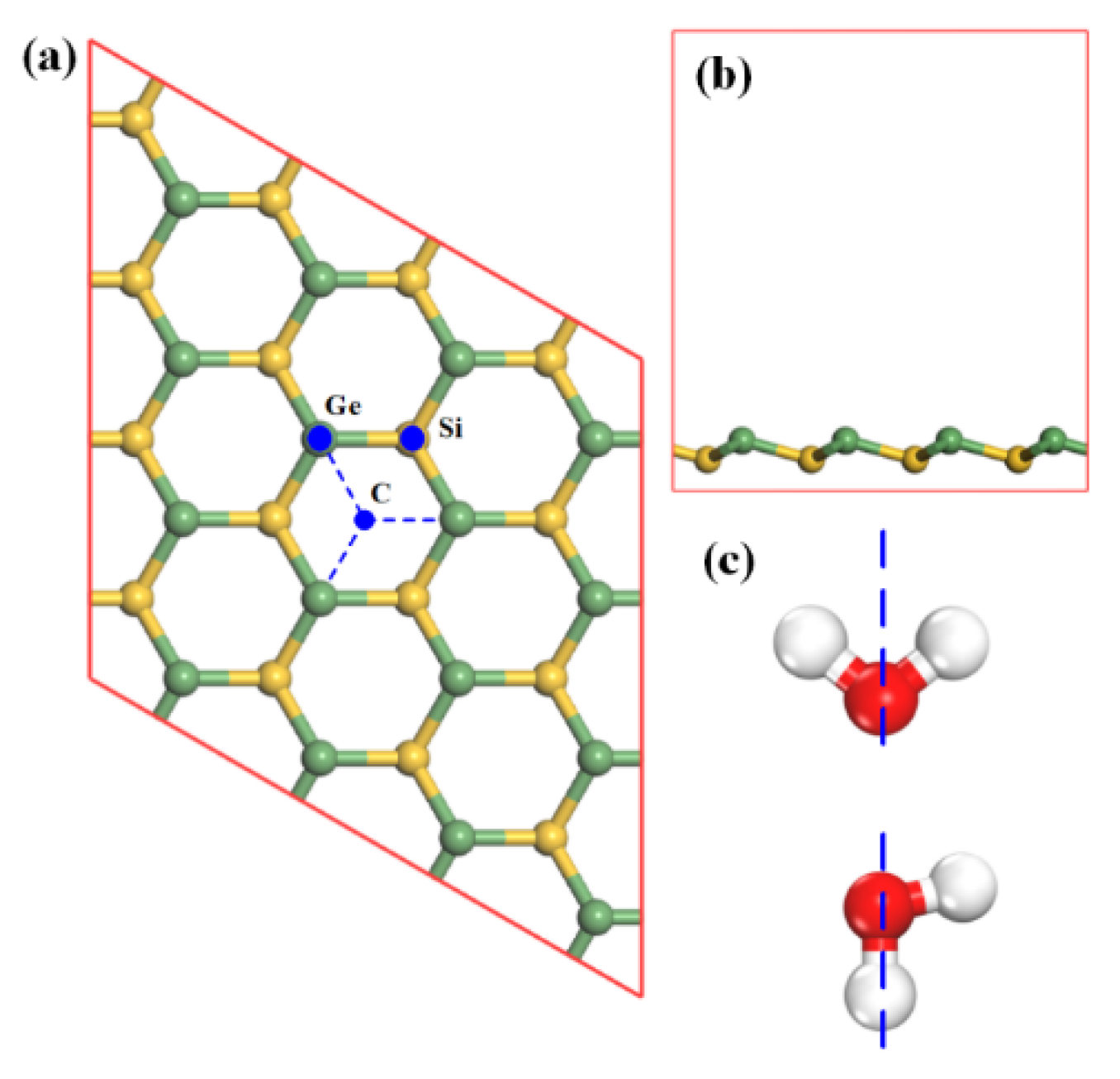
In order to find the most stable adsorption configurations, the gas molecule was placed at three selected positions above SiGe monolayer with different orientations [
20]. As illustrated in
Figure 1a, the three selected sites, namely Ge site, Si site, and center (C) site, were considered. The Ge site was directly above the Ge atom, the Si site directly above the Si atom, and the C site at the center of a hexagon composed by Ge and Si atoms. Intuitively, when the molecule was adsorbed on the substrate, different gas molecule orientations (presented in
Figure 1c) would produce different adsorption effects. The situation also appeared in other gases’ adsorption [
21]. Therefore, in this work, the effect was also considered. In
Figure 1c, the blue dashed line represents the
z-axis where the lower atoms would be placed close to the substrate. For the sake of brevity and calculation source limitation, guaranteeing the accuracy of calculation at the same time, the paper just considers single gas molecule adsorption.
To explore the different adsorption effects between SiGe substrate and calculated gas molecules including CO, CO
2, SO
2, NO
2, NO, NH
3, H
2, H
2O, and O
2, the detailed information about the most stable adsorption sites is provided in
Table 1, containing equilibrium substrate-gas distance (
D), adsorption energy, and charge transfer (
Q).
D is defined as the center-to-center distance of the nearest atoms between SiGe and the gas molecule;
Q is defined as the total Mulliken charge transfer between the model SiGe monolayer and the gas molecule, and a negative number means charge transfer from SiGe to the gas molecule.
3.1. CO, CO2, H2, and H2O Adsorption
For CO and CO2 on SiGe, the adsorption effects were obviously different for different adsorption sites. The largest region for CO was around −0.24 eV, while the largest Ead for CO2 was −0.2 eV, which meant the SiGe monolayer was slightly more sensitive to CO. Then, for CO adsorption, the C orientation (the C atom points directly to the SiGe substrate) was more suitable than the O orientation adsorption models (O points directly to the adsorption site). The most stable adsorption site of CO was the center site. The shortest distance (D) was 3.41 Å, which existed between the C and Ge atoms. The considerably large D corroborated the smallest value of Ead. Moreover, after the adsorption, electrons transferred from the substrate to CO, which may induce a doping behavior. The most stable adsorption site for CO2 was different. The smallest Ead of −0.2 eV emerged in the Si site when the C atom of the gas molecule pointed to the substrate. The adsorption induced a larger Ead compared to that of CO adsorption. The distance between C and Si was 3.89 Å, and the amount of electrons that transferred to the gas molecule was smaller than the CO adsorption, which was consistent with the adsorption energies.
The H2 adsorption at the center site showed an Ead as −0.15 eV, which was really small to make us believe that it was a physical adsorption. The electrons transferred from the substrate to the H2 molecule were few, and the distance was relatively large, presenting as 2.89 Å between the center to the H atom, the distance between the H and Ge/Si atoms being even larger, at least 3.66 Å (H-Ge). For H2O adsorption, Ead = −0.35 eV was a relatively suitable physical adsorption value for gas detecting. The O and Ge distance was 3.00 Å, which makes building the covalence bond difficult. The electrons moved from the gas molecule to the substrate, and the large amount of electrons (0.03 |e|) just corresponded to the relatively large adsorption energy.
3.2. NO, SO2, and NH3 Adsorption
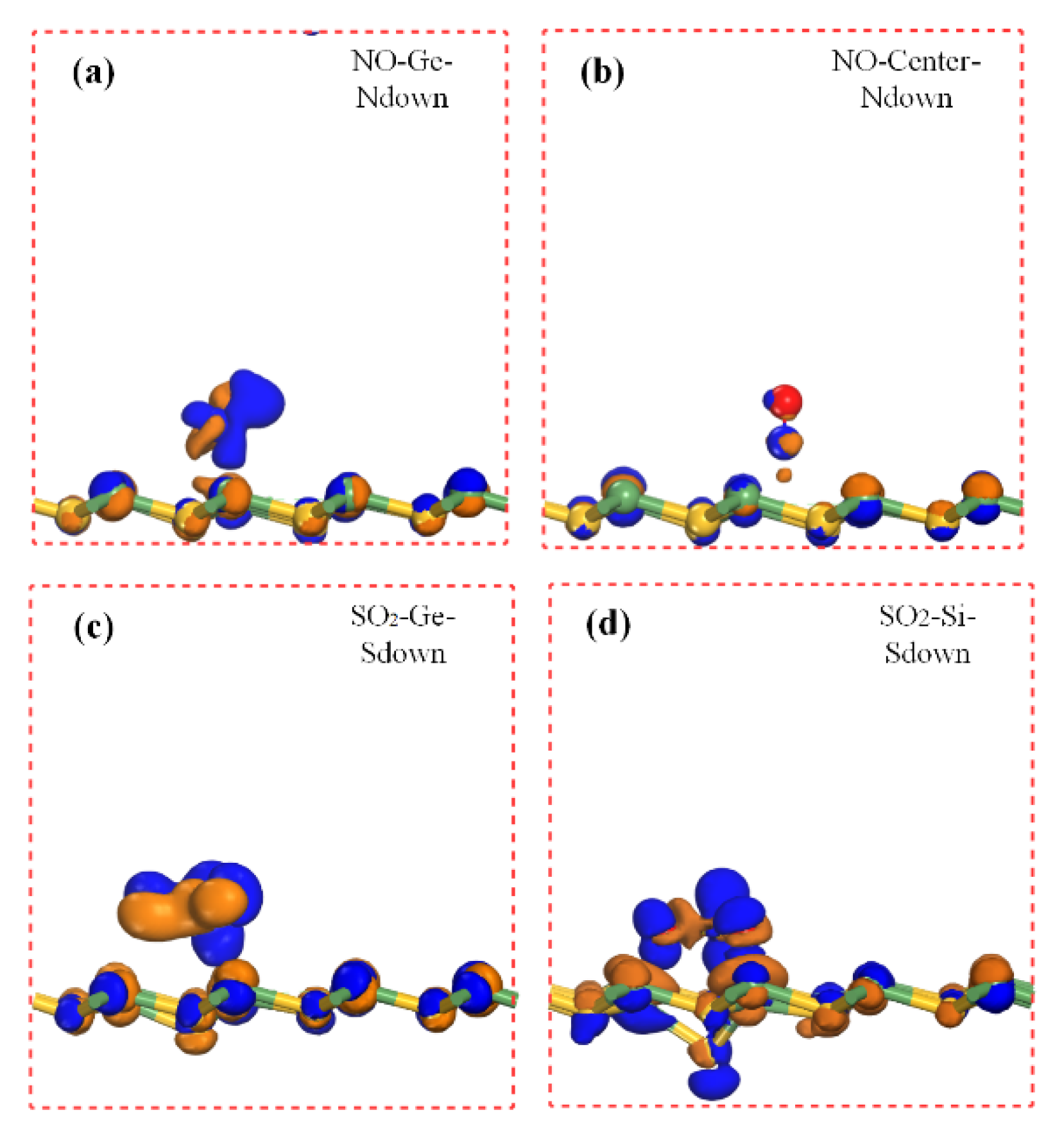
For NO adsorption, the center and Ge sites presented the most attractive adsorption performances with the N atom of NO molecule all closing to the Ge atom. The center site exhibited a smaller value of Ead of −0.48 eV, and the shortest distance existed between the N and Ge atoms, which reached 2.80 Å. Meanwhile, the Ge site presented a slightly smaller absolute value of Ead of 0.40 eV. These two values seemed to be in attractive physical adsorption ranges. To ensure the preliminary guess, the adsorption distance was also explored. In order to consider the possible built bond, the distance between N and the atom around the hexagon was investigated. The shortest distance of N and Ge is mentioned above. Regarding the covalent radius, the N covalent radius was 0.71 Å, and that of Ge was 1.20 Å. The existing N-Ge distance (2.80 Å) was larger than the covalent radius sum (1.91 Å) of separate N and Ge atoms, which might indicate that the adsorption could be a kind of physical adsorption. Charge transfer demonstrated that the NO molecule obtained charge as 0.03 |e|. For Ge site adsorption of NO, the adsorption energy was −0.40 eV, and the D was 2.28 Å (distance of N-Ge). The D of the Ge site was smaller than that of the center site; however, the center site adsorption exhibited a smaller Ead and a smaller amount of |Q|. This may be attributed to the center adsorption site that can be influenced by too many atoms surrounding especially the three nearest Ge atoms. Every Ge atom of the hexagonal ring contributing to the adsorption made the D relatively average to Ge atoms and may also reduce the charge transfer from SiGe to NO. Considering the calculated parameters, the physical adsorption could be verified for NO adsorbing on the center and Ge sites.
Similar to the NO adsorption, there were two sensitive sites for SO
2 adsorption that drew our attention. The Ge and Si sites with the S atom pointing directly to them initially possessed two attractive values of adsorption energy as −0.57 and −0.78 eV, respectively. The S-Ge distance was 2.76 Å of Ge site adsorption. For Si site adsorption with S pointed to, the shortest distance became O-Ge as 2.11 Å, while the distance of S-Si was 3.61 Å. This adsorption was accomplished with obvious Si site deformation. However, the
Ead of SO
2 adsorbed on the Ge site with the O atom pointed to was just −0.11 eV with a distance of 3.19 Å for O-Ge. This interesting scene could be explained through the adsorption structure. When the calculation is ready for S pointing directly to the Si site, the Si atom of SiGe monolayer became deformed. Si seemed to be repelled by the S atom, inducing bond elongation between Si and Ge; at the same time, the O atom gradually became close to the Ge atom, and SO
2 finally became almost parallel to the SiGe plane, which was different from
Figure 1c. The two sites adsorptions showed a large number of electrons transferring to SO
2, and physical/chemical adsorptions might exist, respectively. That will be investigated later.
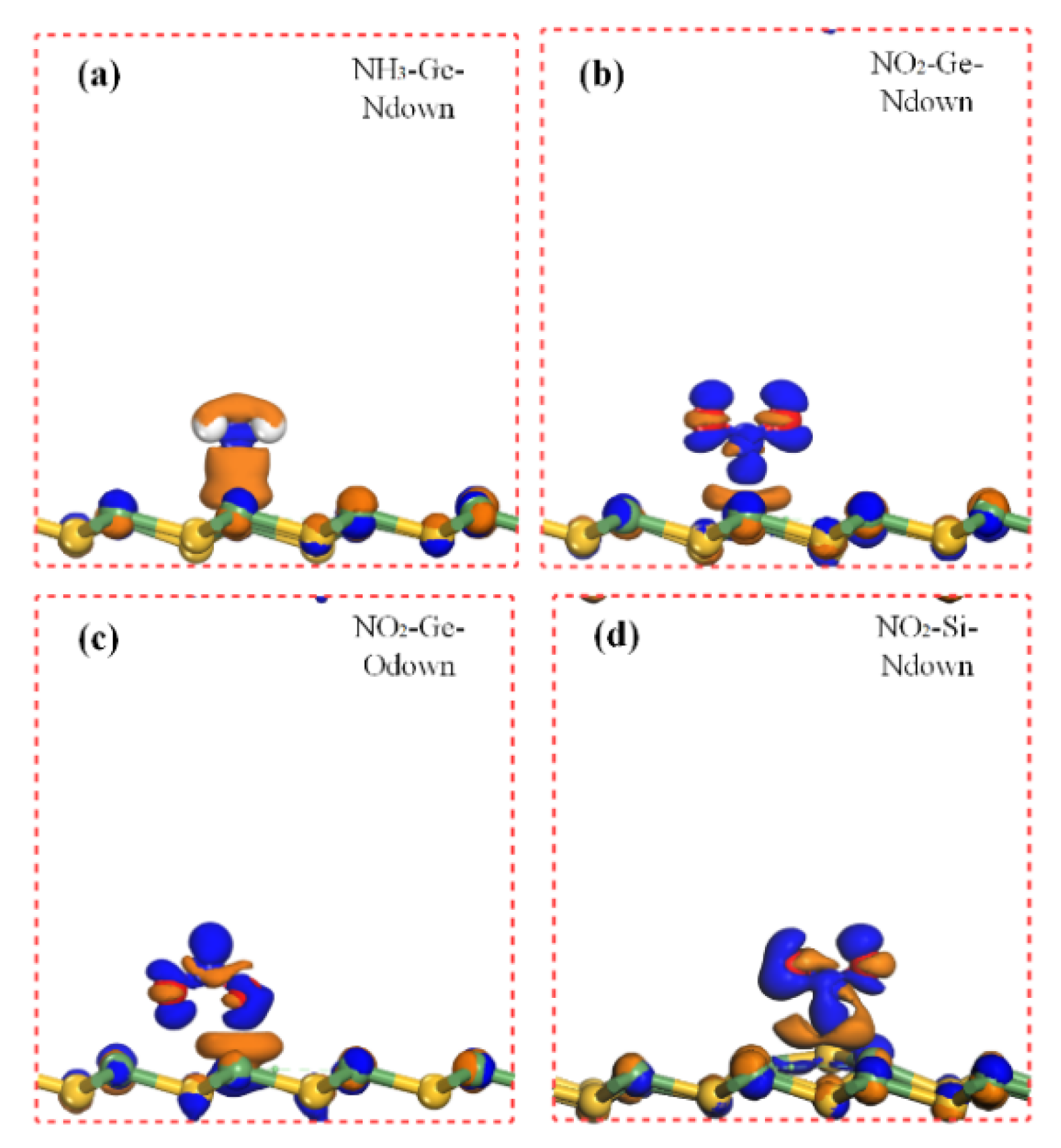
For NH3 adsorbed at SiGe monolayer, the three adsorption models were all calculated, and two larger adsorption energy models were selected as the Ge and Si sites. The same adsorption energies of −0.62 eV excited our interest. More importantly, it was an attractive value for physical adsorption as a gas sensing application. Hence, the calculation results were tested, and it was found that the NH3 placed at the Si site moved to the Ge site finally. Therefore, the Ge site must be the most attractive sensing site for NH3. The distance between N and Ge was 2.32 Å, which was larger than the covalent radius sum (1.91 Å) of separate N and Ge atoms. As a consequence of all the above, NH3 adsorption on the Ge site might be physical adsorption with a moderate adsorption energy.
3.3. NO2 and O2 Adsorption
In the case of NO2 adsorption, the most energetic favorable site where the NO2 molecule preferred to reside was the Ge and Si sites. After full relaxation, the N and O atoms all preferred to point closely to SiGe monolayer. The adsorption energies for N close to the Ge site, O close to the Ge site, O close to the Si site, and N close to the Si site were −1.00 eV, −1.14 eV, −1.140 eV, and −1.24 eV, respectively. The super large adsorption energies made us pay attention to all these adsorption sites. When the O atom of NO2 pointed to the Ge and Si sites, the same adsorption energy made us wonder if the situation was similar to the NH3 adsorption mentioned above. Through our calculation, the O atom firstly pointing directly to the Si site would move to the nearest Ge site and finally kept the same distance (2.03 Å) of Ge-O compared to the situation of O pointing directly to the Ge site. This indicated that the Ge site was the most sensitive site when the O of NO2 pointed directly to SiGe monolayer. As for the N of NO2 pointing to the Ge site and Si site, the adsorption energies were still very large as −1.00 eV and −1.24 eV. The distances of the two situations were 2.16 Å and 1.96 Å, respectively. The shortest distance corresponded to the largest adsorption energy, which simply confirmed the correctness of our work. As for the adsorption model, the covalent radius of N-Si was 1.82 Å, which is very close to the 1.96 Å of the largest Ead adsorption (Si site adsorption with N atom pointing to it). The largest adsorption energies and small distances might imply chemical adsorption activities; meanwhile, the physical or chemical adsorption species of the other NO2 adsorptions were hard to determine considering the large Ead and relatively small distances (the covalent radii of O-Ge and N-Ge were 1.86 Å and 1.91 Å, respectively). The charges moved from SiGe to NO2, reaching about 0.3 ~ 0.4 |e|. Analyzing the band gap of NO2 adsorption, the Fermi level moved down to the valence band, which showed a p-type doping. As for O2 adsorption, the adsorption energy was −0.93 eV; meanwhile, the shortest distance between O2 and SiGe monolayer was the O-Ge distance, which was 2.93 Å. As mentioned above, the covalent radius of O-Ge was 1.86 Å, which was nearly 1 Å smaller than that of O2 adsorption. It was hard to distinguish the adsorption type because of the large adsorption energy and relatively large atom distance. The uncertain gas adsorption models (physical/chemical adsorption) of NO2 and O2 will be studied later.
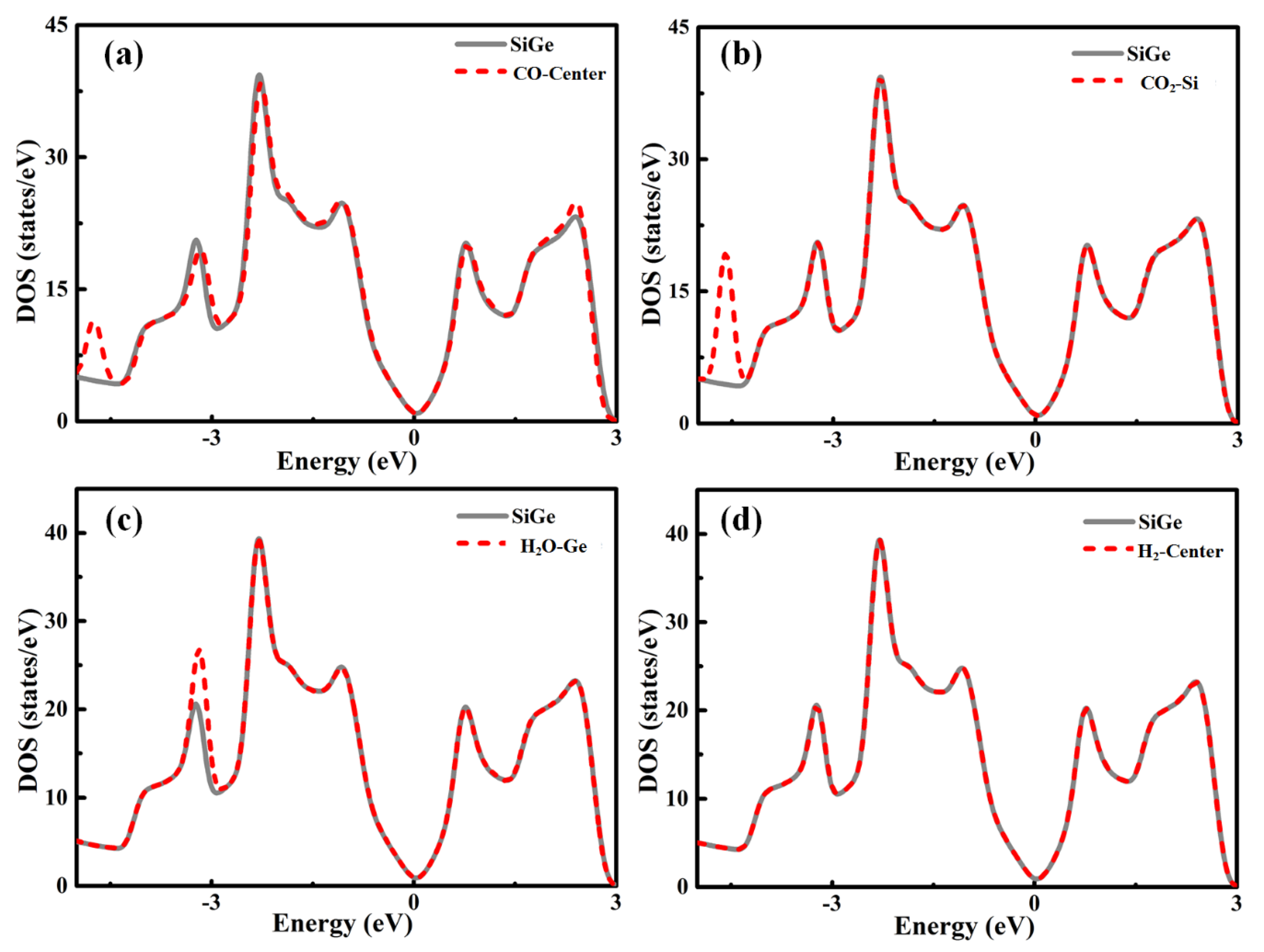
In order to explore the fundamental interaction of Gas-SiGe adsorption, the total electronic density of states is presented in
Figure 2 [
22]. The spin polarization effect was considered for some specific gases, and the results indicated that there were no significant spin polarizations in the total density of state (DOS) plots. Therefore, the DOS plots all adopted no spin polarization effect pattern.
The DOS plots of CO, CO
2, H
2O, and H
2 adsorption are presented in
Figure 2a–d, respectively. From the pictures above, these gases’ adsorptions induced some changes in the trend of the DOS lines. However, during the critical area around the Fermi level, the lines of separate SiGe and gas-SiGe systems fit each other perfectly. This phenomenon suggested that the interaction between the gas molecule and SiGe was negligible. This phenomenon was the same as the result of physical adsorption. These results revealed the same conclusion as the adsorption energy and charge transfer.
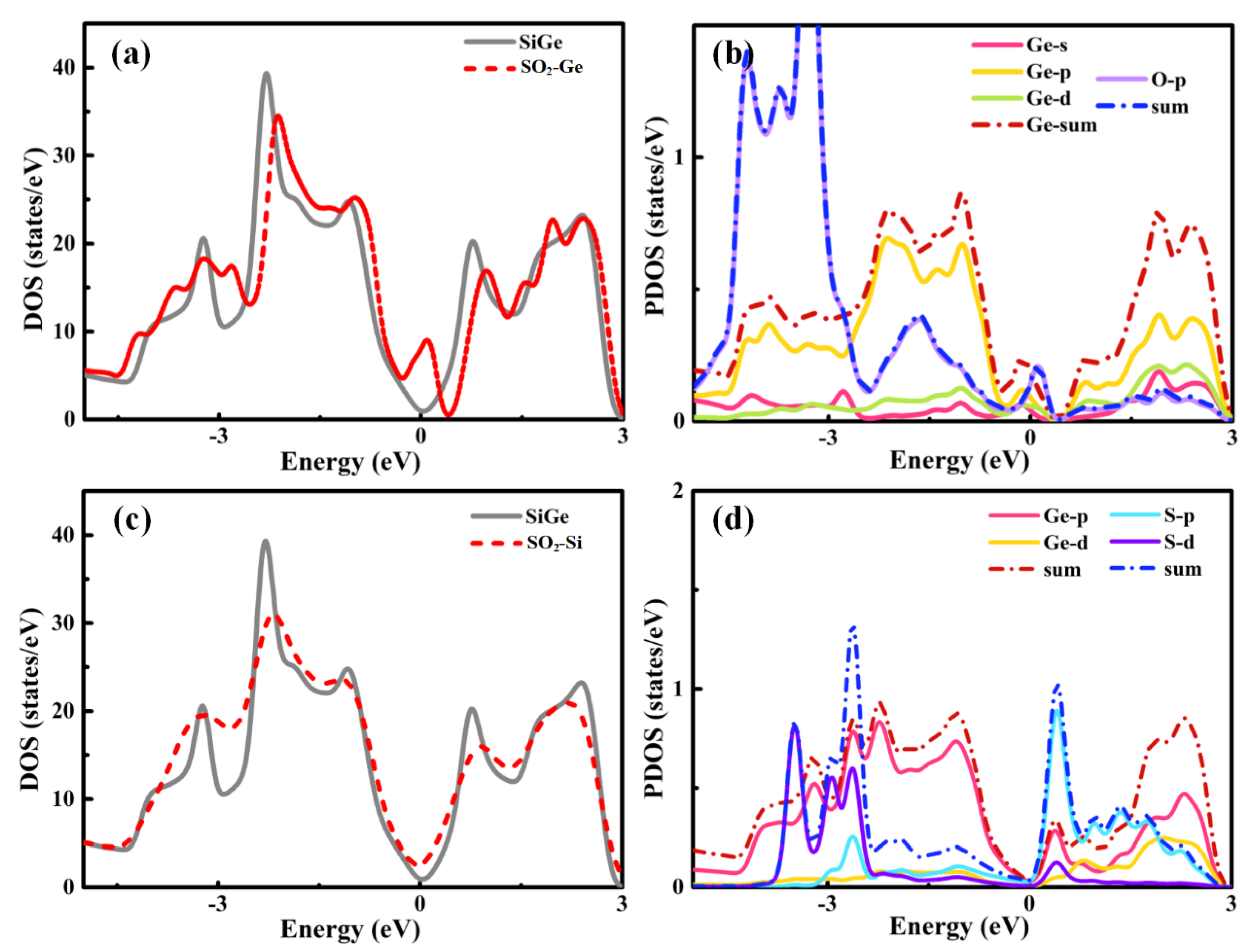
Analyzing the DOS plots of SO
2 in
Figure 3, the differences between SO
2 and those above were clear. The obvious difference around the Fermi level between the separate SiGe monolayer and SO
2-SiGe system presented a relatively strong interaction between the gas molecule and substrate material. Ge site adsorption induced a small peak at the right side of the Fermi level and the little shifts of DOS toward VBM corresponding to the p-type doping that corresponded to the charge transfer for SiGe to the SO
2 molecule as 0.29 |
e|. The p orbital of Ge and p orbital of S shared a hybridization and contributed to the small peak near the Fermi level. In the meantime, the Si site adsorption induced a large peak around the Fermi level, and the DOS shift seemed more serious than Ge site adsorption with larger charge transfer to the SO
2 molecule as 0.477 |
e|. The partial density of states (PDOS) plot implied that the p and d orbitals of Ge and the p orbital of O led to the strong interactions.
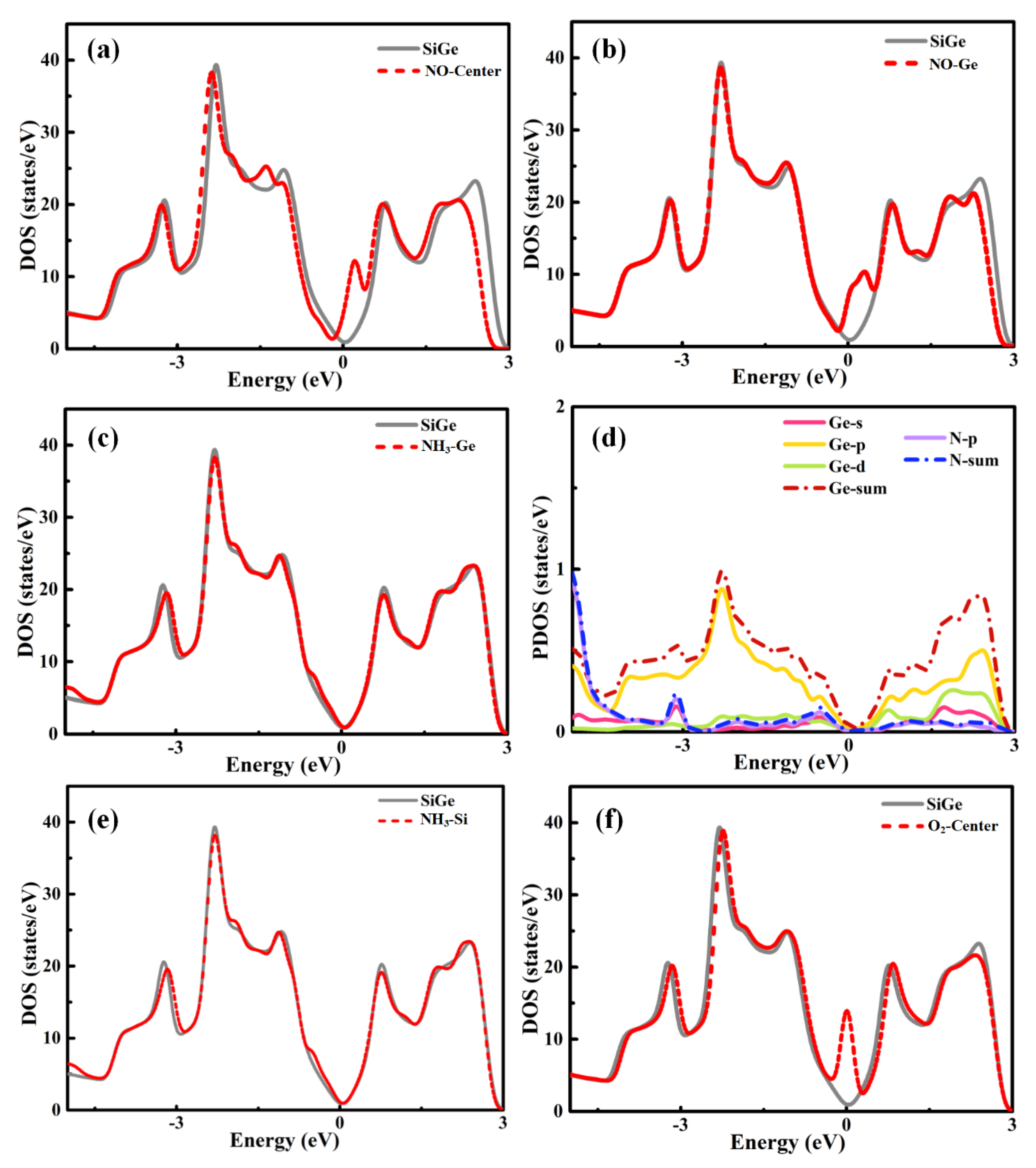
For NO, NH
3, and O
2 adsorption, the DOS and PDOS figures are presented in
Figure 4. The NO adsorptions on SiGe monolayer at the center and Ge sites caused quite an increase of DOS around the Fermi level. This implied the relatively strong interaction between the gas molecule and SiGe monolayer, which was similar to SO
2 adsorption. The slight movement of the Fermi level, especially N-center adsorption, was in good agreement with the charge transfer to the gas molecule. When NH
3 was adsorbing in SiGe monolayer, the same final adsorption site mentioned above was confirmed again through the same DOS plots in
Figure 4c,e. The PDOS plot announced that the s, p, and d orbitals of Ge plus the p orbital of N mainly contributed to the attractive adsorption, while the s and p orbitals of Ge together with the p orbital of N contributed to the slight increase of the valence band gap area near the Fermi level. The DOS in
Figure 4f of the O-SiGe model was consistent with the strong adsorption behavior. A strong peak appearing around the Fermi level suggested the intense interaction in the adsorption. According to the small distance and large adsorption energy, it must be a chemical adsorption.
Taking into account the four situations of NO
2 adsorption, the plots of b, d, f and g are the PDOS patterns of
Figure 5.
Figure 5a,b,e,g are the DOS patterns. First, the same DOSs of O pointed directly to Ge and Si, respectively, implying that the final adsorption structures of the two situations became the same one: the O atom pointing to the Ge site adsorption model (shown in
Figure 5c,d,g,h). Thus, the DOS plots confirmed the result mentioned above. The peaks around the Fermi level during these situations showed the interaction. Moreover, the relatively small peak, shown in
Figure 5e, of the Si site adsorption (N orientation) during the four situations related to the smallest adsorption energy. The similar Fermi level movements implied p-type doping where the charges transferred to the gas molecules. By analyzing the other figures in detail,
Figure 5a,b show that the s and d orbitals of Ge together with the s and p orbitals of N contributed to the large increase in the Fermi level, which was consistent with the largest adsorption energy. At the same time, the pretty large increase in
Figure 5c,g for O-Ge adsorption patterns was mainly induced by the s and d orbitals of Ge coupled with the p orbital of O. The interaction between the gas molecule and substrate layer was slightly smaller than that in
Figure 5a.
3.4. Charge Density Difference
Charge transfer is an important parameter to determine the interaction between the gas molecule and the substrate material. The charge density difference (CDD) [
23] can intuitively help us figure it out.
Figure 6,
Figure 7 and
Figure 8 elucidate the CDD images of these Gas-SiGe systems, calculated by the formula [
24]
△ρ =
ρSiGe+molecule – (
ρSiGe +
ρmolecule), where
ρSiGe+molecule,
ρSiGe, and
ρmolecule are the charge density of the gas-adsorbed SiGe monolayer, the pristine SiGe monolayer, and the gas molecule, respectively.
ρSiGe and
ρmolecule must be calculated in the same adsorbed configuration.
The charge redistributions caused by gas adsorptions are clearly described in
Figure 6,
Figure 7 and
Figure 8. After CO adsorption, the charge transfer apparently moved from SiGe to the CO molecule analyzed in
Table 1. At the same time, the CDD plot in
Figure 6a also verified this, which implied a charge redistribution in the CO molecule. The charges concentrated in the O atom. The CO
2 adsorption exhibited smaller charge transfer to the CO
2 molecule mainly remaining in the O atoms. Moreover, the charge movement in H
2 adsorption was comparably small, so that there was barely any charge image in the H
2 molecule under this isosurface value. For H
2O adsorption, there was a positive charge state in H
2O after adsorption, while the O atom owned the sole negative charge. For NO adsorption at center and Ge sites, the negative charge scattered on the N and O atoms. Considering the SO
2 adsorption, the charge redistribution was obviously larger than other gas adsorption mentioned above. Since the Si site adsorption (
Figure 7d) caused obvious SiGe monolayer deformation. The positive and negative charge redistributions between O atoms and Ge atom demonstrated the bond existing as chemical adsorption. However, the comparatively little charge redistribution displayed a smaller adsorption strength. It was still hard to confirm the adsorption style for SO
2-Ge (the S atom pointed to the Ge site) adsorption, and it will be ascertained in the next part. A similar situation was also found when NH
3 adsorbed on SiGe at the Ge site with N pointing directly to the Ge atom (shown in
Figure 8a). Many electrons are depleted in NH
3 and the area between the gas molecule and SiGe monolayer. H atoms carry a positive charge, and the negative charges concentrated on the N atom at the same time. To date, NO
2 adsorption style has been identified as chemical adsorption. The CDD plots and Mulliken charge analysis shared similar results that the NO
2 molecule totally carried a negative charge and a strong charge transfer occurred between the gas molecule and the nearest atoms of the substrate monolayer.
3.5. Electron Localization Function
In order to confirm the bond existing between some high adsorption energies and short distance adsorption models, the electron localization function (ELF) [
25,
26,
27] was used. ELF is an effective tool for understanding the characteristics of chemical bonding and the lone electron pairs (non-bonding electron pairs) by qualitatively describing the degree of electron localization. The function has a value between zero and one, where ELF = 1 indicates the prefect localization characteristics of the covalent bonding or the lone electron pairs, ELF = 0.5 represents the electron-gas-like pair probability, and ELF = 0 corresponds to delocalization. For brevity, NO-center, NH
3-Ge, SO
2-Si, O
2-center, and NO
2-Ge (N pointed to) were considered. For NO-center adsorption shown in
Figure 9a, the value between N and the nearest Ge atom was smaller than 0.5, close to zero. There was no bond between N and the nearest Ge. For NH
3 and SO
2 adsorption shown in
Figure 9b,c, the situations were different. The values were larger than 0.5, which represented the electron localized and the interactions between gas molecules and substrate atoms were strong. The new bonds were built during the two gases’ adsorption. They were considered as chemical adsorption. As shown in
Figure 9d, O
2 adsorption at the center site showed an obvious separation between the O and Ge area in the ELF plot. The nearly zero value of ELF is shown as the blue area, and electrons were evenly distributed around the O and Ge atoms, which ensured that O
2 adsorbed at the center site in a physical adsorption model. However, NO
2 adsorption at the Ge site with the N atom orientation possessed the smallest adsorption energy compared with other NO
2 adsorptions. If it were verified as chemical adsorption, the other NO
2 adsorptions could be considered as chemical adsorption naturally. In
Figure 9, the ELF value at the area between N and Ge was really close to one. The covalent characteristic bond was obvious. Therefore, there must be a new bond built between N and Ge when NO
2 adsorbed at the Ge site with N pointing to it. Moreover, the other NO
2 adsorptions mentioned above also would be chemical adsorption.
3.6. Effect of an Electric Field on SiGe-Gas Interaction
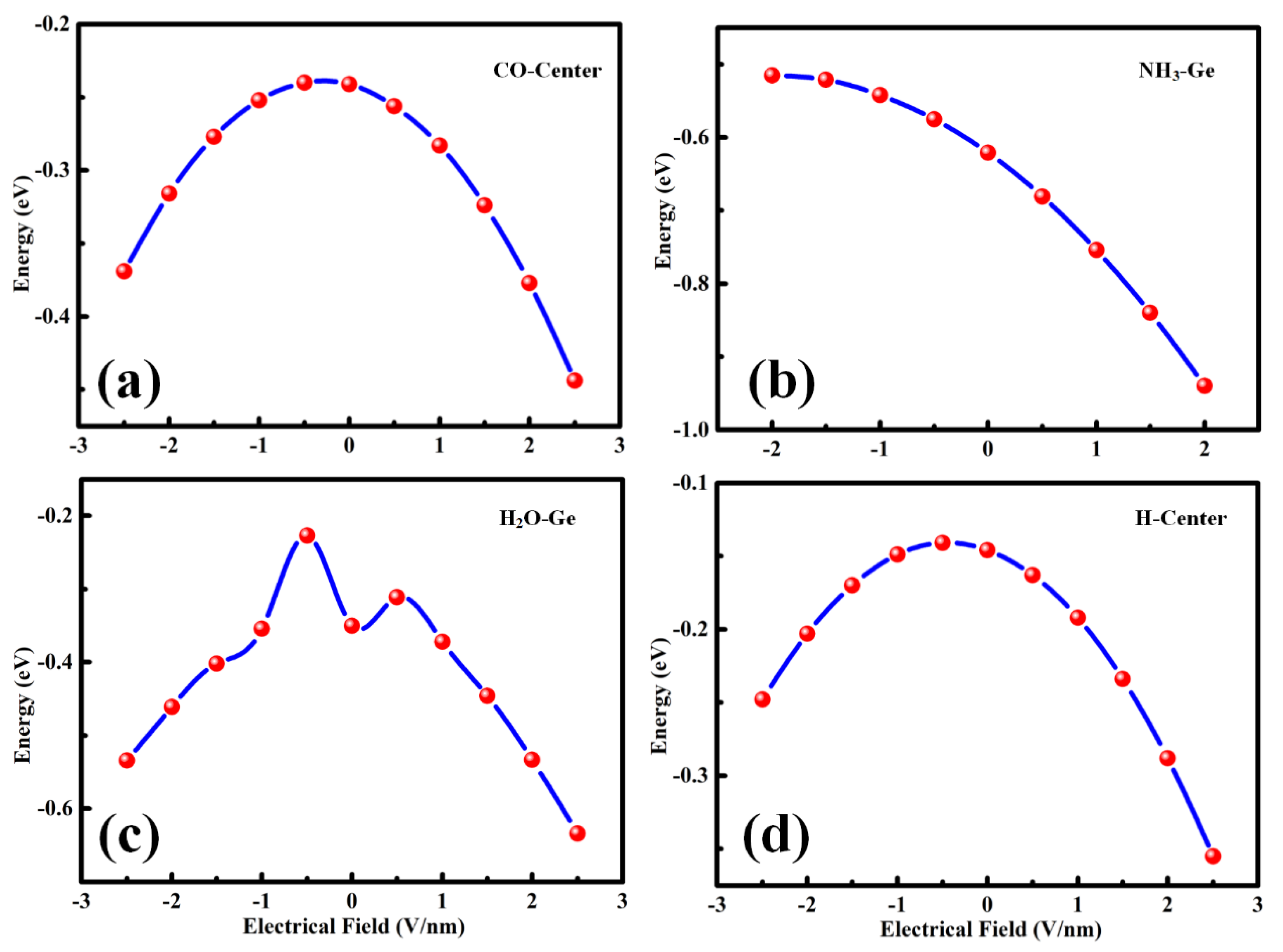
The charge transfer between the gas molecule and the monolayer material played a crucial role in interaction and adsorption strength. An external electric field is a common method to adjust the property of the material. It can effectively alter the charge transfer. Therefore, the E-field [
28,
29] was used to modify the adsorption effect. As shown in
Figure 10, the small
Ead adsorption models of CO, H
2O, and H
2, plus the attractive
Ead adsorption of the NH
3-Ge-site were calculated. The CO and H
2 models shown in
Figure 10a,d exhibited the same trend under the E-field. The E-field produced an obvious enhancement in gas adsorption. As for H
2O adsorption, the increasing values of |E-field| in 0 ~
and 0 ~ −0.5 made |
Ead| decrease, and the negative direction E-field possessed a more obvious phenomenon. Then, the adsorption energy became larger when the E-field increased. Moreover, in
Figure 10b, there was a different trend. The positive direction of the E-field similarly enhanced |
Ead|, making the adsorption stronger, while the negative direction of E-field displayed a trend of reducing |
Ead|. This was an interesting effect that might be used in the desorption process, which may improve the utilization rate of the material.
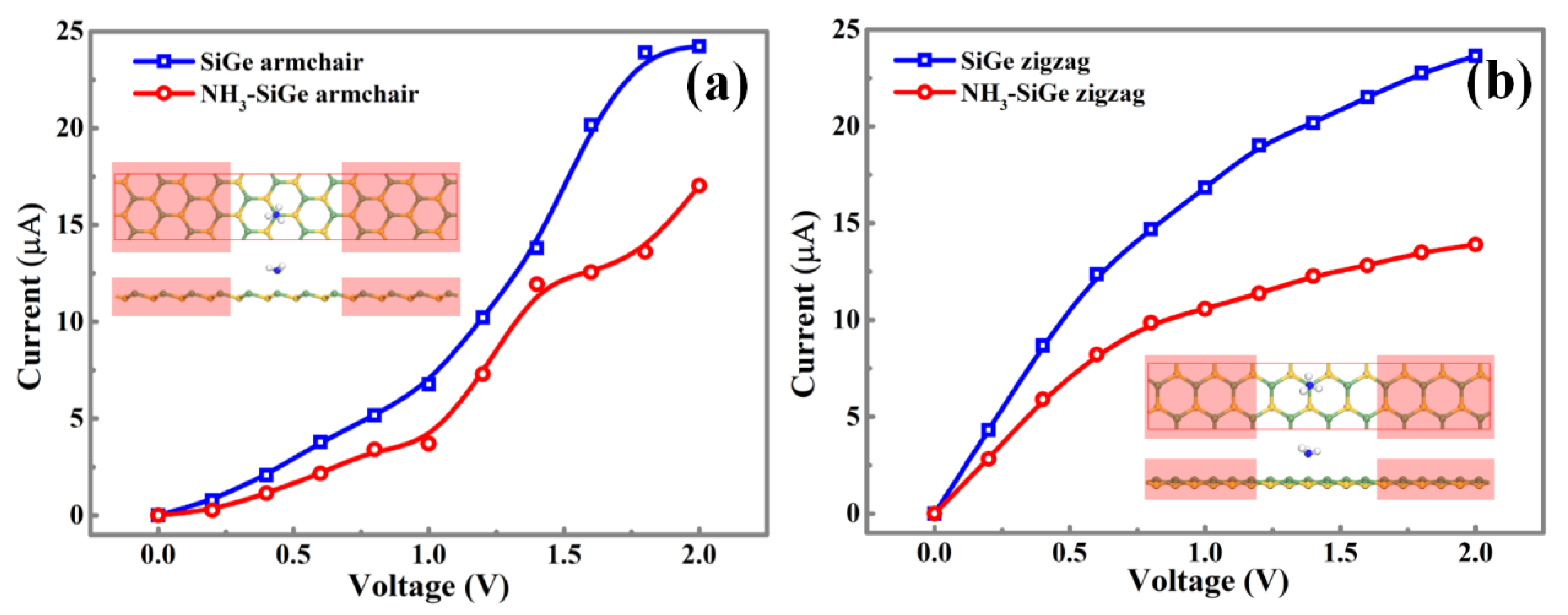
To explore the performance of the SiGe monolayer, the NH
3-Ge adsorption model was chosen to calculate the current-voltage [
30,
31,
32] (I-V) relation before and after the gas adsorption through the NEGF (Non-Equilibrium Green Function) method showing in
Figure 11. The armchair direction of the intrinsic SiGe monolayer showed a small irregular increase, and the curve reached the extremum at around 1.8 V. The NH
3 adsorption made the I-V curve more irregular, but it still showed an increasing trend. For the zigzag direction of the intrinsic SiGe monolayer, the I-V curves increased along with the rising bias from 0 to 2.0 V with a decreased slope, and it would be its extremum when 2.0 V was applied. The NH
3 adsorption in Ge site reduced the conductive ability, which might be beneficial for the application of gas sensing.