Bioluminescence-Based Energy Transfer Using Semiconductor Quantum Dots as Acceptors
Abstract
1. Introduction
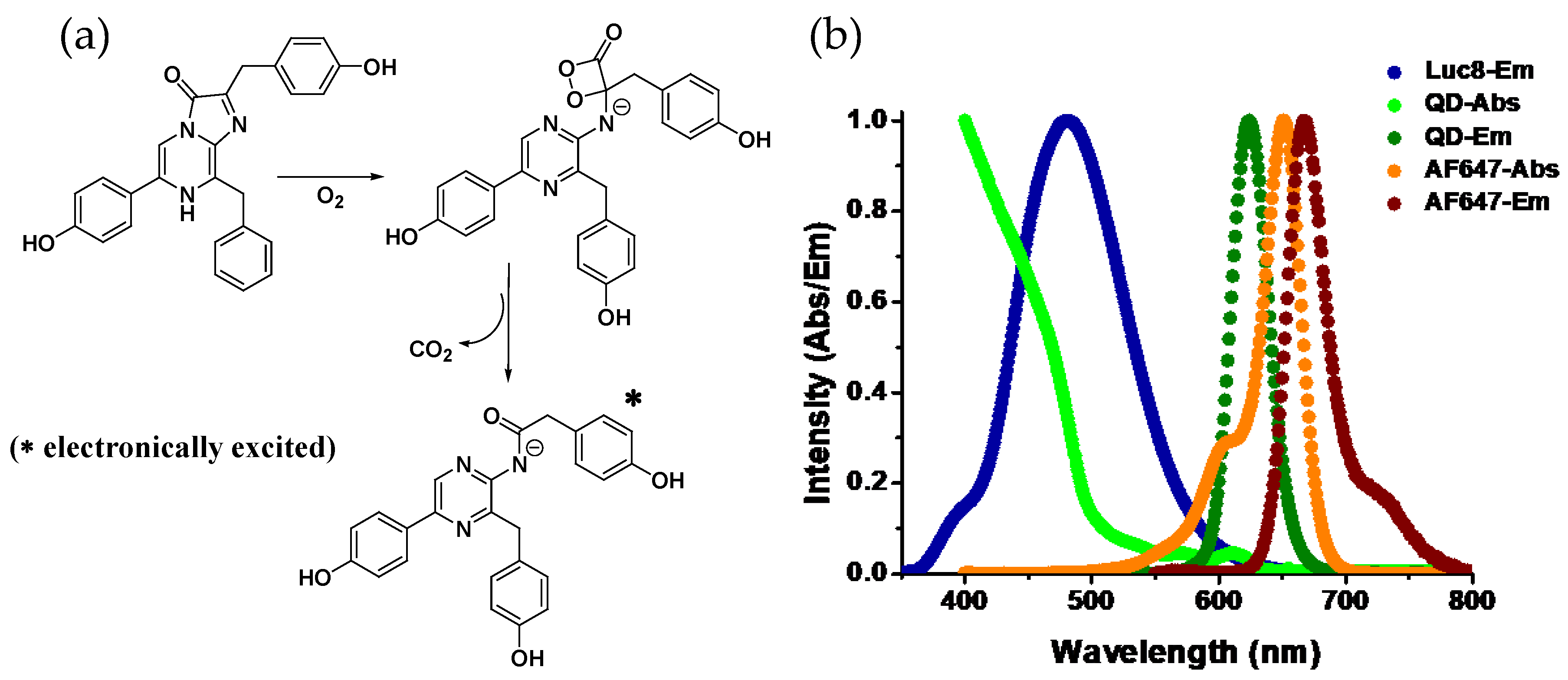
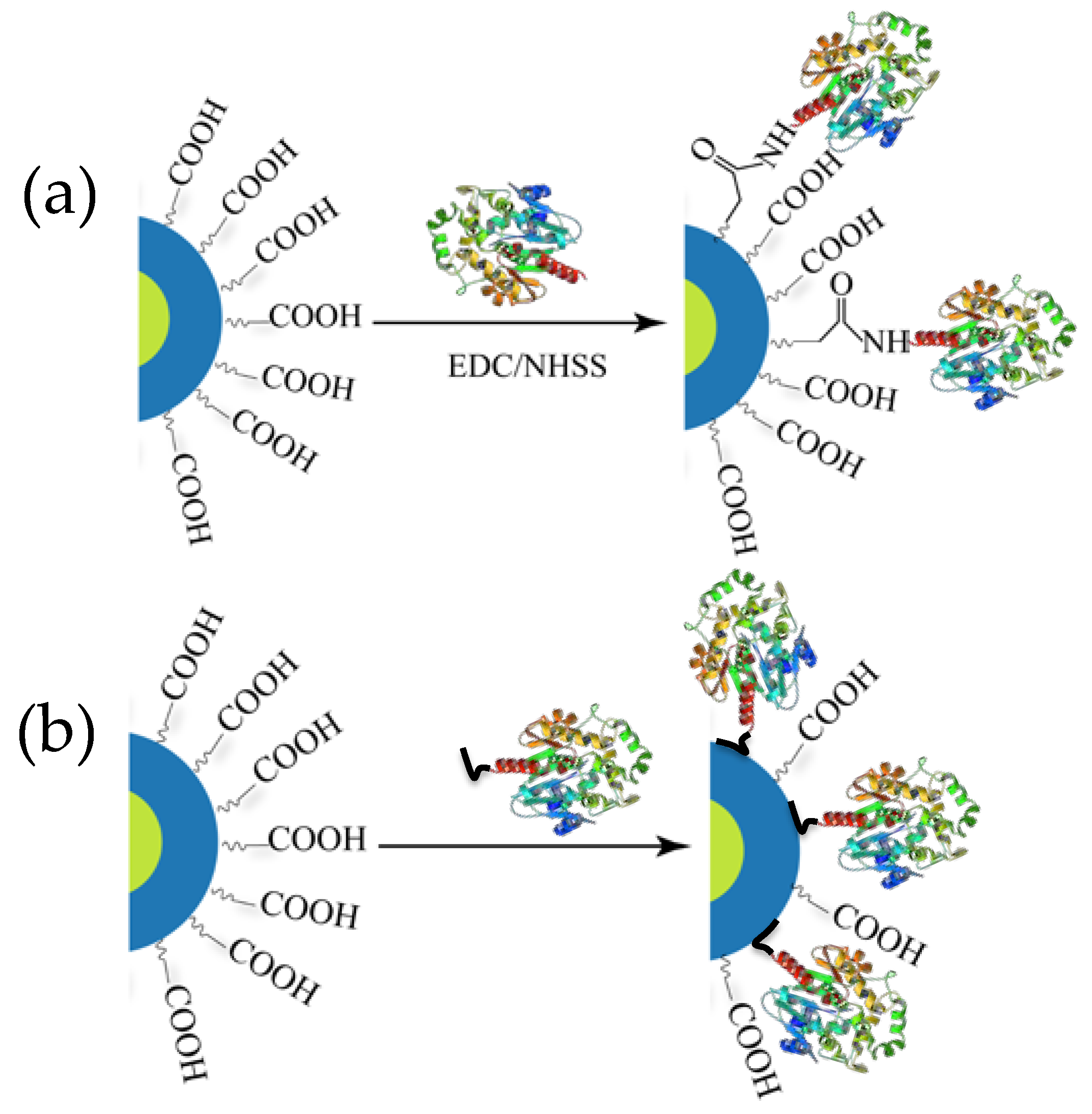
2. System Design and Ligation Strategy
3. Applications
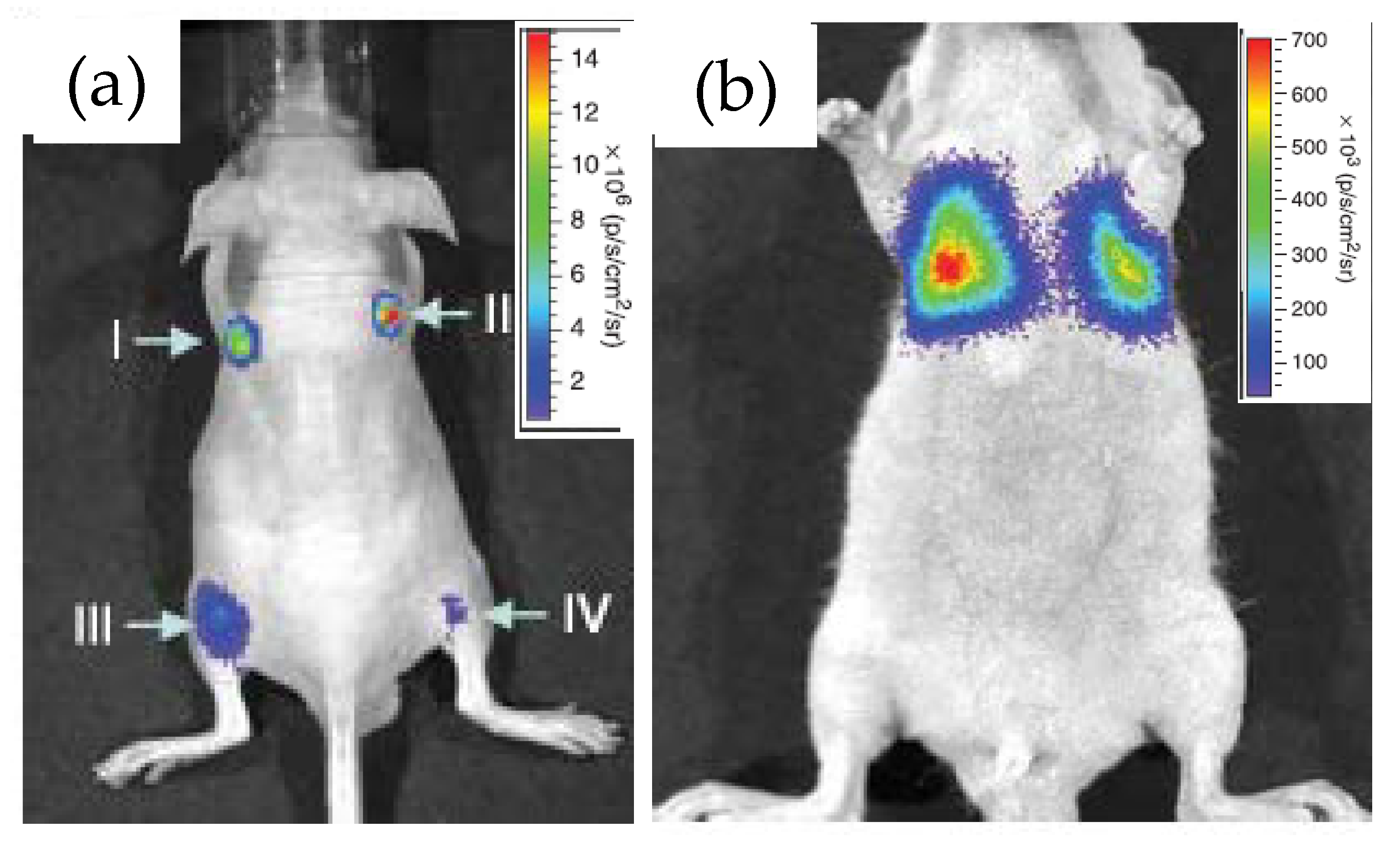
3.1. Bioimaging
3.2. Biosensing
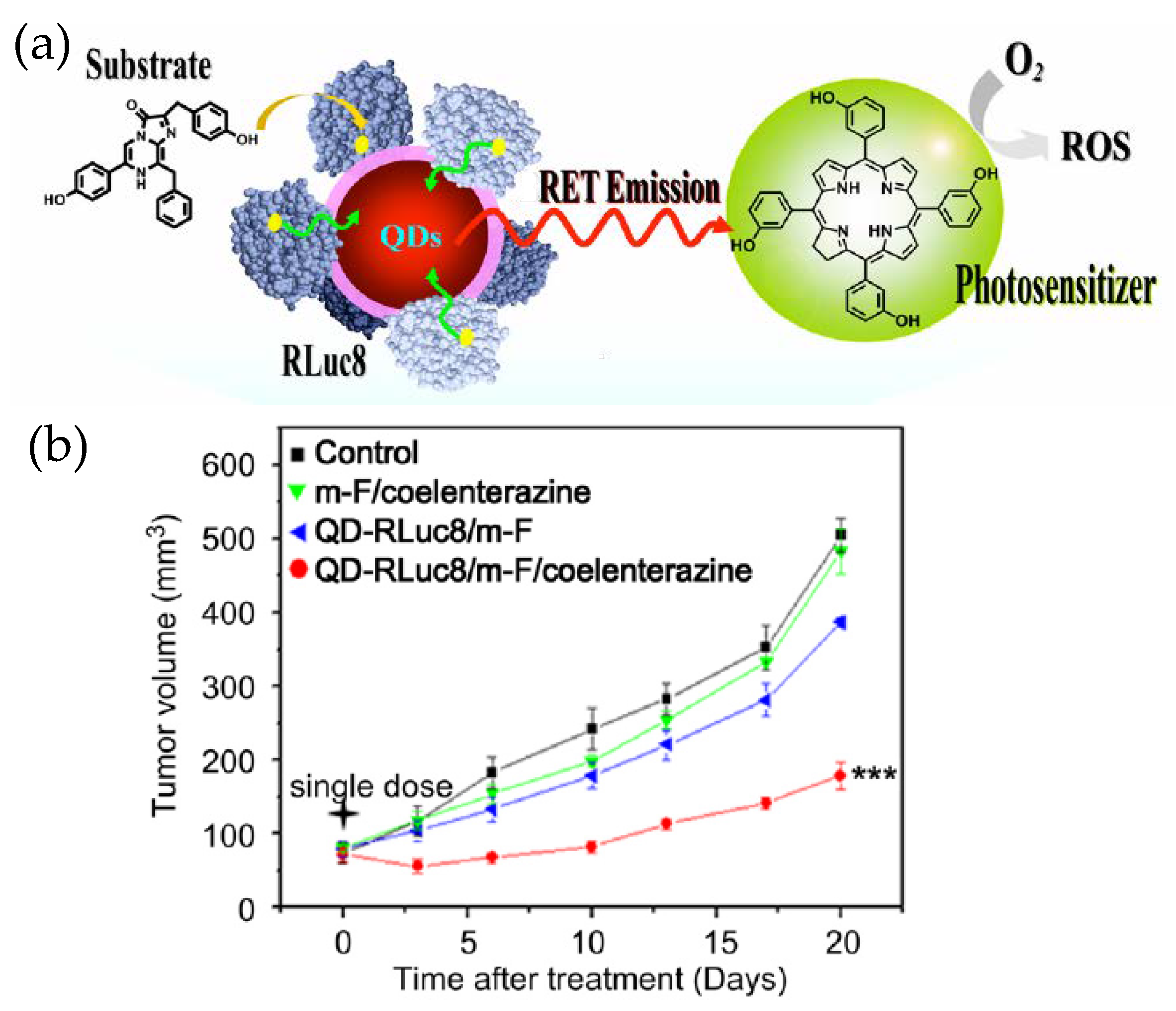
3.3. Therapeutics
4. Developing New Designs
4.1. Anisotropic-QD–FLuc Constructs
4.2. BRET-Multistep FRET Constructs
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Navizet, I.; Liu, Y.J.; Ferré, N.; Roca-Sanjuán, D.; Lindh, R. The chemistry of bioluminescence: An analysis of chemical functionalities. ChemPhysChem 2011, 12, 3064–3076. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.; Fdez Galván, I.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferre, N.; Liu, Y.J.; Navizet, I.; Roca-Sanjuan, D.; et al. Chemi- and bioluminescence of cyclic peroxide. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M. Advanced chemistry of dioxetane-based chemiluminescent substrates originating from bioluminescence. J. Photochem. Photobiol. C 2004, 5, 27–53. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.; Pinto da Silva, L. Chemiluminescence and bioluminescence as an excitation source in the photodynamic therapy of cancer: A critical review. ChemPhysChem 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- Nakatsu, T.; Ichiyama, S.; Hiratake, J.; Saldanha, A.; Nobuyuki, K.; Sakata, K.; Kato, H. Structural basis for the spectral difference in luciferase bioluminescence. Nature 2006, 440, 372–376. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Murtiashaw, M.H.; Boije, H.; Fleet, S.E. A mutagenesis study of the putative luciferin binding site residues of firefly luciferase. Biochemistry 2003, 42, 10429–10436. [Google Scholar] [CrossRef]
- Morin, J.G.; Hastings, J.W. Energy transfer in a bioluminescent system. J. Cell. Physiol. 1971, 77, 313–318. [Google Scholar] [CrossRef]
- Medinitz, I.L.; Hildebrandt, N. FRET–Förster Resonance Energy Transfer from Theory to Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Stryer, L.; Haugland, R.P. Energy transfer: A spectroscopic ruler. Proc. Nat. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef]
- Pfleger, K.D.; Eidne, K.A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 2006, 3, 165–174. [Google Scholar] [CrossRef]
- Milligan, G. Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur. J. Pharm. Sci. 2004, 21, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Moore, J.A.; Shreder, K. Quenching of biotinylatedaequorin bioluminescence by dye-labeled avidin conjugates: Application to homogeneous bioluminescence resonance energy transfer assays. Org. Lett. 2001, 3, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef]
- Hildebrandt, N.; Geissler, D. Semiconductor quantum dots as FRET acceptors for multiplexed diagnostics and molecular ruler application. Adv. Exp. Med. Biol. 2012, 733, 75–86. [Google Scholar] [PubMed]
- Hildebrandt, N.; Spillmann, C.M.; Algar, W.R.; Pons, T.; Stewart, M.H.; Oh, E.; Susumu, K.; Diaz, S.A.; Delehanty, J.B.; Medintz, I.L. Energy transfer with semiconductor quantum dot bioconjugates: A versatile platform for biosensing, energy harvesting, and other developing applications. Chem. Rev. 2017, 117, 536–711. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Wegner, D.; Huston, A.L.; Blanco-Canosa, J.B.; Armstrong, A.; Dawson, P.E.; Hildebrandt, N.; Medintz, I.L. Quantum dots as simultaneous acceptors and donors in time-gated Förster resonance energy transfer relays: Characterization and biosensing. J. Am. Chem. Soc. 2012, 134, 1876–1891. [Google Scholar] [CrossRef]
- Loening, A.M.; Fenn, T.D.; Wu, A.M.; Gambhir, S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. 2006, 19, 391–400. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Samburski, S.S.; Jalkanen, S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophsosphatase and phosphotransfer reactions. FASEB J. 2003, 17, 1328–1330. [Google Scholar] [CrossRef]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A small luciferase is brightening up the field of bioluminescence. Bioconjug. Chem. 2016, 27, 1175–1187. [Google Scholar] [CrossRef]
- Susumu, K.; Field, L.D.; Oh, E.; Hunt, M.; Delehanty, J.B.; Palomo, V.; Dawson, P.E.; Huston, A.L.; Medintz, I.L. Purple-, blue-, and green-emitting multishell alloyed quantum dots: Synthesis, characterization, and application for ratiometric extracellular pH sensing. Chem. Mater. 2017, 17, 7330–7344. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Boeneman Gemill, K.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Green, M. The nature of quantum dot capping ligands. J. Mater. Chem. 2010, 20, 5797–5809. [Google Scholar] [CrossRef]
- Blanco-Canosa, J.B.; Wu, M.; Susumu, K.; Petryayeva, E.; Jennigs, T.L.; Dawson, P.E.; Algar, W.R.; Medintz, I.L. Recent progress in the bioconjugation of quantum dots. Coord. Chem. Rev. 2014, 263, 101–137. [Google Scholar] [CrossRef]
- So, M.K.; Xu, C.; Loening, A.M.; Gambhir, S.S.; Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 2006, 24, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Buckhout-White, S.; Oh, E.; Susumu, K.; Medintz, I.L. Exploring attachment chemistry with FRET in hybrid quantum dot dye-labeled DNA dendrimer composites. Mol. Sys. Des. Eng. 2018, 3, 314–327. [Google Scholar] [CrossRef]
- Knecht, S.; Ricklin, D.; Eberle, A.N.; Ernst, B. Oligohis-tags: Mechanisms of binding to Ni2+-NTA surfaces. J. Mol. Recognit. 2009, 22, 270–279. [Google Scholar] [CrossRef]
- Schmitt, J.; Hess, H.; Stunnenberg, H.G. Affinity purification of histidine-tagged proteins. Mol. Biol. Rep. 1993, 18, 223–230. [Google Scholar] [CrossRef]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The controlled display of biomolecules on nanoparticles: A challenge suited to bioorthogonal chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Higashiya, S.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Kinetics of metal-affinity driven self-assembly between proteins or peptides and CdSe-ZnS quantum dots. J. Phys. Chem. C 2007, 111, 11528–11538. [Google Scholar] [CrossRef]
- Diaz, S.A.; Breger, J.C.; Medintz, I.L. Monitoring enzymatic proteolysis using either enzyme- or substrate bioconjugated quantum dots. Methods Enzymol. 2016, 571, 19–54. [Google Scholar] [PubMed]
- Algar, W.R.; Blanco-Canosa, J.B.; Manthe, R.L.; Susumu, K.; Stewart, M.H.; Dawson, P.E.; Medintz, I.L. Synthesizing and modifying peptides for chemoselective ligation and assembly into quantum dot-peptide bioconjugates. Methods Mol. Biol. 2013, 1025, 47–73. [Google Scholar] [PubMed]
- Spillmann, C.M.; Ancona, M.G.; Buckhout-White, S.; Algar, W.R.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Goldman, E.R.; Medintz, I.L. Achieving effective terminal exciton delivery in quantum dot antenna-sensitized multistep DNA photonic wires. ACS Nano 2013, 7, 7101–7118. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Yoshimura, H.; Kim, S.B. Advances in fluorescence and bioluminescence imaging. Anal. Chem. 2013, 85, 590–609. [Google Scholar] [CrossRef]
- Boulnois, J.L. Photophysical processes in recent medical laser developments: A review. Lasers Med. Sci. 1986, 1, 47–66. [Google Scholar] [CrossRef]
- Ma, Q.; Su, X. Near-infrared quantum dots: Synthesis, functionalization and analytical applications. Analyst 2010, 135, 1867–1877. [Google Scholar] [CrossRef]
- Aswathy, R.G.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Near-infrared quantum dots for deep tissue imaging. Anal. Bioanal. Chem. 2010, 397, 1417–1435. [Google Scholar] [CrossRef]
- Boeneman, K.; Delehanty, J.B.; Blanco-Canosa, J.; Susumu, K.; Stewart, M.H.; Oh, E.; Huston, A.L.; Dawson, G.; Ingale, S.; Walters, R.; et al. Selecting improved peptidyl motifs for cytosolic delivery of disparate protein and nanoparticle materials. ACS Nano 2013, 7, 3778–3796. [Google Scholar] [CrossRef]
- Field, L.D.; Delehanty, J.B.; Chen, Y.C.; Medintz, I.L. Peptides for specifically targeting nanoparticles to cellular organelles: Quo vadis? Acc. Chem. Res. 2015, 48, 1380–1390. [Google Scholar] [CrossRef]
- Breger, J.C.; Muttenthaler, M.; Delehanty, J.B.; Thompson, D.; Oh, E.; Susumu, K.; Deschamps, J.R.; Anderson, G.P.; Field, L.D.; Walper, S.A.; et al. Nanoparticle cellular uptake by dendritic wedge peptides: Achieving single peptide facilitated delivery. Nanoscale 2017, 9, 10447–10464. [Google Scholar] [CrossRef]
- Kosaka, N.; Mitsunaga, M.; Bhattacharyya, S.; Miller, S.C.; Choyke, P.L.; Kobayashi, H. Self-illuminating in vivo lymphatic imaging using a bioluminescence resonance energy transfer quantum dot nano-particle. Contrast Media Mol. Imaging 2011, 6, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Sun, H.; England, C.G.; Cheng, L.; Liu, Z.; Caia, W. Quantum dot-NanoLuc bioluminescence resonance energy transfer enables tumor imaging and lymph node mapping in vivo. Chem. Commun. 2016, 52, 6997–7000. [Google Scholar] [CrossRef] [PubMed]
- Bilan, R.; Fleury, F.; Nabiev, I.; Sukhanova, A. Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjug. Chem. 2015, 26, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, S.; Jin, T. Bioluminescence resonance energy transfer (BRET)-coupled annexin V-functionalized quantum dots for near-infrared optical detection of apoptotic cells. ChemBioChem 2017, 18, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, S.; Jin, T. Recombinant protein (Luciferase-IgG binding domain) conjugated quantum dots for BRET-coupled near-infrared imaging of epidermal growth factor receptors. Bioconjug. Chem. 2018, 29, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.; Xiao, F.; Xia, Z.; Rao, J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed. 2007, 46, 4346–4349. [Google Scholar] [CrossRef]
- Xia, Z.; Xing, Y.; So, M.K.; Koh, A.L.; Sinclair, R.; Rao, J. Multiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugation. Anal. Chem. 2008, 80, 8649–8655. [Google Scholar] [CrossRef]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA–a powerful and growing functional combination in bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef]
- Cissell, K.A.; Campbell, S.; Deo, S.K. Rapid, single-step nucleic acid detection. Anal. Bioanal. Chem. 2008, 391, 2577–2581. [Google Scholar] [CrossRef]
- Yu, X.; Wen, K.; Wang, Z.; Zhang, X.; Li, C.; Zhang, S.; Shen, J. General bioluminescence resonance energy transfer homogeneous immunoassay for small molecules based on quantum dots. Anal. Chem. 2016, 88, 3512–3520. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, C.W.; Yu, H.P.; Lin, Y.F.; Lai, P.S. Bioluminescence resonance energy transfer using luciferase-immobilized quantum dots for self-illuminated photodynamic therapy. Biomaterials 2013, 34, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Sitt, A.; Salant, A.; Menagen, G.; Banin, U. Highly emissive nano rod-in-rod heterostructures with strong linear polarization. Nano Lett. 2011, 11, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Halivni, S.; Sitt, A.; Hadar, I.; Banin, U. Effect of nanoparticle dimensionality on fluorescence resonance energy transfer in nanoparticle-dye conjugated systems. ACS Nano 2012, 6, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Fontaine, D.M.; Branchini, B.R.; Maye, M.M. Designing quantum rods for optimized energy transfer with firefly luciferase enzymes. Nano Lett. 2012, 12, 3251–3256. [Google Scholar] [CrossRef]
- Alam, R.; Karam, L.M.; Doane, T.L.; Coopersmith, K.; Fontaine, D.M.; Branchini, B.R.; Maye, M.M. Probing bioluminescence resonance energy transfer in quantum rod-luciferase nanoconjugates. ACS Nano 2016, 10, 1969–1977. [Google Scholar] [CrossRef]
- Samanta, A.; Walper, S.A.; Susumu, K.; Dwyer, C.L.; Medintz, I.L. An enzymatically-sensitized sequential and concentric energy transfer relay self-assembled around semiconductor quantum dots. Nanoscale 2015, 7, 7603–7614. [Google Scholar] [CrossRef]
- Alam, R.; Karam, L.M.; Doane, T.L.; Zylstra, J.; Fontaine, D.M.; Branchini, M.R.; Maye, M.M. Near infrared bioluminescence resonance energy transfer from firefly luciferase-quantum dot bionanoconjugates. Nanotechnology 2014, 25, 495606. [Google Scholar] [CrossRef]
- Dwyer, C.L.; Díaz, S.A.; Walper, S.A.; Samanta, A.; Susumu, K.; Oh, E.; Buckhout-White, S.; Medintz, I.L. Chemoenzymatic sensitization of DNA photonic wires mediated through quantum dot energy transfer relays. Chem. Mater. 2015, 27, 6490–6494. [Google Scholar] [CrossRef]
- Zhang, F.; Nangreave, J.; Liu, Y.; Yan, H. Structural DNA nanotechnology: State of the art and future perspective. J. Am. Chem. Soc. 2014, 136, 11198–11211. [Google Scholar] [CrossRef]
- Samanta, A.; Banerjee, S.; Liu, Y. DNA nanotechnology for nanophotonic applications. Nanoscale 2015, 7, 2210–2220. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Cheng, E.; Zhao, M.; Meng, H.; Liu, D.; Zhou, D. A pH-driven, reconfigurable DNA nanotriangle. Chem. Commun. 2009, 7, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Zhang, H.; Govorov, A.O.; Liedl, T.; Liu, N. Reconfigurable 3D plasmonic metamolecules. Nat. Mater. 2014, 13, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Maksoudian, C.; Soenen, S.J.; Susumu, K.; Oh, E.; Medintz, I.L.; Manshian, B.B. A multiparametric evaluation of quantum dot size and surface-grafted peptide density on cellular uptake and cytotoxicity. Bioconj. Chem. 2013, 24, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Bradburne, C.E.; Delehanty, J.B.; Boeneman Gemmill, K.; Mei, B.C.; Mattoussi, H.; Susumu, K.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. Cytotoxicity of quantum dots used for in vitro cellular labeling: Role of QD surface ligand, delivery modality, cell type, and direct comparison to organic fluorophores. Bioconj. Chem. 2020, 31, 1570–1583. [Google Scholar]
- Bilal, M.; Oh, E.; Liu, R.; Breger, J.C.; Medintz, I.L.; Cohen, Y. Bayesian network resource for meta-analysis: Cellular toxicity of quantum dots. Small 2019, 15, 1900510. [Google Scholar] [CrossRef]
- Buckhout-White, S.; Spillmann, C.M.; Algar, W.R.; Khachatrian, A.; Melinger, J.S.; Goldman, E.R.; Ancona, M.G.; Medintz, I.L. Assembling programmable FRET-based photonic networks using designer DNA scaffolds. Nat. Comm. 2014, 5, 5615. [Google Scholar] [CrossRef]
- Klein, W.P.; Diaz, S.A.; Chiriboga, M.; Walper, S.A.; Medintz, I.L. Dendrimeric DNA-based nanoscaffolded BRET-FRET optical encryption keys. ACS Appl. Nano Mat. 2019, 2, 7459–7465. [Google Scholar] [CrossRef]



| QD Acceptor Property | Effect on BRET |
|---|---|
| Non-trivial size with large surface-to-volume (s/v) ratio. | Allows for multiple luciferases to be displayed around the QD. 1 Allows for display of other biologicals on the QD surface. |
| Display of multiple Luc around the QD. | Increases the probability that BRET will occur. |
| Absorption increases to the blue. | Large spectral overlap with Luc emission. |
| Long excited lifetime, high quantum yield. | Bright QD acceptor PL. QD can act as donor or FRET relay to ternary or downstream acceptors. |
| Size-tunable PL. | Choice of QD PL emission window with large spectral separation from Luc emission. |
| Resistance to photo- and chemical degradation. | Allows for long-term robust use. |
| Can be surface functionalized with many different ligands | Provides access to different bioconjugation chemistries. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, A.; Medintz, I.L. Bioluminescence-Based Energy Transfer Using Semiconductor Quantum Dots as Acceptors. Sensors 2020, 20, 2909. https://doi.org/10.3390/s20102909
Samanta A, Medintz IL. Bioluminescence-Based Energy Transfer Using Semiconductor Quantum Dots as Acceptors. Sensors. 2020; 20(10):2909. https://doi.org/10.3390/s20102909
Chicago/Turabian StyleSamanta, Anirban, and Igor L. Medintz. 2020. "Bioluminescence-Based Energy Transfer Using Semiconductor Quantum Dots as Acceptors" Sensors 20, no. 10: 2909. https://doi.org/10.3390/s20102909
APA StyleSamanta, A., & Medintz, I. L. (2020). Bioluminescence-Based Energy Transfer Using Semiconductor Quantum Dots as Acceptors. Sensors, 20(10), 2909. https://doi.org/10.3390/s20102909






