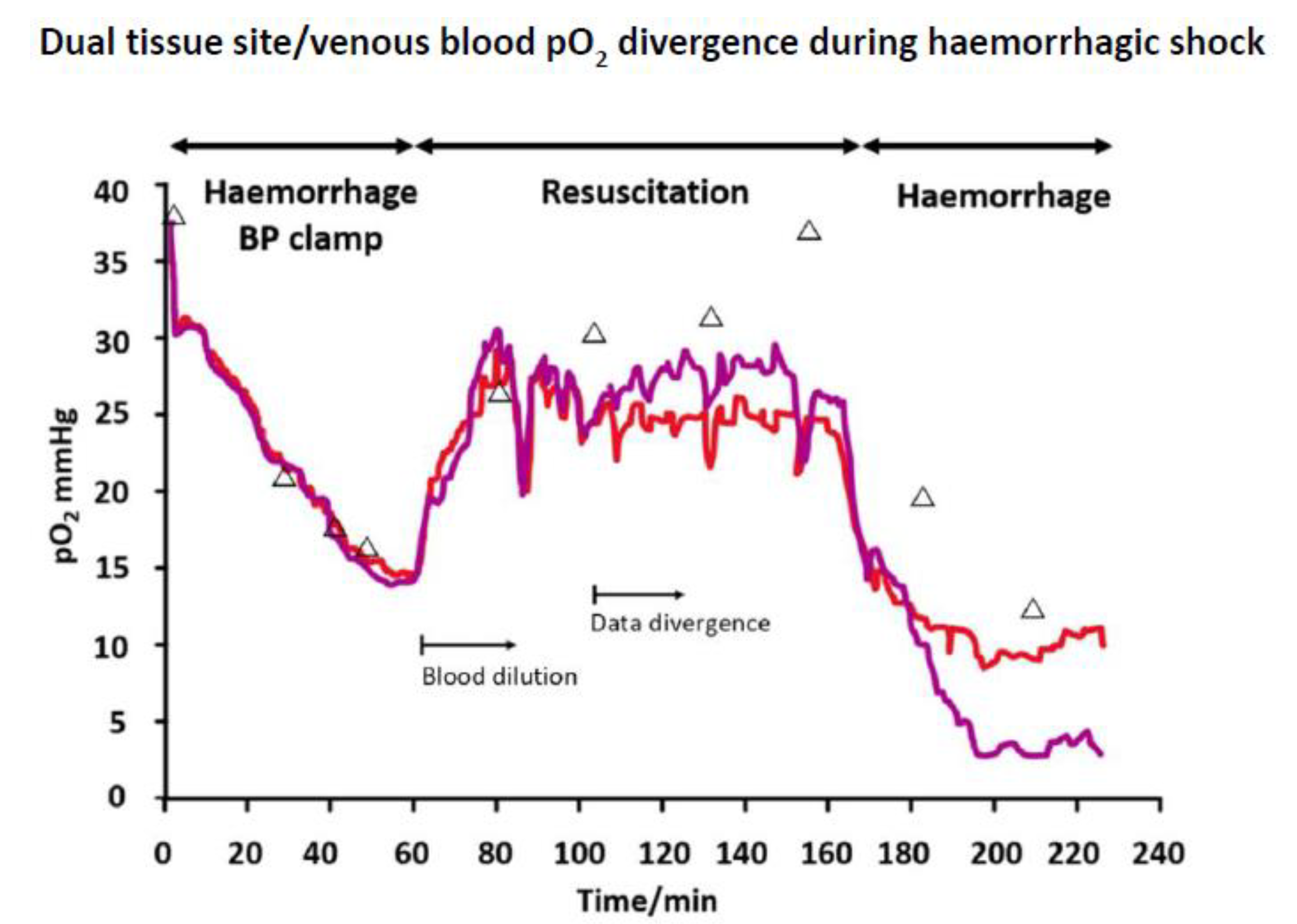
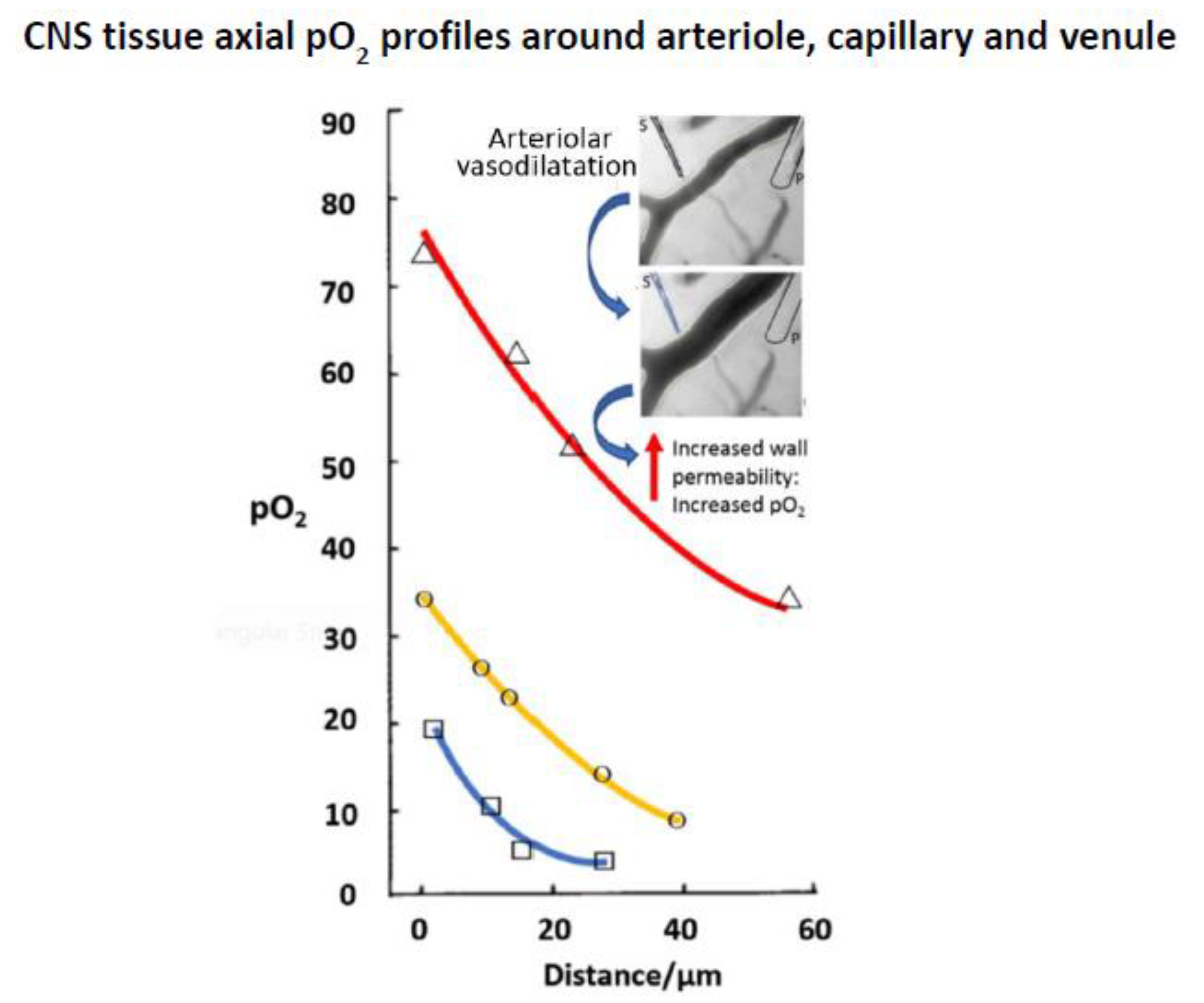
4.1. Compartmental Difference
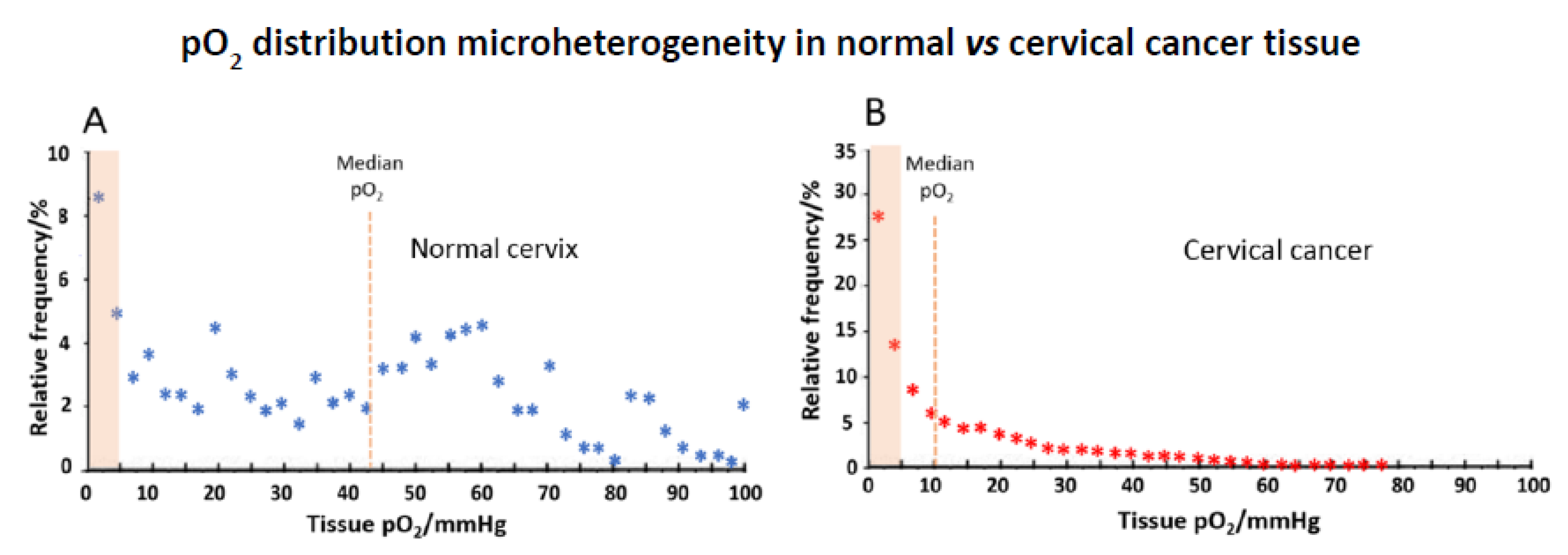
4.2. Tissue Micro-Heterogeneity
4.3. Cancer Tissue
5. Glucose Electrodes
5.1. Monitoring Needs
5.2. Clinical Realities
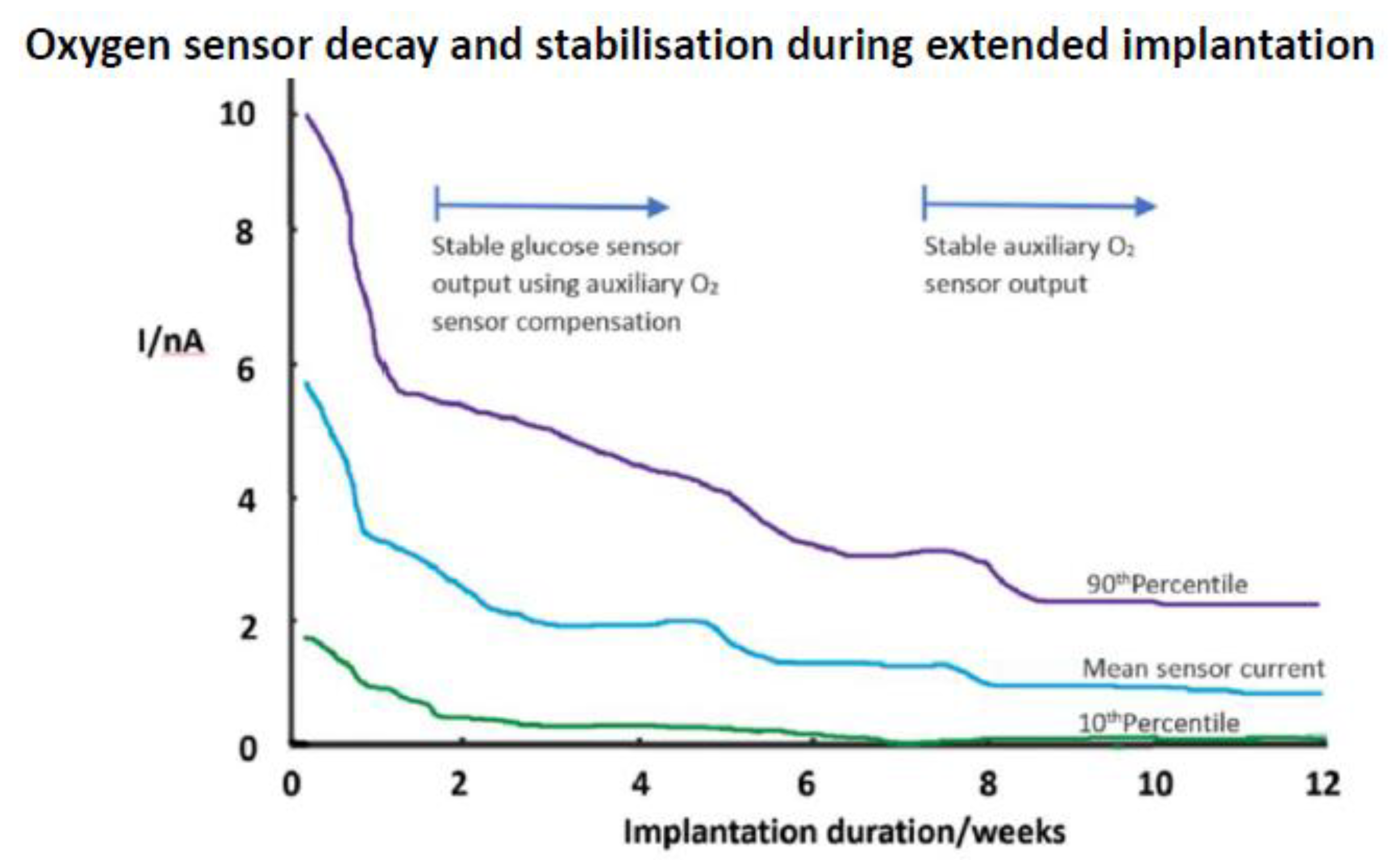
5.3. Membranes and Coatings
5.4. Bioactive Molecule Release for Biocompatibility
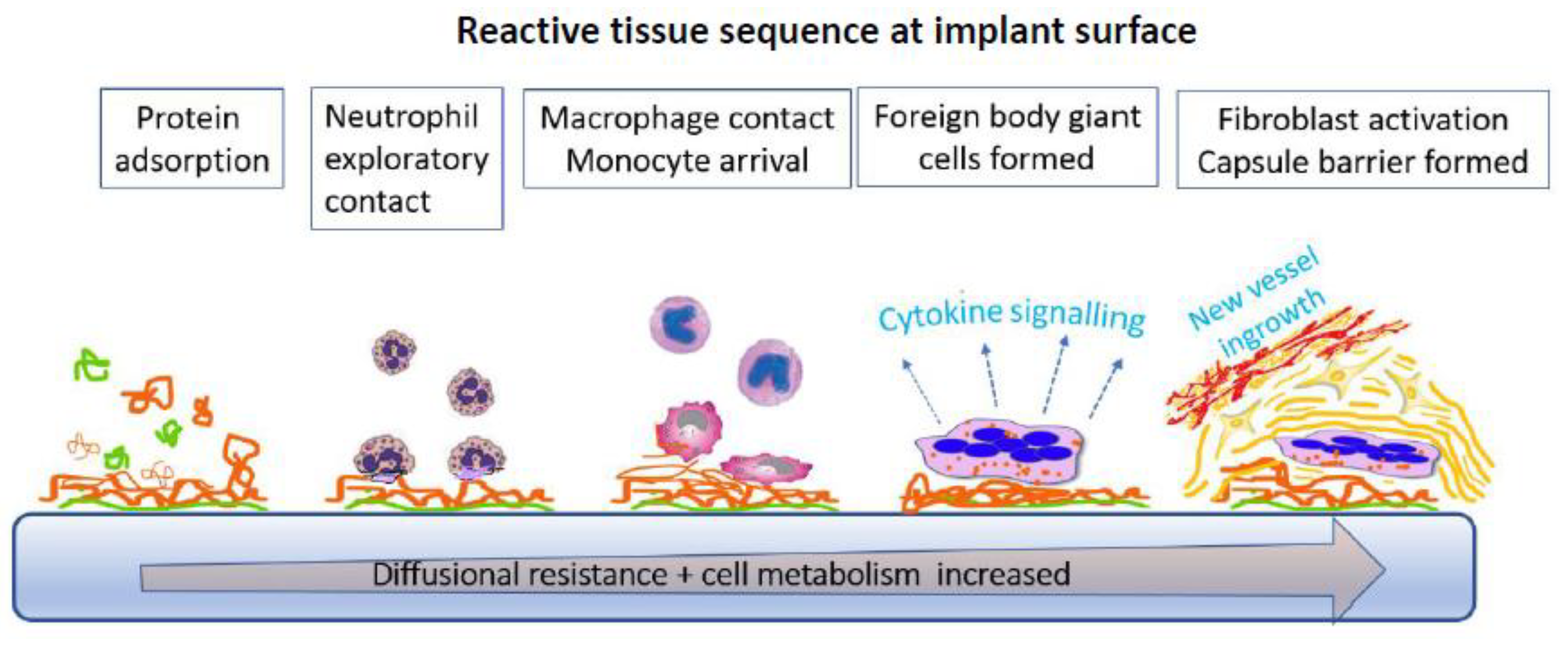
5.5. Tissue Reactivity to Implants
5.6. Tissue Reaction Implications for Glucose Sensors
6. Lactate
7. Conclusions
Funding
Conflicts of Interest
References
- Walker, J.L.; Brown, H.M. Intracellular ionic activity measurements in nerve and muscle. Physiol. Rev. 1977, 57, 729–778. [Google Scholar] [CrossRef] [PubMed]
- Land, S.C.; Porterfield, D.M.; Sanger, R.H.; Smith, P.J.S. The self-referencing oxygen-selective microelectrode: Detection of transmembrane oxygen flux from single cells. J. Exp. Biol. 1999, 202, 211–218. [Google Scholar] [PubMed]
- Wians, E.H. Clinical Laboratory Tests: Which, Why, and What Do The Results Mean? Labmedicine 2009, 40, 105–113. [Google Scholar] [CrossRef]
- Rodenburg, R.J.T.; Schoonderwoerd, G.C.; Tiranti, V.; Taylor, R.W.; Rotig, A.; Valente, L.; Invernizzi, F.; Chretien, D.; He, L.; Backx, G.; et al. A multi-center comparison of diagnostic methods for the biochemical evaluation of suspected mitochondrial disorders. Mitochondrion 2013, 13, 36–43. [Google Scholar] [CrossRef]
- Laje, R.; Agostino, P.V.; Golombek, D.A. The Times of Our Lives: Interaction among Different Biological Periodicities. Front. Integr. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.L.; Fedullo, P.F.; Clausen, J.L. Rapid changes in arterial blood gas levels after exercise in pulmonary patients. Chest 1983, 83, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Oropello, J.M.; Manasia, A.; Hannon, E.; Leibowitz, A.; Benjamin, E. Continuous fiberoptic arterial and venous blood gas monitoring in hemorrhagic shock. Chest 1996, 109, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Heining, M.P.D.; Linton, R.A.F.; Band, D.M. Continuous intravascular monitoring of plasma ionized calcium. In Ion Measurements in Physiology and Medicine, 1st ed.; Kessler, M., Harrison, D.K., Hoper, J., Eds.; Springer: Berlin, Germany, 1985; pp. 292–296. [Google Scholar]
- Linton, R.A.F.; Lim, M.; Band, D.M. Continuous intravascular monitoring of plasma potassium using potassium-selective electrodes. Crit. Care Med. 1982, 10, 337–340. [Google Scholar] [CrossRef]
- Lim, M.; Linton, R.A.F.; Band, D.M. Continuous intravascular monitoring of ephinephrine-induced changes in plasma potassium. Anesthesiology 1982, 57, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.F.; Smith, M.; Corfield, D.R.; Treasure, T. Continuous multi-channel intravascular monitoring of the effects of dopamine and dobutamine on plasma potassium in dogs. Intensive Care Med. 1989, 15, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Band, D.M.; Semple, S.J. Continuous measurement of blood pH with an indwelling arterial glass electrode. J. Appl. Physiol. 1967, 22, 584–587. [Google Scholar] [CrossRef]
- Lang, D.A.; Matthews, D.R.; Peto, J.; Turner, R.C. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 1979, 301, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Cater, D.B.; Silver, I.A. Quantitative measurements of oxygen tension in normal tissues and in the tumours of patients before and after radiotherapy. Acta Radiol. 1960, 53, 233–256. [Google Scholar] [CrossRef]
- Tracinski, M.; Silver, I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001, 128, 263–276. [Google Scholar] [CrossRef]
- Lubbers, D.W.; Baumgartl, H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO(2) distribution in the living tissue. Kidney Int. 1997, 51, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, D.W.; Baumgartl, H.; Zimelka, W. Heterogeneity and stability of local PO2 distribution within the brain tissue. Adv. Exp. Med. Biol. Oxyg. Transp. Tissue XV 1994, 345, 567–574. [Google Scholar]
- Hill, J.L.; Gettes, L.S.; Lynch, M.R.; Hebert, N.C. Flexible valinomycin electrodes for online determination of intravascular and myocardial K+. Am. J. Physiol. 1978, 235, H455–H459. [Google Scholar] [PubMed]
- Hallen, J.; Sejersted, O.M. Intravasal use of pliable K+-selective electrodes in the femoral vein of humans during exercise. J. Appl. Physiol. 1993, 75, 2318–2325. [Google Scholar] [CrossRef]
- Paterson, D.J.; Estavillo, J.A.; Nye, P.C.G. The effect of hypoxia on plasma potassium concentration and the excitation of the arterial chemoreceptors in the cat. Q. J. Exp. Physiol. CMS 1988, 73, 623–625. [Google Scholar] [CrossRef]
- Webb, S.C.; Canepaanson, R.; Rickards, A.F.; Poole-Wilson, P.A. Myocardial potassium loss after acute coronary occlusion in humans. J. Am. Coll. Cardiol. 1987, 9, 1230–1234. [Google Scholar] [CrossRef]
- Watanabe, I.; Saito, S.; Ozawa, Y.; Hatano, M.; Gettes, L.S. Continuous coronary venous K+ monitoring in myocardial ischaemia in swine heart. Jpn. Circ. J. 1988, 52, 1019–1020. [Google Scholar]
- Bourdillon, P.D.; Bettmann, M.A.; McCracken, S.; Poole-Wilson, P.A.; Grossman, W. Effects of a new non-ionic and a conventional ionic contrast agent on coronary sinus ionised calcium and left ventricular hemodynamics in dogs. J. Am. Coll. Cardiol. 1985, 6, 845–853. [Google Scholar] [CrossRef]
- Meruva, R.K.; Meyerhoff, M.E. Catheter-Type sensor for potentiometric monitoring of oxygen, pH and carbon dioxide. Biosens. Bioelectron. 1998, 13, 201–212. [Google Scholar] [CrossRef]
- Cobbe, S.M.; Poole-Wilson, P.A. Continuous coronary sinus and arterial pH monitoring during pacing induced ischaemia in coronary artery disease. Br. Heart J. 1982, 47, 369–374. [Google Scholar] [CrossRef]
- Khalid, A.; Peng, L.; Arman, A.; Warren-Smith, S.C.; Schartner, E.P.; Sylvia, G.M.; Hutchinson, M.R.; Ebendorff-Heidepriem, H.; McLaughlin, R.A.; Gibson, B.C.; et al. Silk: A bio-derived coating for optical fibre sensing applications. Sens. Actuators B Chem. 2020, 311, 127864. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, J.J.; Wang, X.; Zhu, X.D.; Wang, G.F.; Song, Y.L.; Bai, C.X. Continuous intra-arterial blood pH monitoring in rabbits with acid-base disorders. Respir. Physiol. Neurobiol. 2011, 177, 183–188. [Google Scholar] [CrossRef]
- Canovas, R.; Sanchez, S.P.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Cytotoxicity Study of Ionophore-Based Membranes: Toward On Body and in Vivo Ion Sensing. ACS Sens. 2019, 4, 2524–2535. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, P.; Liang, R.N.; Qin, W. Improving the Biocompatibility of Polymeric Membrane Potentiometric Ion Sensors by Using a Mussel-Inspired Polydopamine Coating. Anal. Chem. 2019, 91, 6424–6429. [Google Scholar] [CrossRef]
- Murphy, S.M.; Hamilton, C.J.; Davies, M.L.; Tighe, B.J. Polymer membranes in clinical sensor applications. 2. The design and fabrication of permselective hydrogels for electrochemical devices. Biomaterials 1992, 13, 979–990. [Google Scholar] [CrossRef]
- Berrocal, M.J.; Johnson, R.D.; Badr, I.H.A.; Liu, M.D.; Gao, D.Y.; Bachas, L.G. Improving the blood compatibility of ion-selective electrodes by employing poly(MPC-co-BMA), a copolymer containing phosphorylcholine, as a membrane coating. Anal. Chem. 2002, 74, 3644–3648. [Google Scholar] [CrossRef]
- Brooks, H.A.; Allen, J.R.; Feldhoff, P.W.; Bachas, L.G. Effect of surface-attached heparin on the response of potassium-selective electrodes. Anal. Chem. 1996, 68, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- EspadasTorre, C.; Oklejas, V.; Mowery, K.; Meyerhoff, M.E. Thromboresistant chemical sensors using combined nitric oxide release ion sensing polymeric films. J. Am. Chem. Soc. 1997, 119, 2321–2322. [Google Scholar] [CrossRef]
- Vassilev, P.; Koper, M.T.M. Electrochemical reduction of oxygen on gold surfaces: A density functional theory study of intermediates and reaction paths. J. Phys. Chem. C 2007, 111, 2607–2613. [Google Scholar] [CrossRef]
- Bratanow, N.; Polk, K.; Bland, R.; Kram, H.B.; Lee, T.S.; Shoemaker, W.C. Continuous polarographic monitoring of intra-arterial oxygen in the peroperative period. Crit. Care Med. 1985, 13, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Green, G.E.; Hassell, K.T.; Mahutte, C.K. Comparison of arterial blood gas with continuous intra-arterial and trans-cutaneous pO2 sensors in adult critically ill patients. Crit. Care Med. 1987, 15, 491–494. [Google Scholar] [CrossRef]
- Mahutte, C.K. On-line arterial blood gas analysis with optodes: Current status. Clin. Biochem. 1998, 31, 119–130. [Google Scholar] [CrossRef]
- Ren, H.; Coughlin, M.A.; Major, T.C.; Aiello, S.; Pena, A.R.; Bartlett, R.H.; Meyerhoff, M.E. Improved in Vivo Performance of Amperometric Oxygen (PO2) Sensing Catheters via Electrochemical Nitric Oxide Generation/Release. Anal. Chem. 2015, 87, 8067–8072. [Google Scholar] [CrossRef]
- Widness, J.A.; Kulhavy, J.C.; Johnson, K.J.; Cress, G.A.; Kromer, I.J.; Acarregui, M.J.; Feld, R.D. Clinical performance of an in-line point-of-care monitor in neonates. Pediatrics 2000, 106, 497–504. [Google Scholar] [CrossRef]
- Norde, W. My voyage of discovery to proteins in flatland ... and beyond. Colloid Surf. B 2008, 61, 1–9. [Google Scholar] [CrossRef]
- Noh, H.; Vogler, E.A. Volumetric interpretation of protein adsorption: Competition from mixtures and the Vroman effect. Biomaterials 2007, 28, 405–422. [Google Scholar] [CrossRef]
- Brash, J.L. Protein Surface Interactions and Biocompatibility: A Forty Year Perspective. ACS Symp. Ser. Proteins Interfaces III State Art 2012, 1120, 277–300. [Google Scholar]
- Pawlak, M.; Bakker, E. Chemical Modification of Polymer Ion-Selective Membrane Electrode Surfaces. Electroanalysis 2014, 26, 1121–1131. [Google Scholar] [CrossRef]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Modinger, Y.; Teixeira, G.Q.; Neidlinger-Wilke, C.; Ignatius, A. Role of the Complement System in the Response to Orthopedic Biomaterials. Int. J. Mol. Sci. 2018, 19, 3367. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, K.N.; Teramura, Y.; Hamad, O.A.; Asif, S.; Duehrkop, C.; Fromell, K.; Gustafson, E.; Hong, J.; Kozarcanin, H.; Magnusson, P.U.; et al. Dangerous liaisons: Complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 2016, 274, 245–269. [Google Scholar] [CrossRef]
- Sotiri, I.; Robichaud, M.; Lee, D.; Braune, S.; Gorbet, M.; Ratner, B.D.; Brash, J.L.; Latour, R.A.; Reviakine, I. BloodSurf 2017: News from the blood-biomaterial frontier. Acta Biomater. 2019, 87, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Chen, L.; Han, D.; Jiang, L. On improving blood compatibility: From bioinspired to synthetic design and fabrication of biointerfacial topography at micro/nano scales. Colloid Surf. B 2011, 85, 2–7. [Google Scholar] [CrossRef]
- Sundaram, S.; Lim, F.; Cooper, S.L.; Colman, R.W. Role of leucocytes in coagulation induced by artificial surfaces: Investigation of expression of Mac-1, granulocyte elastase release and leucocyte adhesion on modified polyurethanes. Biomaterials 1996, 17, 1041–1047. [Google Scholar] [CrossRef]
- Ward, W.K.; Wood, M.D.; Slobodzian, E.P. Continuous amperometric monitoring of subcutaneous oxygen in rabbit by telemetry. J. Med. Eng. Technol. 2002, 26, 158–167. [Google Scholar] [CrossRef]
- Ward, W.K.; Van Albert, S.; Bodo, M.; Pearce, F.; Gray, R.; Harlson, S.; Rebec, M.V. Design and Assessment of a Miniaturized Amperometric Oxygen Sensor in Rats and Pigs. IEEE Sens. J. 2010, 10, 1259–1265. [Google Scholar] [CrossRef]
- Makale, M.T.; Jablecki, M.C.; Gough, D.A. Mass transfer and gas-phase calibration of implanted oxygen sensors. Anal. Chem. 2004, 76, 1773–1777. [Google Scholar] [CrossRef]
- Kumosa, L.S.; Routh, T.L.; Lin, J.T.; Lucisano, J.Y.; Gough, D.A. Permeability of subcutaneous tissues surrounding long-term implants to oxygen. Biomaterials 2014, 35, 8287–8296. [Google Scholar] [CrossRef]
- Adatia, K.; Raja, M.; Vadgama, P. An electrochemical study of microporous track-etched membrane permeability and the effect of surface protein layers. Colloids Surf. B 2017, 158, 84–92. [Google Scholar] [CrossRef]
- Intaglietta, M.; Johnson, P.C.; Winslow, R.M. Microvascular and tissue oxygen distribution. Cardiovasc. Res. 1996, 32, 632–643. [Google Scholar] [CrossRef]
- Vovenko, E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: An experimental study on rats. Pflug. Arch. Eur. J. Physiol. 1999, 437, 617–623. [Google Scholar] [CrossRef]
- Sharan, M.; Vovenko, E.P.; Vadapalli, A.; Popel, A.S.; Pittman, R.N. Experimental and theoretical studies of oxygen gradients in rat pial microvessels. J. Cerebr. Blood Flow Metab. 2008, 28, 1597–1604. [Google Scholar] [CrossRef]
- Finnerty, N.J.; Bolger, F.B. In Vitro development and In Vivo application of a platinum-based electrochemical device for continuous measurements of peripheral tissue oxygen. Bioelectrochemistry 2018, 119, 124–135. [Google Scholar] [CrossRef]
- Russell, D.M.; Garry, E.M.; Taberner, A.J.; Barrett, C.J.; Paton, J.F.R.; Budgett, D.M.; Malpas, S.C. A fully implantable telemetry system for the chronic monitoring of brain tissue oxygen in freely moving rats. J. Neurosci. Methods 2012, 204, 242–248. [Google Scholar] [CrossRef]
- Weltin, A.; Ganatra, D.; Konig, K.; Joseph, K.; Hofmann, U.G.; Urban, G.A.; Kieninger, J. New life for old wires: Electrochemical sensor method for neural implants. J. Neural Eng. 2019, 17, 016007. [Google Scholar] [CrossRef]
- Lee, G.J.; Kim, S.K.; Kang, S.W.; Kim, O.K.; Chae, S.J.; Choi, S.; Shin, J.H.; Park, H.K.; Chung, J.H. Real time measurement of myocardial oxygen dynamics during cardiac ischemia-reperfusion of rats. Analyst 2012, 137, 5312–5319. [Google Scholar] [CrossRef]
- Valadka, A.B.; Gopinath, S.P.; Contant, C.F.; Uzura, M.; Robertson, C.S. Relationship of brain tissue Po-2 to outcome after severe head injury. Crit. Care Med. 1998, 26, 1576–1581. [Google Scholar] [CrossRef]
- Nortje, J.; Gupta, A.K. The role of tissue oxygen monitoring in patients with acute brain injury. Br. J. Anaesth. 2006, 97, 95–106. [Google Scholar] [CrossRef]
- Kennedy, A.; Ng, C.T.; Chang, T.C.; Biniecka, M.; O’Sullivan, J.N.; Heffernan, E.; Fearon, U.; Veale, D.J. Tumor Necrosis Factor Blocking Therapy Alters Joint Inflammation and Hypoxia. Arthritis Rheum. 2011, 63, 923–932. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Klitzman, B.; Braun, R.D.; Brizel, D.M.; Haroon, Z.A.; Secomb, T.W. Review of methods used to study oxygen transport at the microcirculatory level. Int. J. Cancer 2000, 90, 237–255. [Google Scholar] [CrossRef]
- Collingridge, D.R.; Young, W.K.; Vojnovic, B.; Wardman, P.; Lynch, E.M.; Hill, S.A.; Chaplin, D.J. Measurement of tumor oxygenation: A comparison between polarographic needle electrodes and a time-resolved luminescence-based optical sensor. Radiat. Res. 1997, 147, 329–334. [Google Scholar] [CrossRef]
- Vaupel, P.; Hockel, M.; Mayer, A. Detection and characterization of tumor hypoxia using pO(2) histography. Antioxid. Redox Signal. 2007, 9, 1221–1235. [Google Scholar] [CrossRef]
- De Santis, V.; Singer, M. Tissue oxygen tension monitoring of organ perfusion: Rationale, methodologies, and literature review. Br. J. Anaesth. 2015, 115, 357–365. [Google Scholar] [CrossRef]
- Ngwenya, L.B.; Burke, J.F.; Manley, G.T. Brain Tissue Oxygen Monitoring and the Intersection of Brain and Lung: A Comprehensive Review. Respir. Care 2016, 61, 1232–1244. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Menke, A.; Orchard, T.J.; Imperatore, G.; Bullard, K.M.; Mayer-Davis, E.; Cowie, C.C. The Prevalence of Type 1 Diabetes in the United States. Epidemiology 2013, 24, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, M.C.; Press, M. Management strategies for brittle diabetes. Ann. Enocinol. (Paris) 2006, 67, 287–294. [Google Scholar] [CrossRef]
- Mao, F.; Mano, N.; Heller, A. Long tethers binding redox centers to polymer backbones enhance electron transport in enzyme “wiring” hydrogels. J. Am. Chem. Soc. 2003, 125, 4951–4957. [Google Scholar] [CrossRef] [PubMed]
- Hoss, U.; Budiman, E.S.; Liu, H.; Christiansen, M.P. Continuous glucose monitoring in the subcutaneous tissue over a 14-day sensor wear period. J. Diabetes Sci. Technol. 2013, 7, 1210–1518. [Google Scholar] [CrossRef]
- Hoss, U.; Budiman, E.S. Factory-Calibrated Continuous Glucose Sensors: The Science behind the Technology. Diabetes Technol. Ther. 2017, 19, S44–S50. [Google Scholar] [CrossRef]
- Vettoretti, M.; Battocchio, C.; Sparacino, G.; Facchinetti, A. Development of an Error Model for a Factory-Calibrated Continuous Glucose Monitoring Sensor with 10-Day Lifetime. Sensors 2019, 19, 5320. [Google Scholar] [CrossRef]
- Mancini, G.; Berioli, M.G.; Santi, E.; Rogari, F.; Toni, G.; Tascini, G.; Crispoldi, R.; Ceccarini, G.; Esposito, S. Flash Glucose Monitoring: A Review of the Literature with a Special Focus on Type 1 Diabetes. Nutrients 2018, 10, 992. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef]
- Nery, E.W.; Kundys, M.; Jelen, P.S.; Jonsson-Niedziolka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef]
- Shichiri, M.; Kawamori, R.; Yamasaki, Y.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 1982, 2, 1129–1131. [Google Scholar] [CrossRef]
- Rigby, G.P.; Crump, P.W.; Vadgama, P. Stabilized needle electrode system for in vivo glucose monitoring based on open flow microperfusion. Analyst 1996, 121, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Rigby, G.P.; Ahmed, S.; Horseman, G.; Vadgama, P. In Vivo glucose monitoring with open microflow—Influences of fluid composition and preliminary evaluation in man. Anal. Chim. Acta 1999, 385, 23–32. [Google Scholar] [CrossRef]
- McGarraugh, G. The Chemistry of Commercial Continuous Glucose Monitors. Diabetes Technol. Ther. 2009, 11, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, B.; Knudsen, B.; Harmon, J.; Moussy, F.; Moussy, Y. Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromolecules 2008, 9, 561–567. [Google Scholar] [CrossRef]
- Galeska, I.; Hickey, T.; Moussy, F.; Kreutzer, D.; Papadimitrakopoulos, F. Characterization and biocompatibility studies of novel humic acids based films as membrane material for an implantable glucose sensor. Biomacromolecules 2001, 2, 1249–1255. [Google Scholar] [CrossRef]
- Yu, B.Z.; Ju, Y.M.; West, L.; Moussy, Y.; Moussy, F. An investigation of long-term performance of minimally invasive glucose biosensors. Diabetes Technol. Ther. 2007, 9, 265–275. [Google Scholar] [CrossRef]
- Dungel, P.; Long, N.; Yu, B.; Moussy, Y.; Moussy, F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J. Biomed. Mater. Res. A 2008, 85, 699–706. [Google Scholar] [CrossRef]
- Turner, R.F.B.; Harrison, D.J.; Rajotte, R.V. Preliminary in vivo biocompatibility studies on perfluorosulfonic acid polymer membranes for biosensor applications. Biomaterials 1991, 12, 361–368. [Google Scholar] [CrossRef]
- Gerritsen, M.; Kros, A.; Sprakel, V.; Lutterman, J.A.; Nolte, R.J.M.; Jansen, J.A. Biocompatibility evaluation of sol-gel coatings for subcutaneously implantable glucose sensors. Biomaterials 2000, 21, 71–78. [Google Scholar] [CrossRef]
- Quinn, C.A.P.; Connor, R.E.; Heller, A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials 1997, 18, 1665–1670. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.F.; Kingston, M.A.; Jones, G.; Wright, G.; Spencer, S.A. Glucose sensor with improved haemocompatibility. Biosens. Bioelectron. 2000, 15, 221–227. [Google Scholar] [CrossRef]
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Commis, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghanis, S.; et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2018, 2, 894–906. [Google Scholar] [CrossRef]
- Burugapalli, K.; Wijesuriya, S.; Wang, N.; Song, W.H. Biomimetic electrospun coatings increase the in vivo sensitivity of implantable glucose biosensors. J. Biomed. Mater. Res. A 2018, 106, 1072–1081. [Google Scholar] [CrossRef]
- Gough, D.A.; Kumosa, L.S.; Routh, T.L.; Lin, J.T.; Lucisano, J.Y. Function of an Implanted Tissue Glucose Sensor for More than 1 Year in Animals. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Lucisano, J.Y.; Routh, T.L.; Lin, J.T.; Gough, D.A. Glucose Monitoring in Individuals With Diabetes Using a Long-Term Implanted Sensor/Telemetry System and Model. IEEE Trans. Biomed. Eng. 2017, 64, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.C.; Barwell, N.P.; Gopal, P.; Gopichand, M.; Higgs, T.; James, T.D.; Jones, C.M.; Mackenzie, A.; Mulavisala, K.P.; Paterson, W. The Development of a Continuous Intravascular Glucose Monitoring Sensor. J. Diabetes Sci. Technol. 2015, 9, 751–761. [Google Scholar] [CrossRef]
- Ahyeon, K.; Scott, P.N.; Schoenfisch, M.H. Glucose sensor membranes for mitigating the foreign body response. J. Diabetes Sci. Technol. 2011, 5, 1052–1059. [Google Scholar]
- Vallejo-Heligon, S.G.; Brown, N.L.; Reichert, W.M.; Klitzman, B. Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 2016, 30, 106–115. [Google Scholar] [CrossRef]
- Norton, L.W.; Koschwanez, H.E.; Wisniewski, N.A.; Klitzman, B.; Reichert, W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. A 2007, 81, 858–869. [Google Scholar] [CrossRef]
- Ward, W.K.; Quinn, M.J.; Wood, M.D.; Tiekotter, K.L.; Pidikiti, S.; Gallagher, J.A. Vascularizing the tissue surrounding a model biosensor: How localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens. Bioelectron. 2003, 19, 155–163. [Google Scholar] [CrossRef]
- Gu, B.; Papadimitrakopoulos, F.; Burgess, D.J. PLGA microsphere/PVA hydrogel coatings suppress the foreign body reaction for 6 months. J. Control. Release 2018, 289, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Wang, Y.; Qiang, L.; Burgess, D.J.; Papadimitrakopoulos, F. Microsphere Erosion in Outer Hydrogel Membranes Creating Macroscopic Porosity to Counter Biofouling-Induced Sensor Degradation. Anal. Chem. 2012, 84, 8837–8845. [Google Scholar] [CrossRef]
- Gifford, R.; Batchelor, M.M.; Lee, Y.; Gokulrangan, G.; Meyerhoff, M.E.; Wilson, G.S. Mediation of in vivo glucose sensor inflammatory response via nitric oxide release. J. Biomed. Mater. Res. A 2005, 75, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Avula, M.; Jones, D.; Rao, A.N.; McClain, D.; McGill, L.D.; Grainger, D.W.; Solzbacher, F. Local release of masitinib alters in vivo implantable continuous glucose sensor performance. Biosens. Bioelectron. 2016, 77, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Ratner, B.D. Biomaterials: Been There, Done That, and Evolving into the Future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Mathur, A.B.; Collier, T.O.; Kao, W.J.; Wiggins, M.; Schubert, M.A.; Hiltner, A.; Anderson, J.M. In Vivo biocompatibility and biostability of modified polyurethanes. J. Biomed. Mater. Res. 1997, 36, 246–257. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Klueh, U.; Frailey, J.T.; Qiao, Y.; Antar, O.; Kreutzera, D.L. Cell based metabolic barriers to glucose diffusion: Macrophages and continuous glucose monitoring. Biomaterials 2014, 35, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Woderer, S.; Kloetzer, H.M.; Staib, A.; Gillen, R.; Li, L.; Yu, X.L.; Gretz, N.; Kraenzlin, B.; Pill, J. Tissue response to subcutaneous implantation of glucose-oxidase-based glucose sensors in rats. Biosens. Bioelectron. 2007, 23, 26–34. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, P.M.; McClain, E.S.; Williams, I.M.; Malabanan, C.M.; James, F.D.; Lord, P.C.; Gregory, J.M.; Cliffel, D.E.; Wasserman, D.H. Fibrotic Encapsulation Is the Dominant Source of Continuous Glucose Monitor Delays. Diabetes 2019, 68, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.T.; Yuan, F.; Reichert, W.M. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal. Bioanal. Chem. 2010, 398, 1695–1705. [Google Scholar] [CrossRef]
- Novak, M.T.; Yuan, F.; Reichert, W.M. Macrophage embedded fibrin gels: An in vitro platform for assessing inflammation effects on implantable glucose sensors. Biomaterials 2014, 35, 9563–9572. [Google Scholar] [CrossRef]
- Wiig, H. Pathophysiology of tissue fluid accumulation in inflammation. J. Physiol. Lond. 2011, 589, 2945–2953. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. L-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Ganesana, M.; Karimi, A.; Leiter, J.C.; Andreescu, S. Platinum-Doped Ceria Based Biosensor for In Vitro and In Vivo Monitoring of Lactate during Hypoxia. Anal. Chem. 2015, 87, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Renehan, K.; Ho, K.K.Y.; Carr, B.D.; Chen, C.V.; Cornell, M.S.; Ye, M.Y.; Rojas-Pena, A.; Chen, H. Evaluation of Continuous Lactate Monitoring Systems within a Heparinized In Vivo Porcine Model Intravenously and Subcutaneously. Biosensors 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.M.; Leitao, E.; Popplewell, J.; Alp, B.; Vadgama, P. Needle enzyme electrode for lactate measurement in vivo. IEEE Sens. J. 2008, 8, 113–120. [Google Scholar] [CrossRef]
- MacLean, D.A.; Bangsbo, J.; Saltin, B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J. Appl. Physiol. 1999, 87, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Gowers, S.A.N.; Curto, V.F.; Seneci, C.A.; Wang, C.; Anastasova, S.; Vadgama, P.; Yang, G.Z.; Boutelle, M.G. 3D Printed Microfluidic Device with Integrated Biosensors for Online Analysis of Subcutaneous Human Microdialysate. Anal. Chem. 2015, 87, 7763–7770. [Google Scholar] [CrossRef] [PubMed]

 ) venous blood glucose, tissue glucose at 60 µL/h microflow (
) venous blood glucose, tissue glucose at 60 µL/h microflow ( ) and at a constrained flow of 10 µL/h (
) and at a constrained flow of 10 µL/h ( ) showing underestimated glucose and total loss of response with clamped flow. Bolus tail vein administration of glucose (G) and insulin (I). Adapted from [83].
) showing underestimated glucose and total loss of response with clamped flow. Bolus tail vein administration of glucose (G) and insulin (I). Adapted from [83].
 ) venous blood glucose, tissue glucose at 60 µL/h microflow (
) venous blood glucose, tissue glucose at 60 µL/h microflow ( ) and at a constrained flow of 10 µL/h (
) and at a constrained flow of 10 µL/h ( ) showing underestimated glucose and total loss of response with clamped flow. Bolus tail vein administration of glucose (G) and insulin (I). Adapted from [83].
) showing underestimated glucose and total loss of response with clamped flow. Bolus tail vein administration of glucose (G) and insulin (I). Adapted from [83].
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadgama, P. Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity. Sensors 2020, 20, 3149. https://doi.org/10.3390/s20113149
Vadgama P. Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity. Sensors. 2020; 20(11):3149. https://doi.org/10.3390/s20113149
Chicago/Turabian StyleVadgama, Pankaj. 2020. "Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity" Sensors 20, no. 11: 3149. https://doi.org/10.3390/s20113149
APA StyleVadgama, P. (2020). Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity. Sensors, 20(11), 3149. https://doi.org/10.3390/s20113149




