Bent DNA Bows as Sensing Amplifiers for Detecting DNA-Interacting Salts and Molecules
Abstract
1. Introduction
2. Materials and Methods
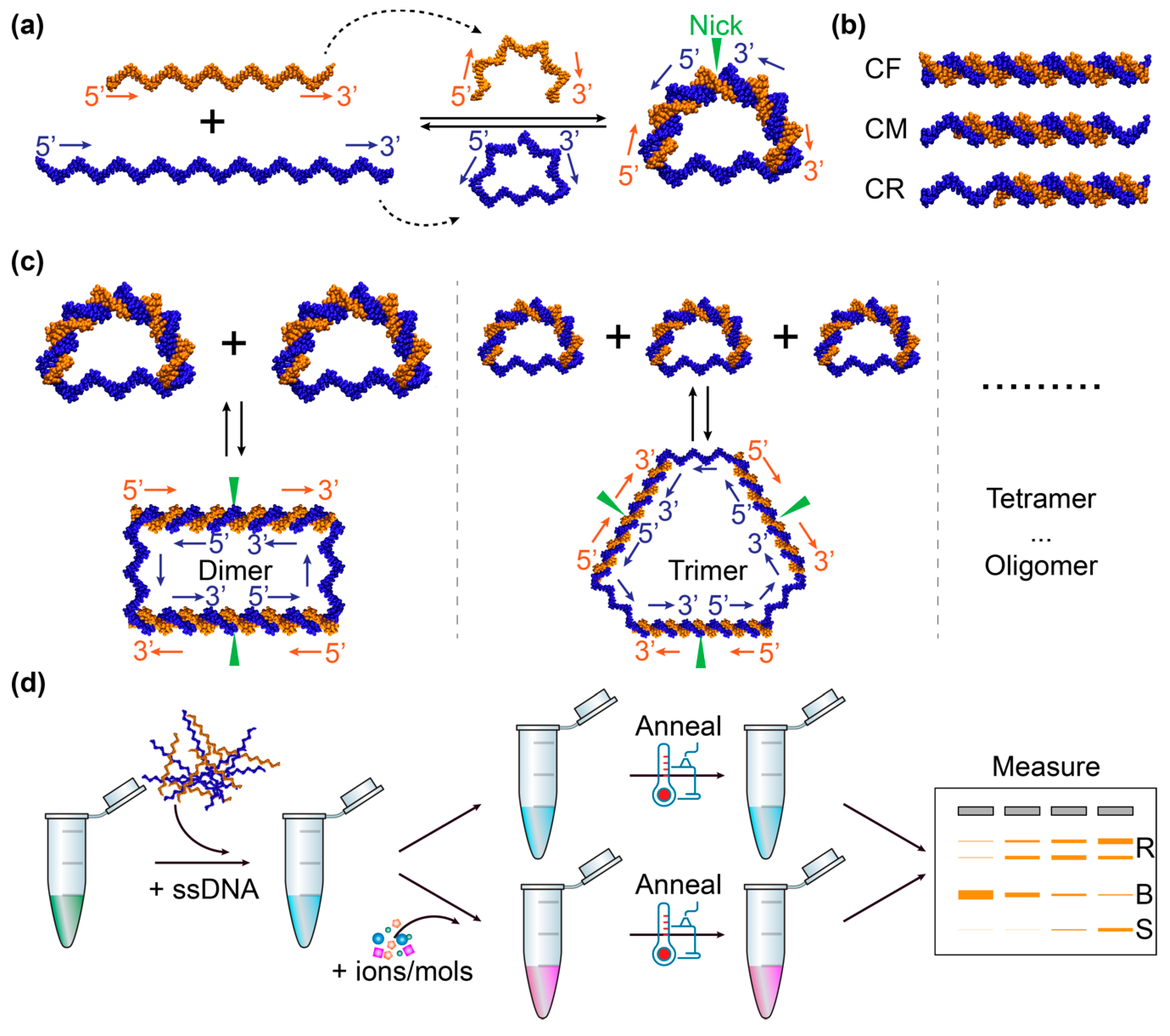
2.1. Construction of DNA Bows
2.2. Detection of DNA-Interacting Salts/Molecules Using DNA Bows
2.3. Gel Electrophoresis
2.4. Visualization of DNA Bows Using Transmission Electron Microscopy (TEM)
3. Results
3.1. Visualization of Bent DNA Bows Using TEM
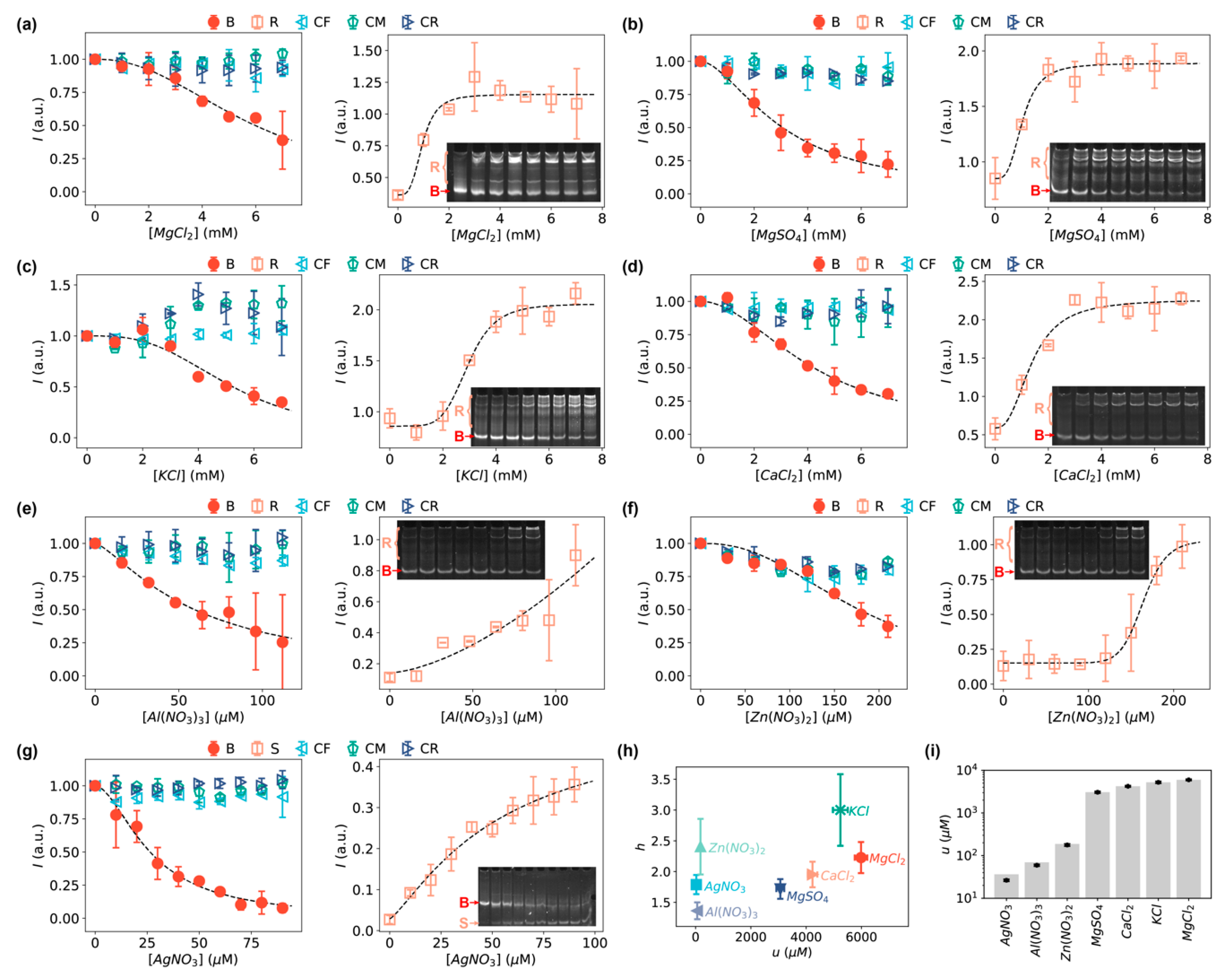
3.2. Detection of Inorganic Ions Using Bent DNA Bows
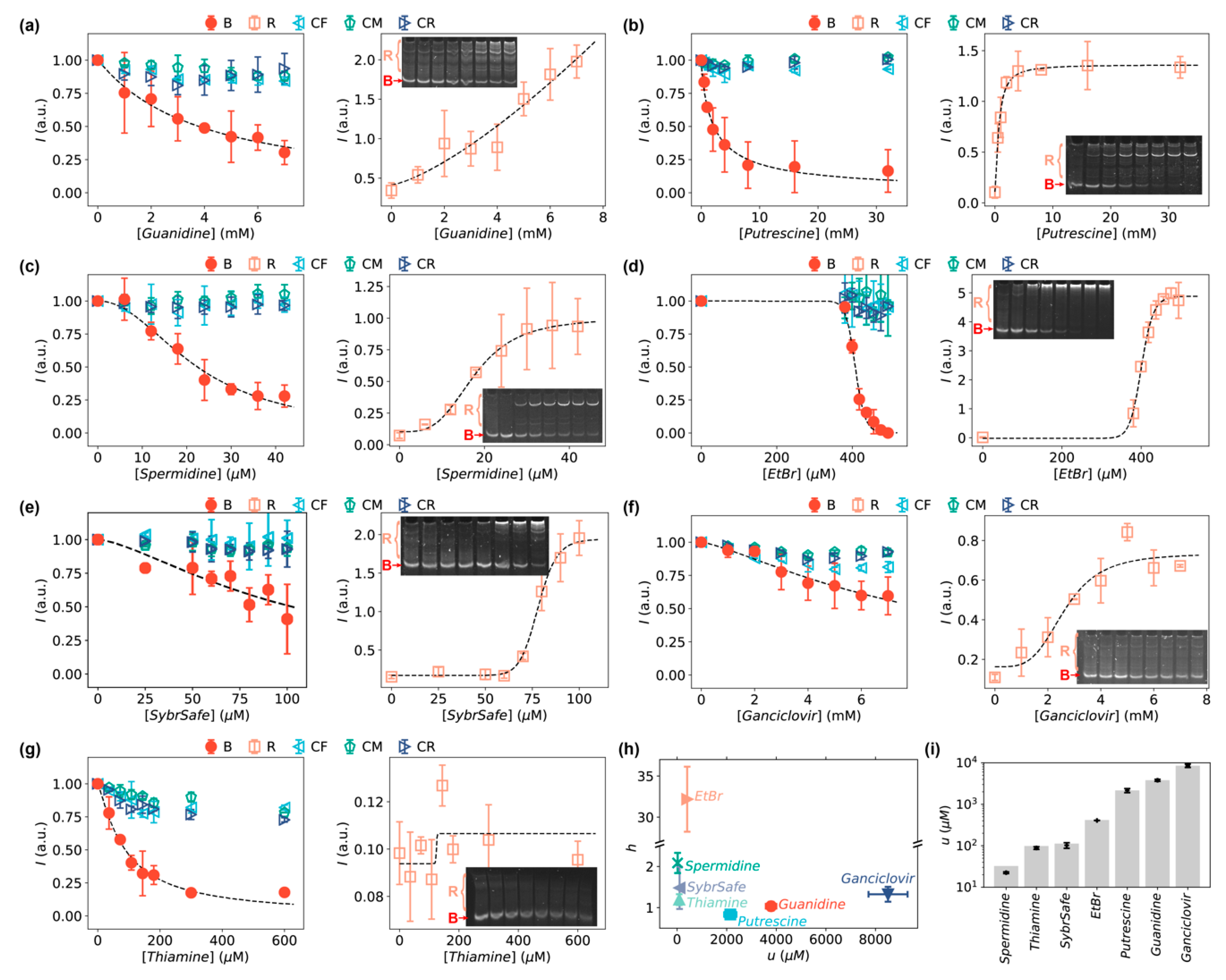
3.3. Detection of Small Organic Molecules Using Bent DNA Bows
4. Discussions and Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lipfert, J.; Doniach, S.; Das, R.; Herschlag, D. Understanding nucleic acid-ion interactions. Annu. Rev. Biochem. 2014, 83, 813–841. [Google Scholar] [CrossRef] [PubMed]
- Wanunu, M.; Tor, Y. Methods for Studying Nucleic Acid/Drug Interactions, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bui, V.-C.; Nguyen, T.-H. DNA aggregation induced by Mg2+ ions under different conditions. J. Mol. Recognit. 2018, 31, e2721. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Choi, S.; Sang Cho, Y.; Yang, S.-J.; Cho, Y.-S.; Kim, K.K. Magnesium ions enhance infiltration of osteoblasts in scaffolds via increasing cell motility. J. Mater. Sci. Mater. Med. 2017, 28, 96. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.E.; Grilley, D.; Soto, A.M. Ions and RNA folding. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 221–243. [Google Scholar] [CrossRef]
- Yang, L.; Arora, K.; Beard, W.A.; Wilson, S.H.; Schlick, T. Critical role of magnesium ions in DNA polymerase beta’s closing and active site assembly. J. Am. Chem. Soc. 2004, 126, 8441–8453. [Google Scholar] [CrossRef]
- Ivanov, I.; Tainer, J.A.; McCammon, J.A. Unraveling the three-metal-ion catalytic mechanism of the DNA repair enzyme endonuclease IV. Proc. Natl. Acad. Sci. USA 2007, 104, 1465–1470. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002, 42, 79–91. [Google Scholar] [CrossRef]
- Fei, B.-L.; Xu, W.-S.; Tao, H.-W.; Li, W.; Zhang, Y.; Long, J.-Y.; Liu, Q.-B.; Xia, B.; Sun, W.-Y. Effects of copper ions on DNA binding and cytotoxic activity of a chiral salicylidene Schiff base. J. Photochem. Photobiol. B Biol. 2014, 132, 36–44. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar]
- Hartwig, A.; Asmuss, M.; Ehleben, I.; Herzer, U.; Kostelac, D.; Pelzer, A.; Schwerdtle, T.; Bürkle, A. Interference by toxic metal ions with DNA repair processes and cell cycle control: Molecular mechanisms. Environ. Health Perspect. 2002, 110 (Suppl. 5), 797–799. [Google Scholar] [CrossRef]
- Asmuss, M.; Mullenders, L.H.; Hartwig, A. Interference by toxic metal compounds with isolated zinc finger DNA repair proteins. Toxicol. Lett. 2000, 112–113, 227–231. [Google Scholar] [CrossRef]
- Bal, W.; Maria Protas, A.; Kasprzak, K.S. Genotoxicity of metal ions: Chemical insights. In Metal Ions in Toxicology: Effects, Interactions, Interdependencies; Metal Ions in Life Sciences; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 319–373. [Google Scholar]
- Liang, L.; Shen, J.-W.; Wang, Q. Molecular dynamics study on DNA nanotubes as drug delivery vehicle for anticancer drugs. Colloids Surf. B Biointerfaces 2017, 153, 168–173. [Google Scholar] [CrossRef]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef]
- Hurley, L.H. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2002, 2, 188–200. [Google Scholar] [CrossRef]
- Agudelo, D.; Bourassa, P.; Bérubé, G.; Tajmir-Riahi, H.-A. Intercalation of antitumor drug doxorubicin and its analogue by DNA duplex: Structural features and biological implications. Int. J. Biol. Macromol. 2014, 66, 144–150. [Google Scholar] [CrossRef]
- Wang, M.D.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Stretching DNA with optical tweezers. Biophys. J. 1997, 72, 1335–1346. [Google Scholar] [CrossRef]
- Bustamante, C.; Smith, S.B.; Liphardt, J.; Smith, D. Single-molecule studies of DNA mechanics. Curr. Opin. Struct. Biol. 2000, 10, 279–285. [Google Scholar] [CrossRef]
- Baumann, C.G.; Smith, S.B.; Bloomfield, V.A.; Bustamante, C. Ionic effects on the elasticity of single DNA molecules. Proc. Natl. Acad. Sci. USA 1997, 94, 6185–6190. [Google Scholar] [CrossRef]
- McCauley, M.; Hardwidge, P.R.; Maher, L.J.; Williams, M.C. Dual binding modes for an HMG domain from human HMGB2 on DNA. Biophys. J. 2005, 89, 353–364. [Google Scholar] [CrossRef]
- Rouzina, I.; Bloomfield, V.A. Force-induced melting of the DNA double helix Thermodynamic analysis. Biophys. J. 2001, 80, 882–893. [Google Scholar] [CrossRef]
- Rouzina, I.; Bloomfield, V.A. Force-induced melting of the DNA double helix. Effect of solution conditions. Biophys. J. 2001, 80, 894–900. [Google Scholar] [CrossRef]
- Scipioni, A.; Anselmi, C.; Zuccheri, G.; Samori, B.; De Santis, P. Sequence-dependent DNA curvature and flexibility from scanning force microscopy images. Biophys. J. 2002, 83, 2408–2418. [Google Scholar] [CrossRef]
- Tinoco, I.; Li, P.T.X.; Bustamante, C. Determination of thermodynamics and kinetics of RNA reactions by force. Q. Rev. Biophys. 2006, 39, 325–360. [Google Scholar] [CrossRef]
- Ceglarek, J.A.; Revzin, A. Studies of DNA-protein interactions by gel electrophoresis. Electrophoresis 1989, 10, 360–365. [Google Scholar] [CrossRef]
- Garner, M.M.; Revzin, A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: Application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981, 9, 3047–3060. [Google Scholar] [CrossRef]
- Guédin, A.; Lacroix, L.; Mergny, J.-L. Thermal melting studies of ligand DNA interactions. Methods Mol. Biol. 2010, 613, 25–35. [Google Scholar]
- LiCata, V.J.; Wowor, A.J. Applications of fluorescence anisotropy to the study of protein–dna interactions. In Biophysical Tools for Biologists, Volume One: In Vitro Techniques; Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 84, pp. 243–262. [Google Scholar]
- Cary, P.D.; Kneale, G.G. Circular dichroism for the analysis of protein-DNA interactions. Methods Mol. Biol. 2009, 543, 613–624. [Google Scholar]
- Garbett, N.C.; Ragazzon, P.A.; Chaires, J.B. Circular dichroism to determine binding mode and affinity of ligand-DNA interactions. Nat. Protoc. 2007, 2, 3166–3172. [Google Scholar] [CrossRef]
- Buurma, N.J.; Haq, I. Advances in the analysis of isothermal titration calorimetry data for ligand-DNA interactions. Methods 2007, 42, 162–172. [Google Scholar] [CrossRef]
- Czapla-Masztafiak, J.; Kwiatek, W.M.; Sá, J.; Szlachetko, J. X-ray spectroscopy on biological systems. In X-ray Scattering; Ares, A.E., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Prosser, K.E.; Walsby, C.J. Electron paramagnetic resonance as a tool for studying the mechanisms of paramagnetic anticancer metallodrugs. Eur. J. Inorg. Chem. 2017, 2017, 1573–1585. [Google Scholar] [CrossRef]
- Duguid, J.G.; Bloomfield, V.A.; Benevides, J.M.; Thomas, G.J. Raman spectroscopy of DNA-metal complexes. II. The thermal denaturation of DNA in the presence of Sr2+, Ba2+, Mg2+, Ca2+, Mn2+, Co2+, Ni2+, and Cd2+. Biophys. J. 1995, 69, 2623–2641. [Google Scholar] [CrossRef]
- Campagne, S.; Gervais, V.; Milon, A. Nuclear magnetic resonance analysis of protein-DNA interactions. J. R. Soc. Interface 2011, 8, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Interplay between Metal, Ions and Nucleic Acids; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal ions in life sciences; Springer Netherlands: Dordrecht, The Netherlands, 2012; Volume 10. [Google Scholar]
- Qu, H.; Tseng, C.-Y.; Wang, Y.; Levine, A.J.; Zocchi, G. The elastic energy of sharply bent nicked DNA. Europhys. Lett. 2010, 90, 18003. [Google Scholar] [CrossRef]
- Qu, H.; Zocchi, G. The complete bending energy function for nicked DNA. EPL 2011, 94, 18003. [Google Scholar] [CrossRef]
- Freeland, J.; Khadka, P.; Wang, Y. Mechanical-energy-based amplifiers for probing interactions of DNA with metal ions. Phys. Rev. E 2018, 98, 062403. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Y.; Tseng, C.-Y.; Zocchi, G. Critical Torque for Kink Formation in Double-Stranded DNA. Phys. Rev. X 2011, 1, 021008. [Google Scholar] [CrossRef]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef]
- Hickenboth, C.R.; Moore, J.S.; White, S.R.; Sottos, N.R.; Baudry, J.; Wilson, S.R. Biasing reaction pathways with mechanical force. Nature 2007, 446, 423–427. [Google Scholar] [CrossRef]
- Beaudet, M.P.; Cox, G.W.; Yue, S. Detection of Immobilized Nucleic Acid. Patent CA2540508A1, 14 April 2005. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.R. Biomedical Image Processing. Computer 1983, 16, 22–34. [Google Scholar] [CrossRef]
- Oliver, R.M. Negative stain electron microscopy of protein macromolecules. In Part D: Enzyme Structure; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1973; Volume 27, pp. 617–672. [Google Scholar]
- Marini, M.; Falqui, A.; Moretti, M.; Limongi, T.; Allione, M.; Genovese, A.; Lopatin, S.; Tirinato, L.; Das, G.; Torre, B.; et al. The structure of DNA by direct imaging. Sci. Adv. 2015, 1, e1500734. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.P.; Maher, L.J. DNA curvature and flexibility in vitro and in vivo. Q. Rev. Biophys. 2010, 43, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Trohalaki, S.; Frisch, H.L.; Lerman, L.S. The effects of lithium, rubidium, cesium and magnesium ions on the close packing of persistence-length DNA fragments. Biophys. Chem. 1991, 40, 197–205. [Google Scholar] [CrossRef]
- Chen, P.; Sjogren, C.A.; Larsen, P.B.; Schnittger, A. A multi-level response to DNA damage induced by aluminium. Plant J. 2019, 98, 479–491. [Google Scholar] [CrossRef]
- Lankoff, A.; Banasik, A.; Duma, A.; Ochniak, E.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Wojcik, A. A comet assay study reveals that aluminium induces DNA damage and inhibits the repair of radiation-induced lesions in human peripheral blood lymphocytes. Toxicol. Lett. 2006, 161, 27–36. [Google Scholar] [CrossRef]
- Yegin, S.Ç.; Dede, S.; Mis, L.; Yur, F. Effects of Zinc Supplementation on DNA Damage in Rats with Experimental Kidney Deficiency. Biol. Trace Elem. Res. 2017, 176, 338–341. [Google Scholar] [CrossRef]
- Nejdl, L.; Ruttkay-Nedecky, B.; Kudr, J.; Krizkova, S.; Smerkova, K.; Dostalova, S.; Vaculovicova, M.; Kopel, P.; Zehnalek, J.; Trnkova, L.; et al. DNA interaction with zinc(II) ions. Int. J. Biol. Macromol. 2014, 64, 281–287. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Haddy, F.J.; Vanhoutte, P.M.; Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R546–R552. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhu, R.; Zhu, L.; Qiu, T.; Cao, Z.; Kang, T. Potassium channels: Structures, diseases, and modulators. Chem. Biol. Drug Des. 2014, 83, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. The role of calcium in the control of cell function. In Integration of Mitochondrial Function; Lemasters, J.J., Hackenbrock, C.R., Thurman, R.G., Westerhoff, H.V., Eds.; Springer: Boston, MA, USA, 1988; pp. 475–485. [Google Scholar]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and potassium in health and disease. Met. Ions Life Sci. 2013, 13, 29–47. [Google Scholar] [PubMed]
- Sharma, N.; Rather, M.A.; Ajima, M.N.O.; Gireesh-Babu, P.; Kumar, K.; Sharma, R. Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nanoparticles. Food Chem. Toxicol. 2016, 96, 122–132. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Casanova-Morales, N.; Alavi, Z.; Wilson, C.A.M.; Zocchi, G. Identifying chaotropic and kosmotropic agents by nanorheology. J. Phys. Chem. B 2018, 122, 3754–3759. [Google Scholar] [CrossRef]
- O’Sullivan, P.; Rozas, I. Understanding the guanidine-like cationic moiety for optimal binding into the DNA minor groove. ChemMedChem 2014, 9, 2065–2073. [Google Scholar] [CrossRef]
- Publio, B.C.; Moura, T.A.; Lima, C.H.M.; Rocha, M.S. Biophysical characterization of the DNA interaction with the biogenic polyamine putrescine: A single molecule study. Int. J. Biol. Macromol. 2018, 112, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Vertino, P.M.; Bergeron, R.J.; Cavanaugh, P.F.; Porter, C.W. Structural determinants of spermidine-DNA interactions. Biopolymers 1987, 26, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Ramana, M.M.V.; Betkar, R.; Nimkar, A.; Ranade, P.; Mundhe, B.; Pardeshi, S. Synthesis of a novel 4H-pyran analog as minor groove binder to DNA using ethidium bromide as fluorescence probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.; Whyte, L.G.; Greer, C.W. Development of a SYBR safe technique for the sensitive detection of DNA in cesium chloride density gradients for stable isotope probing assays. J. Microbiol. Methods 2008, 73, 199–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prütz, W.A. Inhibition of DNA-ethidium bromide intercalation due to free radical attack upon DNA. Radiat. Environ. Biophys. 1984, 23, 7–18. [Google Scholar] [CrossRef] [PubMed]
- St Clair, M.H.; Lambe, C.U.; Furman, P.A. Inhibition by ganciclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Antimicrob. Agents Chemother. 1987, 31, 844–849. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Battiwalla, M.; Wu, Y.; Bajwa, R.P.S.; Radovic, M.; Almyroudis, N.G.; Segal, B.H.; Wallace, P.K.; Nakamura, R.; Padmanabhan, S.; Hahn, T.; et al. Ganciclovir inhibits lymphocyte proliferation by impairing DNA synthesis. Biol. Blood Marrow Transplant. 2007, 13, 765–770. [Google Scholar] [CrossRef]
- de Hernandez-Vazquez, A.J.; Garcia-Sanchez, J.A.; Moreno-Arriola, E.; Salvador-Adriano, A.; Ortega-Cuellar, D.; Velazquez-Arellano, A. Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: Findings are parallel to those of biotin deficiency and have implications for energy disorders. J. Nutrigenet. Nutrigenom. 2016, 9, 287–299. [Google Scholar] [CrossRef]
- Wang, J.; Cai, R.; Xu, J.; Liu, Z. Study on the effect of thiamine on the metabolism of yeast by intrinsic fluorescence. Luminescence 2005, 20, 216–219. [Google Scholar] [CrossRef]
- Winkler, W.; Nahvi, A.; Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 2002, 419, 952–956. [Google Scholar] [CrossRef]
- Sadoon, A.A.; Khadka, P.; Freeland, J.; Gundampati, R.K.; Manso, R.H.; Ruiz, M.; Krishnamurthi, V.R.; Thallapuranam, S.K.; Chen, J.; Wang, Y. Silver Ions Caused Faster Diffusive Dynamics of Histone-Like Nucleoid-Structuring Proteins in Live Bacteria. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Njagi, J.I.; Kagwanja, S.M. The interface in biosensing: Improving selectivity and sensitivity. In Interfaces and Interphases in Analytical Chemistry; Helburn, R., Vitha, M.F., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1062, pp. 225–247. [Google Scholar]
- Wild, E.J.; Tabrizi, S.J. Therapies targeting DNA and RNA in Huntington’s disease. Lancet Neurol. 2017, 16, 837–847. [Google Scholar] [CrossRef]
- Eberle, R.P.; Hari, Y.; Schürch, S. Transition Metal-based Anticancer Drugs Targeting Nucleic Acids: A Tandem Mass Spectrometric Investigation. Chimia 2017, 71, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Vafabakhsh, R.; Ha, T. Extreme bendability of DNA less than 100 base pairs long revealed by single-molecule cyclization. Science 2012, 337, 1097–1101. [Google Scholar] [CrossRef]
- Crothers, D.M.; Drak, J.; Kahn, J.D.; Levene, S.D. DNA bending, flexibility, and helical repeat by cyclization kinetics. In DNA Structures Part B: Chemical and Electrophoretic Analysis of DNA; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 212, pp. 3–29. [Google Scholar]
- Du, Q.; Smith, C.; Shiffeldrim, N.; Vologodskaia, M.; Vologodskii, A. Cyclization of short DNA fragments and bending fluctuations of the double helix. Proc. Natl. Acad. Sci. USA 2005, 102, 5397–5402. [Google Scholar] [CrossRef]
- Hagerman, P.J.; Ramadevi, V.A. Application of the method of phage T4 DNA ligase-catalyzed ring-closure to the study of DNA structure. I. Computational analysis. J. Mol. Biol. 1990, 212, 351–362. [Google Scholar] [CrossRef]
- Kahn, J.D.; Crothers, D.M. Protein-induced bending and DNA cyclization. Proc. Natl. Acad. Sci. USA 1992, 89, 6343–6347. [Google Scholar] [CrossRef]
- Podtelezhnikov, A.A.; Mao, C.; Seeman, N.C.; Vologodskii, A. Multimerization-cyclization of DNA fragments as a method of conformational analysis. Biophys. J. 2000, 79, 2692–2704. [Google Scholar] [CrossRef]
- Shore, D.; Langowski, J.; Baldwin, R.L. DNA flexibility studied by covalent closure of short fragments into circles. Proc. Natl. Acad. Sci. USA 1981, 78, 4833–4837. [Google Scholar] [CrossRef]
- Taylor, W.H.; Hagerman, P.J. Application of the method of phage T4 DNA ligase-catalyzed ring-closure to the study of DNA structure. II. NaCl-dependence of DNA flexibility and helical repeat. J. Mol. Biol. 1990, 212, 363–376. [Google Scholar] [CrossRef]
- Vologodskaia, M.; Vologodskii, A. Contribution of the intrinsic curvature to measured DNA persistence length. J. Mol. Biol. 2002, 317, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Crothers, D.M. High-throughput approach for detection of DNA bending and flexibility based on cyclization. Proc. Natl. Acad. Sci. USA 2003, 100, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Yamakawa, H. Ring-closure probabilities for twisted wormlike chains. Application to DNA. Macromolecules 1984, 17, 689–698. [Google Scholar] [CrossRef]
- Fields, A.P.; Meyer, E.A.; Cohen, A.E. Euler buckling and nonlinear kinking of double-stranded DNA. Nucleic Acids Res. 2013, 41, 9881–9890. [Google Scholar] [CrossRef]
- Slater, G.W. DNA gel electrophoresis: The reptation model(s). Electrophoresis 2009, 30 (Suppl. 1), S181–S187. [Google Scholar] [CrossRef]
- Stellwagen, N.C. Electrophoresis of DNA in agarose gels, polyacrylamide gels and in free solution. Electrophoresis 2009, 30 (Suppl. 1), S188–S195. [Google Scholar] [CrossRef]
- Saucedo, S.R. Modeling the dynamics of gel electrophorresis in the high school classroom. Phys. Teach. 2013, 51, 28–31. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Zwieb, C.; Wu, C.; Adhya, S. Bending of DNA by gene-regulatory proteins: Construction and use of a DNA bending vector. Gene 1989, 85, 15–23. [Google Scholar] [CrossRef]
- Cong, P.; Dai, L.; Chen, H.; van der Maarel, J.R.C.; Doyle, P.S.; Yan, J. Revisiting the anomalous bending elasticity of sharply bent DNA. Biophys. J. 2015, 109, 2338–2351. [Google Scholar] [CrossRef]
- Dame, R.T.; Wyman, C.; Goosen, N. Structural basis for preferential binding of H-NS to curved DNA. Biochimie 2001, 83, 231–234. [Google Scholar] [CrossRef]
- Yamada, H.; Muramatsu, S.; Mizuno, T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J. Biochem. 1990, 108, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, H.D. Base-Pair Mismatch Can Destabilize Small DNA Loops through Cooperative Kinking. Phys. Rev. Lett. 2019, 122, 218101. [Google Scholar] [CrossRef] [PubMed]
- Shirude, P.S.; Okumus, B.; Ying, L.; Ha, T.; Balasubramanian, S. Single-molecule conformational analysis of G-quadruplex formation in the promoter DNA duplex of the proto-oncogene c-kit. J. Am. Chem. Soc. 2007, 129, 7484–7485. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Okumus, B.; Kim, D.S.; Ha, T. Extreme conformational diversity in human telomeric DNA. Proc. Natl. Acad. Sci. USA 2005, 102, 18938–18943. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, H.D. Determinants of cyclization-decyclization kinetics of short DNA with sticky ends. Nucleic Acids Res. 2020, 48, 5147–5156. [Google Scholar] [CrossRef]
- Jeong, J.; Le, T.T.; Kim, H.D. Single-molecule fluorescence studies on DNA looping. Methods 2016, 105, 34–43. [Google Scholar] [CrossRef]

| Salt/Molecule | Concentrations |
|---|---|
| MgCl2 | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| MgSO4 | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| KCl | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| CaCl2 | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| Al(NO3)3 | 0, 16, 32, 48, 64, 80, 96, 112 µM |
| Zn(NO3)2 | 0, 30, 60, 90, 120, 150, 180, 210 µM |
| AgNO3 | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90 µM |
| Guanidine | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| Putrescine | 0, 0.5, 1, 2, 4, 8, 16, 32 mM |
| Spermidine | 0, 6, 12, 18, 24, 30, 36, 42 µM |
| Ganciclovir | 0, 1, 2, 3, 4, 5, 6, 7 mM |
| Thiamine | 0, 36, 72, 108, 144, 180, 300, 600 µM |
| Ethidium Bromide | 0, 381, 400, 419, 438, 457, 476, 495 µM |
| SYBR Safe * | 0, 25, 50, 60, 70, 80, 90, 100 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeland, J.; Zhang, L.; Wang, S.-T.; Ruiz, M.; Wang, Y. Bent DNA Bows as Sensing Amplifiers for Detecting DNA-Interacting Salts and Molecules. Sensors 2020, 20, 3112. https://doi.org/10.3390/s20113112
Freeland J, Zhang L, Wang S-T, Ruiz M, Wang Y. Bent DNA Bows as Sensing Amplifiers for Detecting DNA-Interacting Salts and Molecules. Sensors. 2020; 20(11):3112. https://doi.org/10.3390/s20113112
Chicago/Turabian StyleFreeland, Jack, Lihua Zhang, Shih-Ting Wang, Mason Ruiz, and Yong Wang. 2020. "Bent DNA Bows as Sensing Amplifiers for Detecting DNA-Interacting Salts and Molecules" Sensors 20, no. 11: 3112. https://doi.org/10.3390/s20113112
APA StyleFreeland, J., Zhang, L., Wang, S.-T., Ruiz, M., & Wang, Y. (2020). Bent DNA Bows as Sensing Amplifiers for Detecting DNA-Interacting Salts and Molecules. Sensors, 20(11), 3112. https://doi.org/10.3390/s20113112





