Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform
Abstract
1. Introduction
2. Materials and Procedures for Development and Testing
2.1. Chemicals
2.2. Apparatus
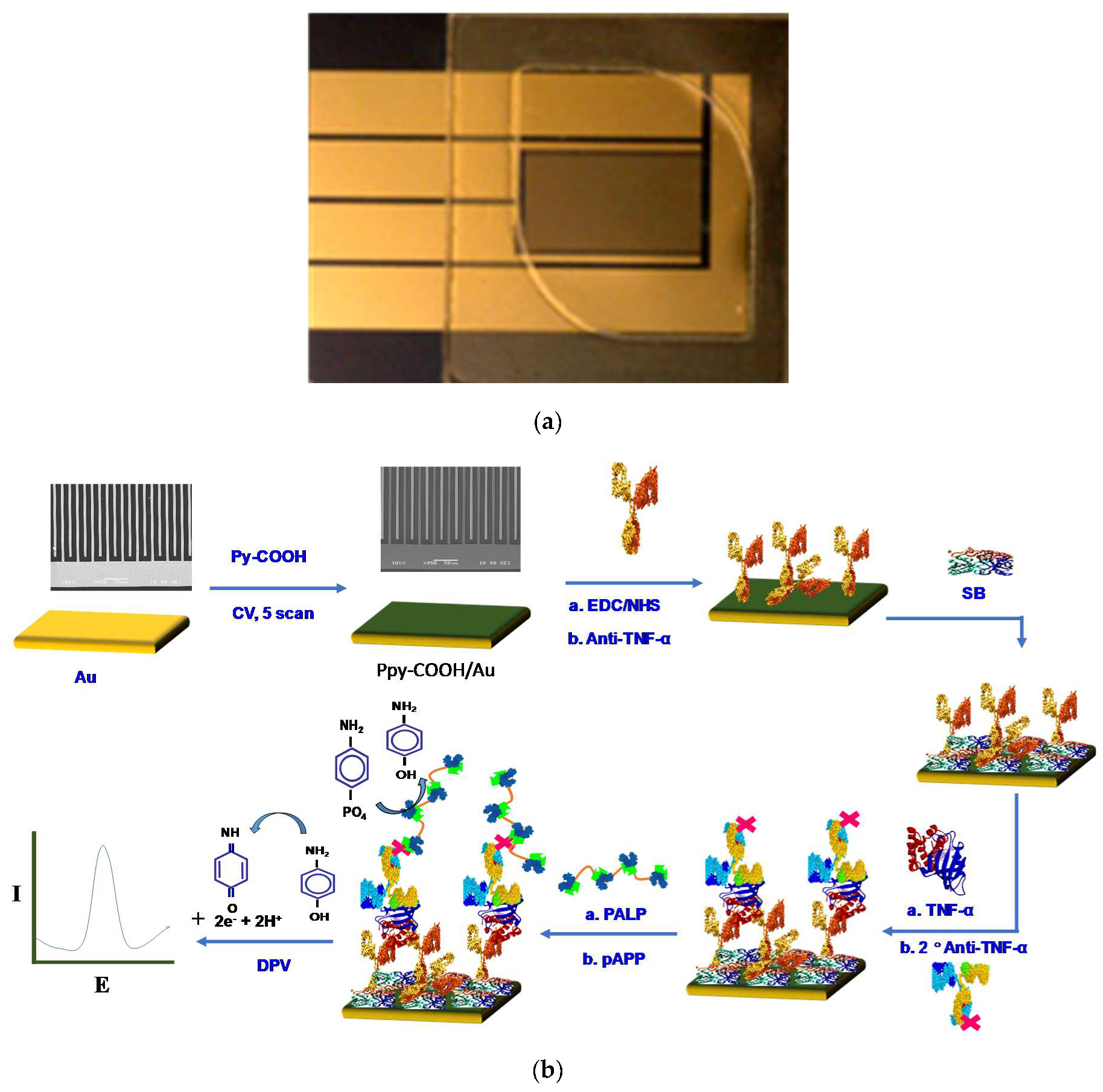
2.3. Gold Electrode Preparation
2.4. PPy-COOH Deposition and Anti-TNF-α Immobilization
2.5. TNF-α Binding and Estimation Studies
- Antigen incubation: 30 min.
- Washing using washing buffer (Femto TBST, 1x): three times.
- Interaction with biotin modified secondary antibody solution (50 µL, 10 µg/mL) in SB-TBST: 30 min.
- Washing using washing buffer (Femto TBST, 1x): three times.
- Interaction with streptavidin modified alkaline phosphatase (ALP) or polymeric alkaline phosphatase (PALP) solution (50 µL, 10 µg/mL) in SB-TBST: 30 min.
- Washing using washing buffer (Femto TBST, 1x): three times.
- Final interaction with 50 µL of 2 mg/mL 4-APP in de-aerated tris-HCl buffer (100 mM, pH 9.0 containing 4 mg/mL MgCl2·6H2O): 20 min.
- Electrochemical oxidation signal recording using differential pulse voltammetry.
3. Results and Discussion
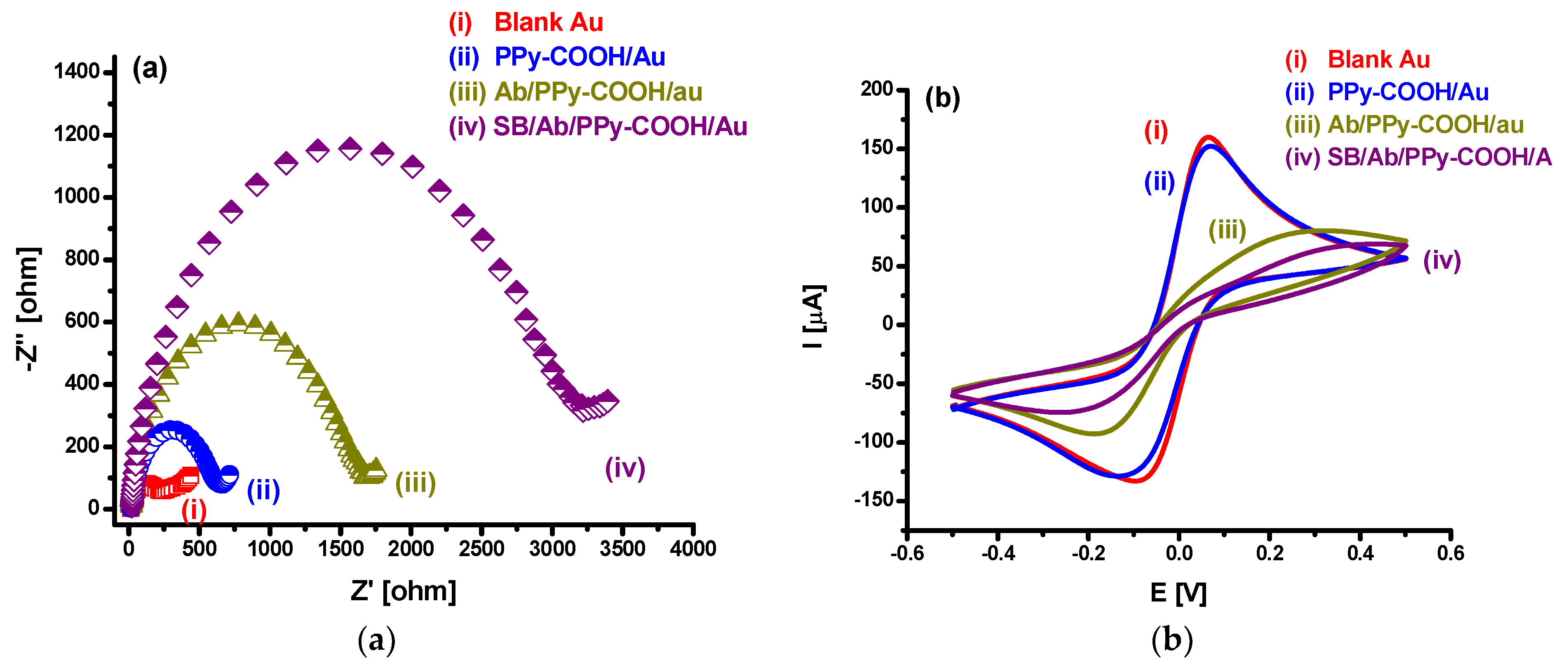
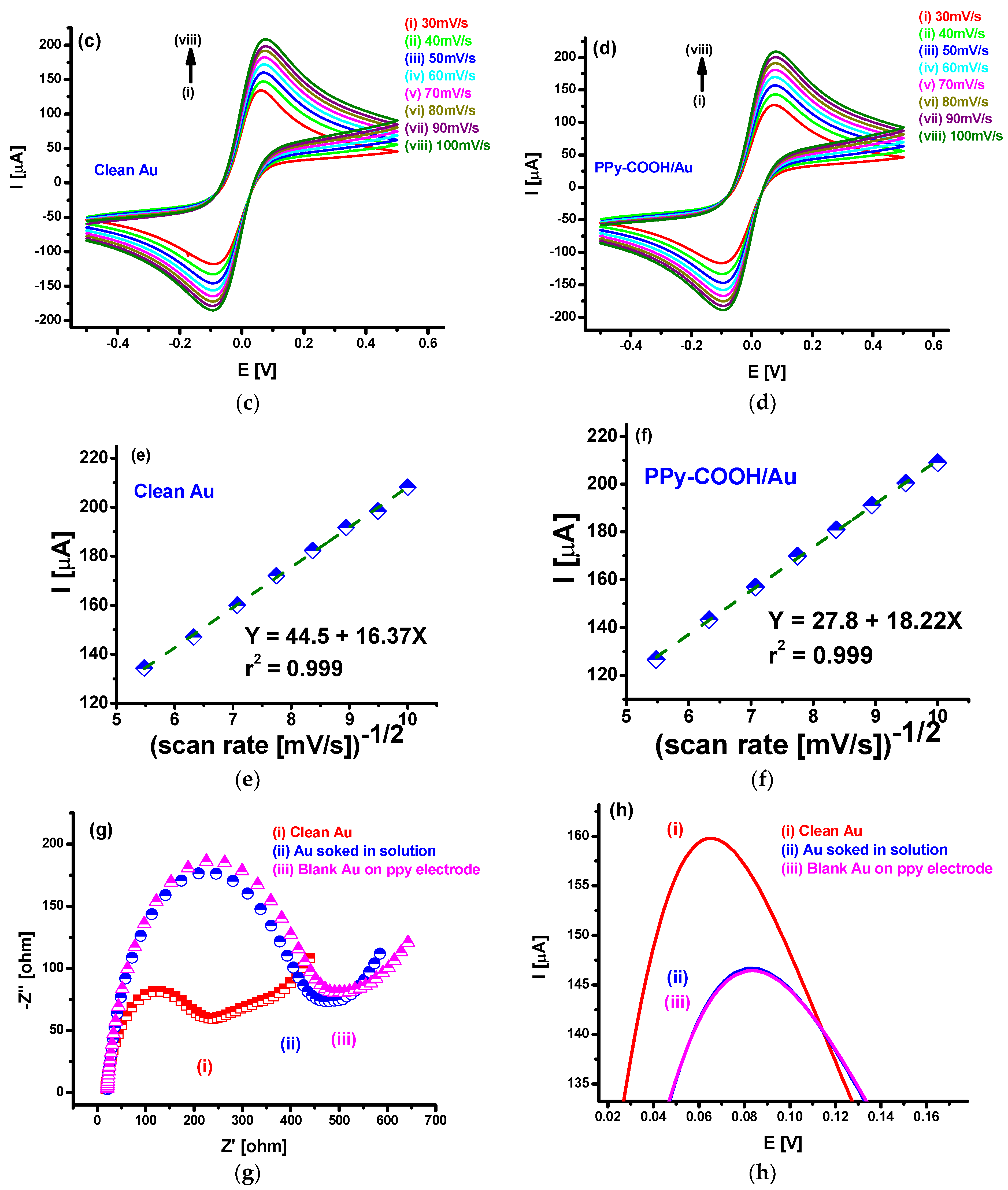
3.1. EIS and CV Investigations
3.2. SEM and AFM Characterization for PPy-COOH Deposition
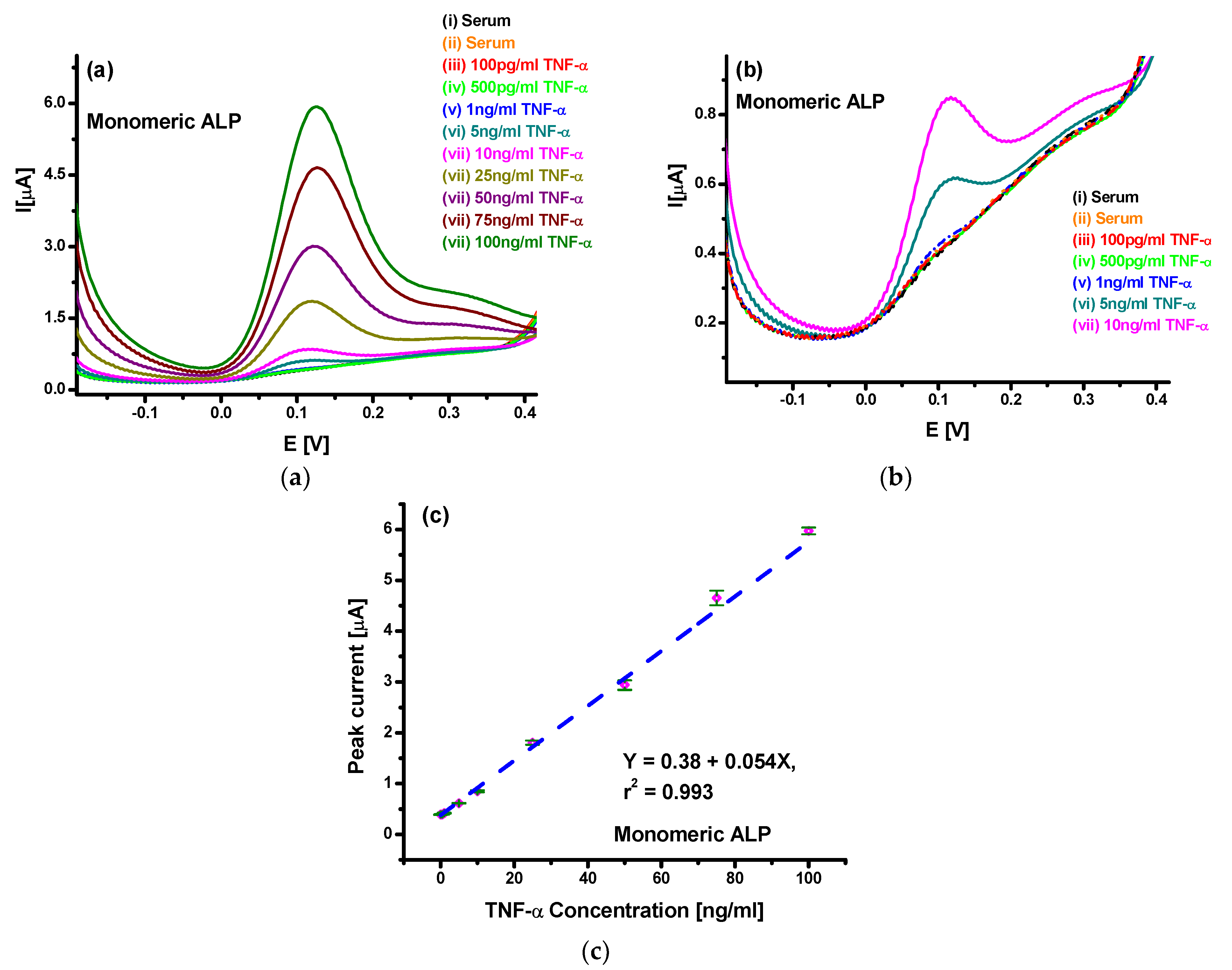
3.3. Differential Pulse Voltammetric Studies for TNF-α Detection
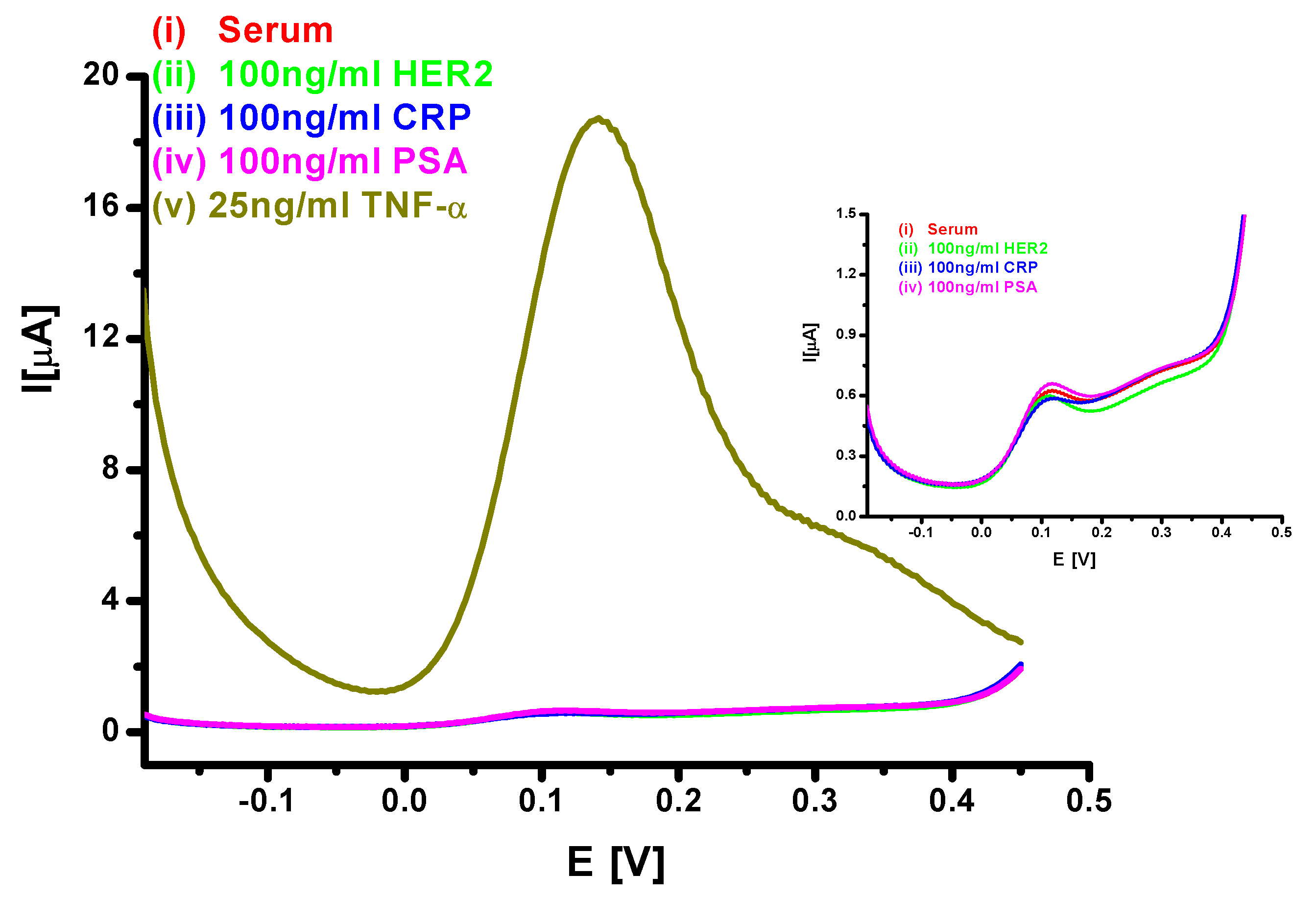
3.4. Interference Studies from Other Proteins
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sankara, V.S.P.K.; Jayanthi, A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar]
- Salek-Maghsoudi, A.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Estrela, P. Recent advances in enhancement strategies for electrochemical ELISA-based immunoassays for cancer biomarker detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Estrela, P. Electrochemical immunosensor for tumor necrosis factor-alpha detection in undiluted serum. Methods 2017, 116, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B. A label-free and sensitive impedimetric immunosensor for TNF α biomarker detection based on epoxysilane-modified disposable ITO-PET electrode. Inter. J. Environ. Anal. Chem. 2020, 100, 363–377. [Google Scholar] [CrossRef]
- Barhoumi, L.; Bellagambi, F.G.; Vivaldi, F.M.; Baraket, A.; Clément, Y.; Zine, N.; Ali, M.B.; Elaissari, A.; Errachid, A. Ultrasensitive immunosensor array for TnF-α detection in artificial saliva using polymer-coated magnetic microparticles onto screen-printed gold electrode. Sensors 2019, 19, 692. [Google Scholar] [CrossRef]
- Barhoumi, L.; Baraket, A.; Bellagambi, F.G.; Karanasiou, G.S.; Ali, M.B.; Fotiadis, D.I.; Bausells, J.; Zine, N.; Sigaud, M.; Errachid, A. A novel chronoamperometric immunosensor for rapid detection of TNF-α in human saliva. Sens. Actuators B 2018, 266, 477–484. [Google Scholar] [CrossRef]
- Bari, S.M.I.; Reis, L.G.; Nestorova, G.G. Calorimetric sandwich-type immunosensor for quantification of TNF-α. Biosen. Bioelectron. 2019, 126, 82–87. [Google Scholar] [CrossRef]
- Yagati, A.K.; Lee, M.H.; Min, J. Electrochemical immunosensor for highly sensitive and quantitative detection of tumor necrosis factor-α in human serum. Bioelectrochemistry 2018, 122, 93–102. [Google Scholar] [CrossRef]
- Bettazzi, F.; Enayati, L.; Sánchez, I.C.; Motaghed, R.; Mascini, M.; Palchetti, I. Electrochemical bioassay for the detection of TNF-α using magnetic beads and disposable screen-printed array of electrodes. Bioanalysis 2013, 5, 11–19. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Kongsuphol, P.; Park, M.K. Off surface matrix based on-chip electrochemical biosensor platform for protein biomarker detection in undiluted serum. Biosens. Bioelectron. 2017, 92, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Dou, W.; Zhao, G. A sandwich electrochemical immunoassay for Salmonella pullorum and Salmonella gallinarum based on a AuNPs/SiO2/Fe3O4 adsorbing antibody and 4 channel screen printed carbon electrode electrodeposited gold nanoparticles. RSC Adv. 2015, 5, 74548–74556. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Laleh, H.; Alireza, K. Ultrasensitive electrochemical immunosensor for detection of tumor necrosis factor-α based on functionalized MWCNT-gold nanoparticle/ionic liquid nanocomposite. Electroanalysis 2015, 27, 2518–2526. [Google Scholar]
- Hu, W.; Li, C.M.; Cui, X.; Dong, H.; Zhou, Q. In situ studies of protein adsorptions on poly(pyrrole-co-pyrrole propylic acid) film by electrochemical surface plasmon resonance. Langmuir 2007, 23, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, E.; Chamsaz, M.; Turner, A.P.F.; Jager, E.W.H.; Beni, V. Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016, 80, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Janmanee, R.; Baba, A.; Phanichphant, S.; Sriwichai, S.; Shinbo, K.; Kato, K.; Kaneko, F. Detection of human IgG on poly(pyrrole-3-carboxylic acid) thin film by electrochemical-surface plasmon resonance spectroscopy. Jpn. J. Appl. Phys. 2011, 50, 01BK02. [Google Scholar] [CrossRef]
- Janmanee, R.; Baba, A.; Phanichphant, S.; Sriwichai, S.; Shinbo, K.; Kato, K.; Kaneko, F. In situ electrochemical-transmission surface plasmon resonance spectroscopy for poly(pyrrole-3-carboxylic acid) thin-film-based biosensor applications. ACS Appl. Mater. Inter. 2012, 4, 4270–4275. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A highly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef]
- Liu, L.; Liu, F.; Jiang, D.; Xiang, G.; Liu, C.; Yang, J.; Pu, X. Hybridization chain reaction and target recycling enhanced tumor necrosis factor alpha aptasensor with host-guest interaction for signal probe collection. Sens. Actuators B 2016, 231, 680–687. [Google Scholar] [CrossRef]
- Baydemir, G.; Bettazzi, F.; Palchetti, I.; Voccia, D. Strategies for the development of an electrochemical bioassay for TNF-alpha detection by using a non-immunoglobulin bioreceptor. Talanta 2016, 151, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kongsuphol, P.; Ng, H.H.; Pursey, J.P.; Arya, S.K.; Wong, C.C.; Stulz, E.; Park, M.K. EIS-based biosensor for ultra-sensitive detection of TNF-α from non-diluted human serum. Biosens. Bioelectron. 2014, 61, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pruna, R.; Palacio, F.; Baraket, A.; Zine, N.; Streklas, A.; Bausell, J.; Errachid, A.; López, M. A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-α by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2018, 100, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.V.; Mehra, M.R.; Endres, S.; Eigler, A.; Cooper, E.S.; Lavie, C.J., Jr.; Ventura, H.O. The clinical relevance of circulating tumor necrosis factor-alpha in acute decompensated chronic heart failure without cachexia. Chest 1996, 110, 992–995. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Khoshroo, A. Label-free electrochemical immunosensor for detection of tumor necrosis factor α based on fullerene-functionalized carbon nanotubes/ionic liquid. J. Electroanal. Chem. 2015, 757, 58–64. [Google Scholar] [CrossRef]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Amperometric magnetoimmunoassay for the direct detection of tumor necrosis factor alpha biomarker in human serum. Anal. Chim. Acta 2014, 838, 37–44. [Google Scholar] [CrossRef]
- Martínez-Borra, J.; López-Larrea, C.; González, S.; Fuentes, D.; Dieguez, A.; Deschamps, E.M.; Pérez-Pariente, J.M.; López-Vázquez, A.; de Francisco, R.; Rodrigo, L.S. High serum tumor necrosis factor-α levels are associated with lack of response to infliximab in fistulizing Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2350–2356. [Google Scholar]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef]
- Palchetti, I. Affinity biosensors for tumor-marker analysis. Bioanalysis 2014, 6, 3417–3435. [Google Scholar] [CrossRef]
- Brunet, M. Cytokines as predictive biomarkers of alloreactivity. Clin. Chim. Acta 2012, 413, 1354–1358. [Google Scholar] [CrossRef]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef]
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical chemistry of cytokines—A review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Bao, B.; Chen, Y.-G.; Zhang, L.; Na Xu, Y.L.; Wang, X.; Liu, J.; Qu, W. Momordica charantia (bitter melon) reduces obesity-associated macrophage and mast cell infiltration as well as inflammatory cytokine expression in adipose tissues. PLoS ONE 2013, 8, e84075. [Google Scholar] [CrossRef] [PubMed]
- Kenison, D.C.; Elsasser, T.H.; Fayer, R. Radioimmunoassay for bovine tumor necrosis factor: Concentrations and circulating molecular forms in bovine plasma. J. Immunoass. 1990, 11, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Leister, K.P.; Huang, R.; Goodwin, B.L.; Chen, A.; Austin, C.P.; Xia, M. Two high throughput screen assays for measurement of TNF-α in THP-1 cells. Curr. Chem. Genom. Transl. Med. 2011, 5, 21–29. [Google Scholar] [CrossRef]
- Zhurauski, P.; Arya, S.K.; Jolly, P.; Tiede, C.; Tomlinson, D.C.; Ko Ferrigno, P.; Estrela, P. Sensitive and selective Affimer-functionalised interdigitated electrode-based capacitive biosensor for Her4 protein tumour biomarker detection. Biosens. Bioelectron. 2018, 108, 1–8. [Google Scholar] [CrossRef]
- Pachauri, N.; Dave, K.; Dinda, A.; Solanki, P.R. Cubic CeO2 implanted reduced graphene oxide-based highly sensitive biosensor for non-invasive oral cancer biomarker detection. J. Mater. Chem. B 2018, 6, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Matharu, Z.; Rahimian, A.; Revzin, A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosens. Bioelectron. 2015, 64, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bahk, Y.K.; Kim, H.H.; Park, D.S.; Chang, S.C.; Go, J.S. A new concept for efficient sensitivity amplification of a QCM based immunosensor for TNF-α by using modified magnetic particles under applied magnetic field. Bull. Korean Chem. Soc. 2011, 32, 4215–4220. [Google Scholar] [CrossRef]
- Pei, H.; Wan, Y.; Li, J.; Hu, H.; Su, Y.; Huang, Q.; Fan, C. Regenerable electrochemical immunological sensing at DNA nanostructure-decorated gold surfaces. Chem. Comm. 2011, 47, 6254–6256. [Google Scholar] [CrossRef] [PubMed]


| Sensor | Sample Medium | Detection Method | Linearity (ng/mL) | Detection Limit (ng/mL) | Ref. |
|---|---|---|---|---|---|
| SB/EA/anti-TNF-α/DTSP SAM/Au | Undiluted serum | DPV | 0.5–100 | 0.06 | [4] |
| MB/TNF-α aptamer SAM/ Au | Cell culture medium | SWV | 9–88 | 5.46 | [39] |
| BSA/anti-TNF-α/MUA SAM/Au | Buffer | QCM | 40–2000 | 25 | [40] |
| BSA/anti-TNF-α/DNA nanostructure/Au | 1% BSA in PBS | Amperometry | 0.1−2.5 | 0.1 | [41] |
| SB/anti-TNF-α/PPy-COOH/Au | Undiluted serum | DPV | 0.1–100 | 0.078 | present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arya, S.K.; Estrela, P. Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform. Sensors 2020, 20, 2857. https://doi.org/10.3390/s20102857
Arya SK, Estrela P. Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform. Sensors. 2020; 20(10):2857. https://doi.org/10.3390/s20102857
Chicago/Turabian StyleArya, Sunil K., and Pedro Estrela. 2020. "Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform" Sensors 20, no. 10: 2857. https://doi.org/10.3390/s20102857
APA StyleArya, S. K., & Estrela, P. (2020). Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform. Sensors, 20(10), 2857. https://doi.org/10.3390/s20102857






