The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia
Abstract
1. Introduction
2. Materials and Methods
2.1. Requirements for the Water Bolus Fluid
2.2. Preparation of the Samples
2.3. MR Relaxometry Measurements
2.4. Electromagnetic Property Measurements
2.5. Effects on Power Deposition Pattern
2.6. Effects on MRT Precision
3. Results
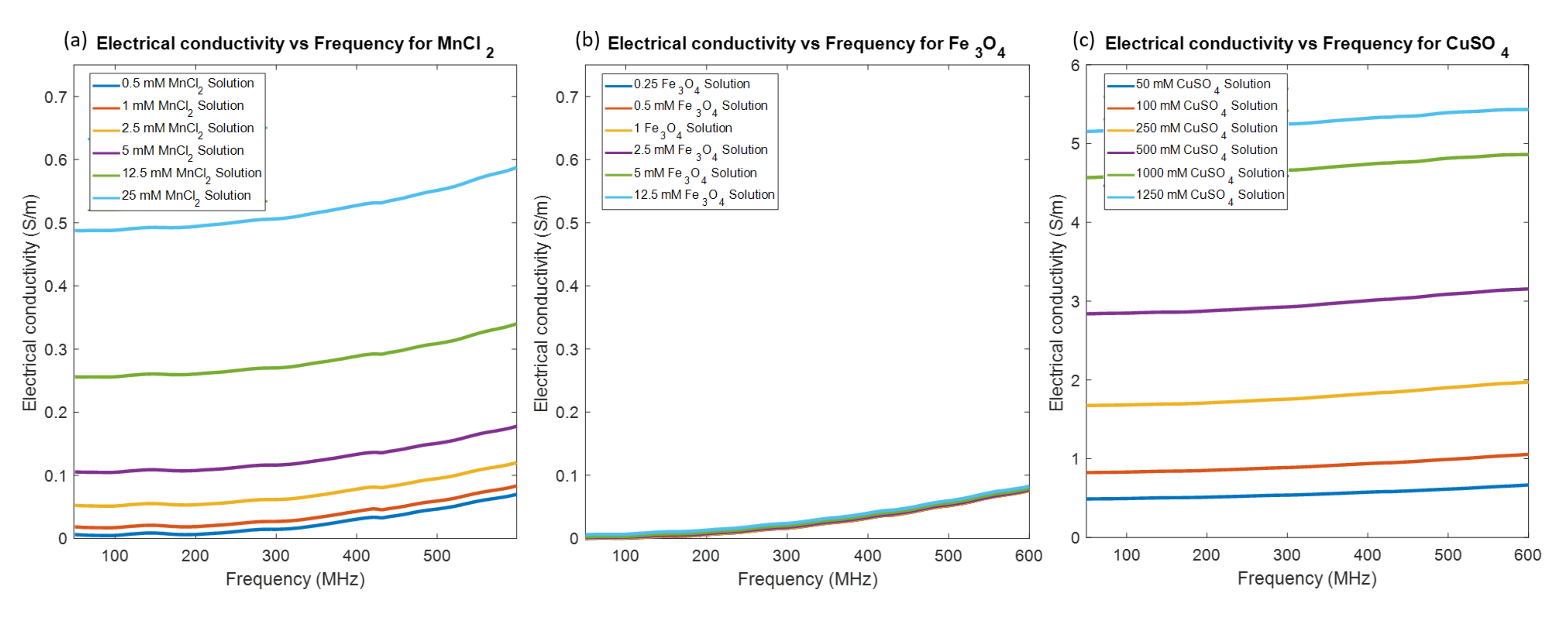
3.1. MR Relaxometry Measurements
3.2. Electromagnetic Property Measurements
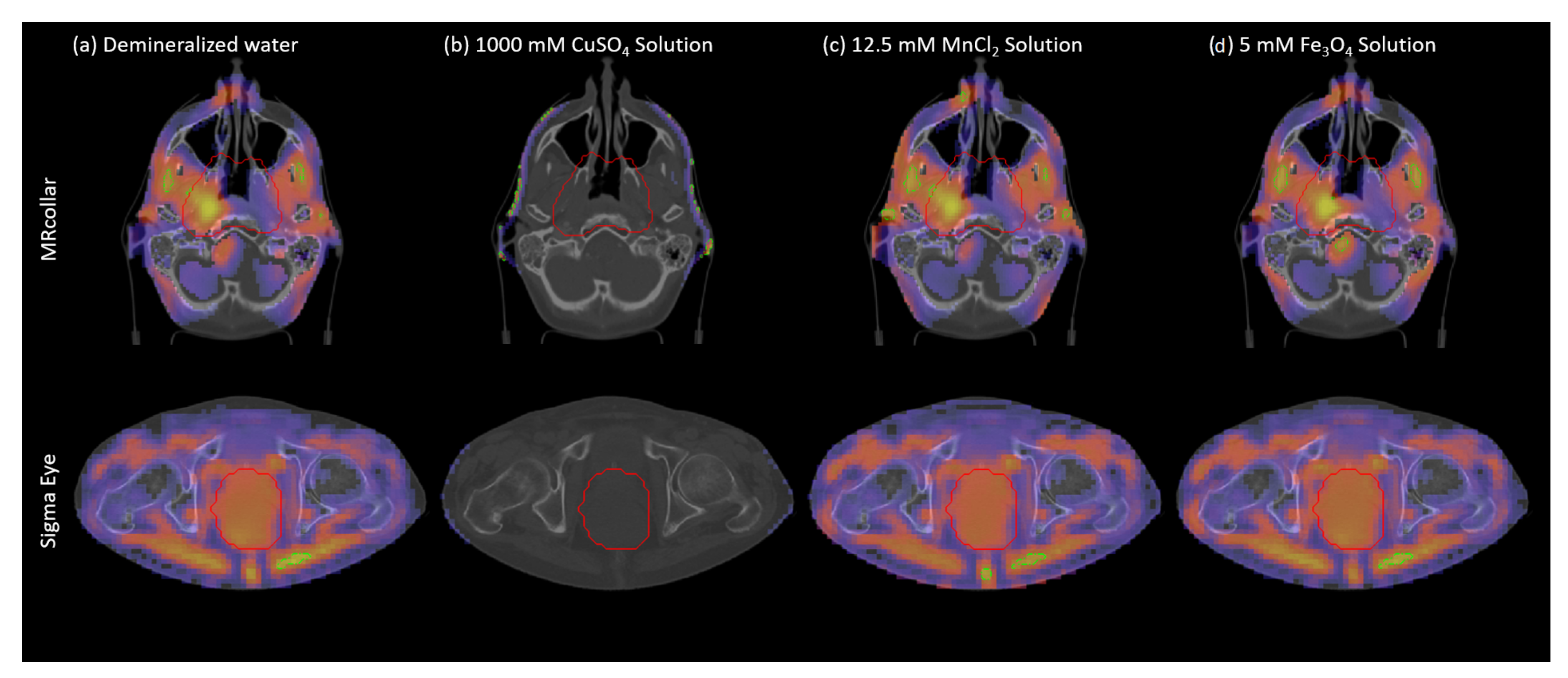
3.3. Effects on SAR Patterns and Applicator Efficiency
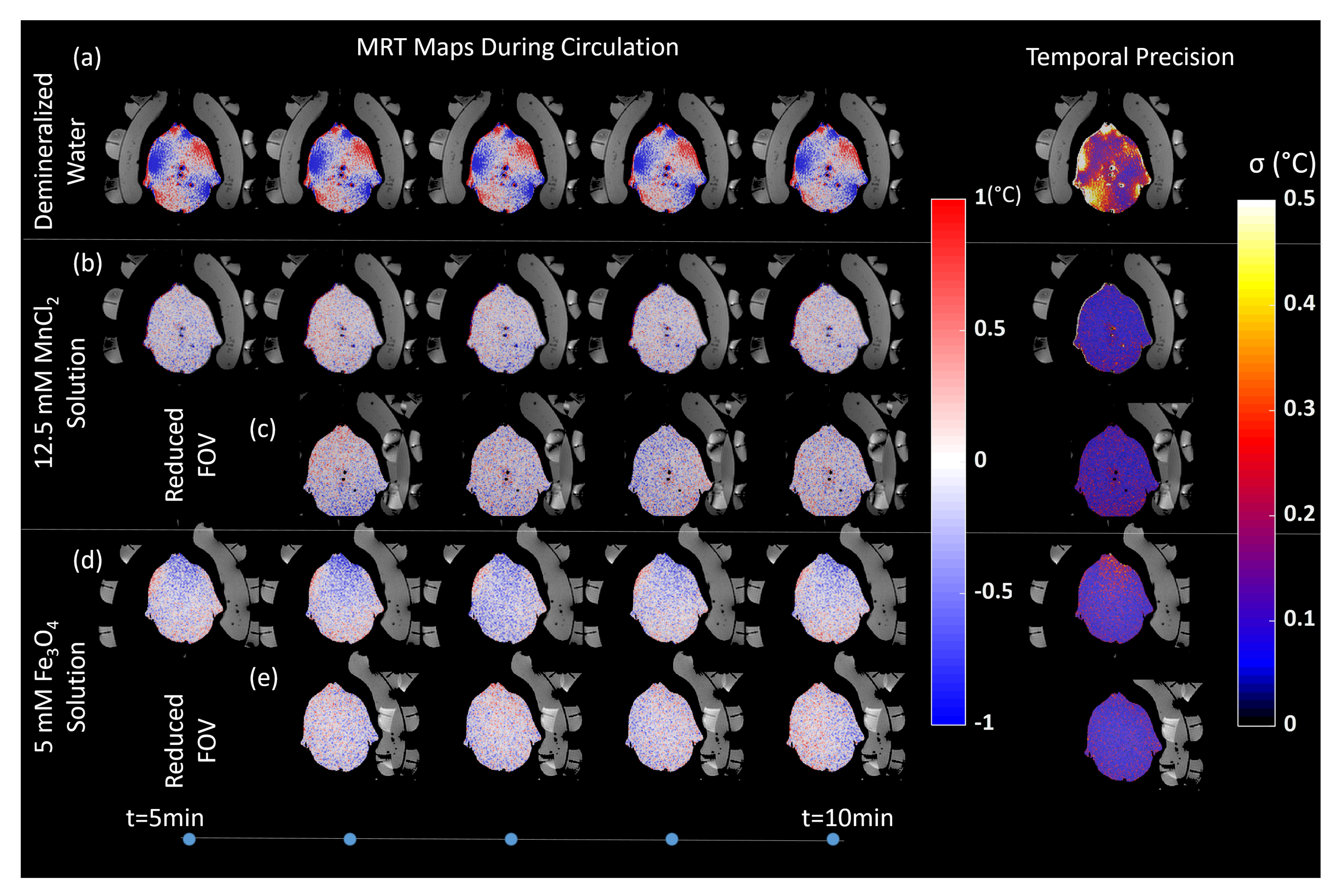
3.4. Effect on MRT Precision
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RF | Radio frequency |
| MRI | Magnetic resonance imaging |
| MRT | Magnetic resonance thermometry |
| WB | Water bolus |
| IR | Inversion recovery |
| MRgFUS | Magnetic resonance guided focused ultrasound surgery |
| HIFU | High intensity focused ultrasound |
| SPIO | Suspending iron oxide nanoparticles |
| SAR | Specific absorption rate |
| SNR | Signal-to-noise ratio |
| THQ | Target-to-hotspot quotinent |
| TC50 | Target coverage of the 50% iso-SAR volume |
References
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials. gov registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Huilgol, N.G.; Gupta, S.; Sridhar, C.R. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: A report of randomized trial. J. Cancer Res. Ther. 2010, 6, 492. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating hyperthermia into modern radiation oncology: What evidence is necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; Van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Drizdal, T.; van Rhoon, G.C.; Crocco, L.; Isernia, T.; Paulides, M.M. The potential of constrained SAR focusing for hyperthermia treatment planning: Analysis for the head & neck region. Phys. Med. Biol. 2018, 64, 015013. [Google Scholar]
- Togni, P.; Rijnen, Z.; Numan, W.C.; Verhaart, R.F.; Bakker, J.F.; Van Rhoon, G.C.; Paulides, M.M. Electromagnetic redesign of the HYPERcollar applicator: Toward improved deep local head-and-neck hyperthermia. Phys. Med. Biol. 2013, 58, 5997. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Paulides, M.M.; Trefna, H.D.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency-and microwave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Rieke, V.; Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological applications-Physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. [Google Scholar] [CrossRef]
- Adibzadeh, F.; Sumser, K.; Curto, S.; Yeo, D.T.; Shishegar, A.A.; Paulides, M.M. Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia. Int. J. Hyperth. 2020, 37, 15–27. [Google Scholar] [CrossRef]
- Delannoy, J.; LeBihan, D.; Hoult, D.I.; Levin, R.L. Hyperthermia system combined with a magnetic resonance imaging unit. Med Phys. 1990, 17, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Özerdem, C.; Hoffmann, W.; Santoro, D.; Müller, A.; Waiczies, H.; Seemann, R.; Graessl, A.; Wust, P.; Niendorf, T. Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: Electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla. PLoS ONE 2013, 8, e61661. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Bakker, J.F.; Hofstetter, L.W.; Numan, W.C.; Pellicer, R.; Fivel, E.W.; Tarasek, M.; Houston, G.C.; van Rhoon, G.C.; Yeo, D.T.; et al. Laboratory prototype for experimental validation of MR-guided radiofrequency head and neck hyperthermia. Phys. Med. Biol. 2014, 59, 2139. [Google Scholar] [CrossRef]
- Paulides, M.M.; Bakker, J.F.; Chavannes, N.; Van Rhoon, G.C. A patch antenna design for application in a phased-array head and neck hyperthermia applicator. IEEE Trans. Biomed. Eng. 2007, 54, 2057–2063. [Google Scholar] [CrossRef]
- Wang, F.N.; Peng, S.L.; Lu, C.T.; Peng, H.H.; Yeh, T.C. Water signal attenuation by D2O infusion as a novel contrast mechanism for 1H perfusion MRI. NMR Biomed. 2013, 26, 692–698. [Google Scholar]
- Rieseberg, S.; Frahm, J.; Finsterbusch, J. Two-dimensional spatially-selective RF excitation pulses in echo-planar imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2002, 47, 1186–1193. [Google Scholar] [CrossRef]
- Grissom, W.A.; Allen, S. Reducing temperature errors in transcranial MR-guided focused ultrasound using a reduced-field-of-view sequence. Magn. Reson. Med. 2020, 83, 1016–1024. [Google Scholar] [CrossRef]
- Chopra, R.; Tang, K.; Burtnyk, M.; Boyes, A.; Sugar, L.; Appu, S.; Klotz, L.; Bronskill, M. Analysis of the spatial and temporal accuracy of heating in the prostate gland using transurethral ultrasound therapy and active MR temperature feedback. Phys. Med. Biol. 2009, 54, 2615. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.P.; Steeves, T.; Fergusson, A.; Moore, D.; Davis, R.M.; Vlaisialjevich, E.; Meyer, C.H. Novel acoustic coupling bath using magnetite nanoparticles for MR-guided transcranial focused ultrasound surgery. Magn. Reson. Med. 2019, 46, 5444–5453. [Google Scholar]
- Paulides, M.M.; Drizdal, T.; Van Rhoon, G.C.; Yeo, D. Novel applicator design for MR guided RF hyperthermia in head and neck cancers: Heating performance and RF coupling. In Proceedings of the ISMRM 26th Annual Meeting & Exhibition, Paris, France, 3–9 May 2018. [Google Scholar]
- Mulder, H.T.; Curto, S.; Paulides, M.M.; Franckena, M.; van Rhoon, G.C. Systematic quality assurance of the BSD2000-3D MR-compatible hyperthermia applicator performance using MR temperature imaging. Int. J. Hyperth. 2018, 35, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.R.; Tung, S.M. Temperature dependence of proton relaxation times in vitro. Magn. Reson. Imaging 1987, 5, 189–199. [Google Scholar] [CrossRef]
- Bucci, O.M.; Bellizzi, G.; Bellizzi, G.G. Microwave broadband characterization of a diluted water-based ferrofluid in presence of a polarizing magnetic field. IEEE Trans. Magn. 2016, 53, 1–8. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Ganter, H.; Nadobny, J.; Fähling, H.; Seebass, M.; Felix, R.; Wust, P. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 267–277. [Google Scholar] [CrossRef]
- Paulides, M.M.; Mestrom, R.M.; Salim, G.; Adela, B.B.; Numan, W.C.; Drizdal, T.; Yeo, D.T.; Smolders, A.B. A printed Yagi–Uda antenna for application in magnetic resonance thermometry guided microwave hyperthermia applicators. Phys. Med. Biol. 2017, 62, 1831. [Google Scholar] [CrossRef]
- Rijnen, Z.; Bakker, J.F.; Canters, R.A.; Togni, P.; Verduijn, G.M.; Levendag, P.C.; Van Rhoon, G.C.; Paulides, M.M. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int. J. Hyperth. 2013, 29, 181–193. [Google Scholar] [CrossRef]
- Canters, R.A.; Wust, P.; Bakker, J.F.; Van Rhoon, G.C. A literature survey on indicators for characterisation and optimisation of SAR distributions in deep hyperthermia, a plea for standardisation. Int. J. Hyperth. 2009, 25, 593–608. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Drizdal, T.; van Rhoon, G.C.; Crocco, L.; Isernia, T.; Paulides, M.M. Predictive value of SAR based quality indicators for head and neck hyperthermia treatment quality. Int. J. Hyperth. 2019, 36, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; Van Der Zee, J. Response to: The Impact of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 1387. [Google Scholar]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; Van Der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Thrall, D.E.; LaRue, S.M.; Yu, D.; Samulski, T.; Sers, L.; Case, B.; Rosner, G.; Azuma, C.; Poulson, J.; Pruitt, A.F.; et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin. Cancer Res. 2005, 11, 5206–5214. [Google Scholar] [CrossRef][Green Version]
- Jones, E.; Thrall, D.; Dewhirst, M.W.; Vujaskovic, Z. Prospective thermal dosimetry: The key to hyperthermia’s future. Int. J. Hyperth. 2006, 22, 247–253. [Google Scholar] [CrossRef]
- Gellermann, J.; Faehling, H.; Mielec, M.; Cho, C.H.; Budach, V.; Wust, P. Image artifacts during MRT hybrid hyperthermia–causes and elimination. Int. J. Hyperth. 2008, 24, 327–335. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [PubMed]
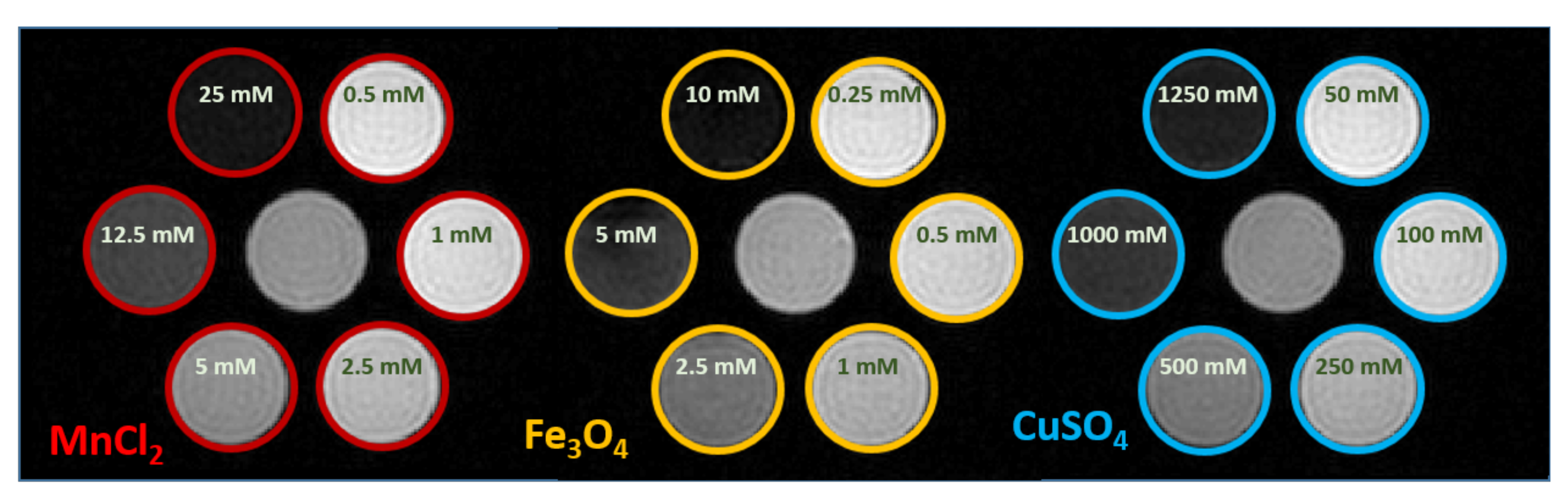
| MRcollar | Sigma Eye | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T2* (ms) at 1.5 T | (S/m) at 434 MHz | at 434 MHz | THQ | TC50 (%) | Power (W) | (S/m) at 100 MHz | at 100 MHz | THQ | TC50 (%) | Power (W) | |
| Demineralized Water | 120 | 0.04 | 79.2 | 0.43 | 23 | 180 | 0.001 | 79.1 | 0.57 | 11 | 1201 |
| 50 mM CuSO Solution | 10.8 | 0.59 | 82.1 | 0.39 | 22 | 570 | 0.495 | 83.5 | 0.57 | 2 | 9564 |
| 100 mM CuSO Solution | 6 | 0.95 | 84.2 | 0.32 | 14 | 1110 | 0.831 | 86.0 | 0.47 | 2 | 23948 |
| 250 mM CuSO Solution | 3.47 | 1.85 | 87.5 | 0.18 | 0 | 4761 | 1.683 | 89.7 | 0.16 | 0 | N/A |
| 500 mM CuSO Solution | 1.94 | 3.03 | 88.1 | 0.10 | 0 | N/A | 2.849 | 91.1 | 0.04 | 0 | N/A |
| 1000 mM CuSO Solution | 1.09 | 4.76 | 83.5 | 0.05 | 0 | N/A | 4.583 | 88.7 | N/A | N/A | N/A |
| 1250 mM CuSO Solution | ≈ | 5.34 | 79.9 | 0.04 | 0 | N/A | 5.171 | 85.4 | N/A | N/A | N/A |
| 0.5 mM MnCl Solution | 11.47 | 0.03 | 78.9 | 0.42 | 23 | 177 | 0.005 | 78.1 | 0.57 | 12 | 1185 |
| 1 mM MnCl Solution | 6.27 | 0.05 | 78.9 | 0.43 | 22 | 187 | 0.017 | 78.2 | 0.57 | 10 | 1337 |
| 2.5 mM MnCl Solution | 3.77 | 0.08 | 79.0 | 0.43 | 22 | 203 | 0.051 | 78.2 | 0.57 | 5 | 1656 |
| 5 mM MnCl Solution | 2.35 | 0.14 | 79.0 | 0.44 | 22 | 230 | 0.105 | 78.3 | 0.58 | 5 | 2181 |
| 12.5 mM MnCl Solution | 1.13 | 0.29 | 78.9 | 0.42 | 21 | 325 | 0.256 | 78.6 | 0.57 | 4 | 4144 |
| 25 mM MnCl Solution | ≈ | 0.53 | 79.0 | 0.40 | 22 | 503 | 0.488 | 78.8 | 0.57 | 2 | 9734 |
| 0.25 mM FeO Solution | 6.42 | 0.04 | 79.2 | 0.42 | 22 | 181 | 0.001 | 78.9 | 0.56 | 10 | 1236 |
| 0.5 mM FeO Solution | 4.1 | 0.04 | 79.2 | 0.42 | 22 | 179 | 0.001 | 78.9 | 0.57 | 7 | 1105 |
| 1 mM FeO Solution | 2.71 | 0.04 | 79.2 | 0.42 | 23 | 181 | 0.001 | 78.9 | 0.57 | 6 | 1132 |
| 2.5 mM FeO Solution | 1.44 | 0.04 | 79.2 | 0.43 | 22 | 183 | 0.002 | 78.9 | 0.57 | 9 | 1159 |
| 5 mM FeO Solution | 1.07 | 0.04 | 79.2 | 0.43 | 22 | 191 | 0.003 | 78.9 | 0.56 | 10 | 1163 |
| 10 mM FeO Solution | ≈ | 0.05 | 79.2 | 0.43 | 22 | 186 | 0.006 | 78.9 | 0.57 | 7 | 1158 |
| Demineralized Water | 12.5 mM MnCl Solution | 5 mM FeO Solution | ||||
|---|---|---|---|---|---|---|
| Full FOV | Full FOV | Reduced FOV | Full FOV | Reduced FOV | ||
| Before | Mean error (C) | −0.06 | −0.06 | −0.06 | ||
| Circulation | Std (C) | 0.17 | 0.15 | 0.09 | ||
| Max error (C) | 1.28 | 1.89 | −1.36 | |||
| During | Mean error (C) | 0.04 | −0.03 | −0.05 | −0.13 | −0.03 |
| Circulation | Std (C) | 0.70 | 0.16 | 0.11 | 0.11 | 0.09 |
| Max error (C) | 41.8 | −2.00 | −2.05 | −1.57 | 1.32 | |
| After | Mean error (C) | 0.20 | −0.16 | −0.05 | ||
| Circulation | Std (C) | 0.29 | 0.26 | 0.11 | ||
| Max error (C) | 28.1 | 5.40 | −2.60 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumser, K.; Bellizzi, G.G.; van Rhoon, G.C.; Paulides, M.M. The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia. Sensors 2020, 20, 2946. https://doi.org/10.3390/s20102946
Sumser K, Bellizzi GG, van Rhoon GC, Paulides MM. The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia. Sensors. 2020; 20(10):2946. https://doi.org/10.3390/s20102946
Chicago/Turabian StyleSumser, Kemal, Gennaro G. Bellizzi, Gerard C. van Rhoon, and Margarethus M. Paulides. 2020. "The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia" Sensors 20, no. 10: 2946. https://doi.org/10.3390/s20102946
APA StyleSumser, K., Bellizzi, G. G., van Rhoon, G. C., & Paulides, M. M. (2020). The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia. Sensors, 20(10), 2946. https://doi.org/10.3390/s20102946







