Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling †
Abstract
1. Introduction
- Interpretation of fNIRS results is not direct given the multiple physiological origins of the hemodynamic modulations [15].
- The presence of a large intersubject variability in the healthy population impairs the detection of a modified hemodynamic response in pathological conditions.
- fNIRS measures are sensitive to optical phenomena occurring within small volumes (of lateral dimension analogous to the source-detector distance) that have the shape of curved spindles (“bananas”); by changing the distance between the source and detector, different depth sensitivities can be obtained. These characteristics, potentially providing a better spatial and depth resolution than EEG, require many overlapping channels with high optode density to obtain a large field of view and spatially resolved brain monitoring, making standard sparse fNIRS systems not appropriate.
- The sensitive fiber-based technology is mechanically bulky whereas fiberless technology does not utilize sensitive detectors, often restricting fiberless fNIRS measurements to hair-free regions, such as the forehead, using a fixed and small interoptode (source-detector) distance [16,17,18,19], severely limiting field of view and depth investigation capabilities of the recordings.
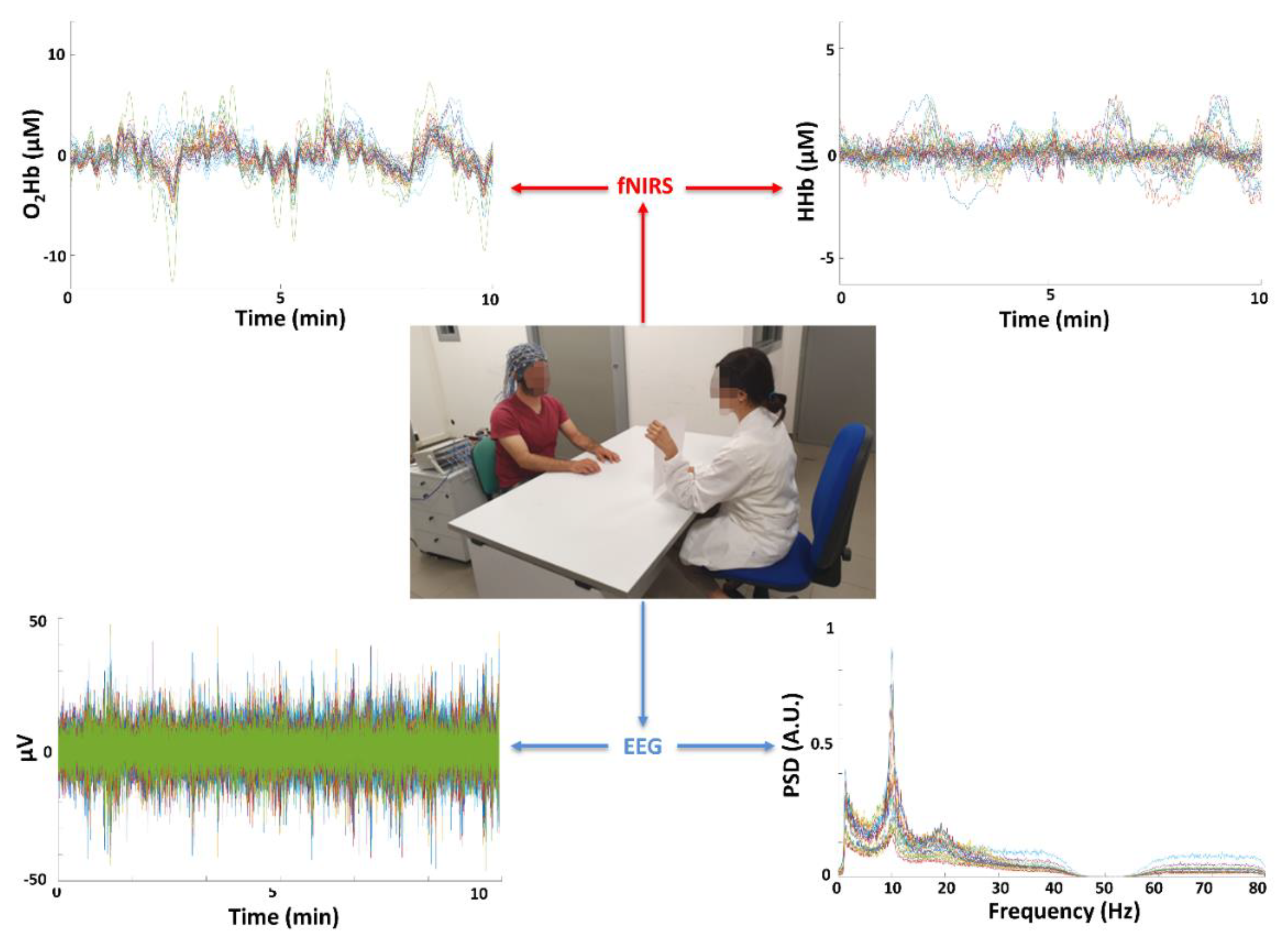
- Integration of fNIRS with EEG is not common in clinical settings.
2. Materials and Methods
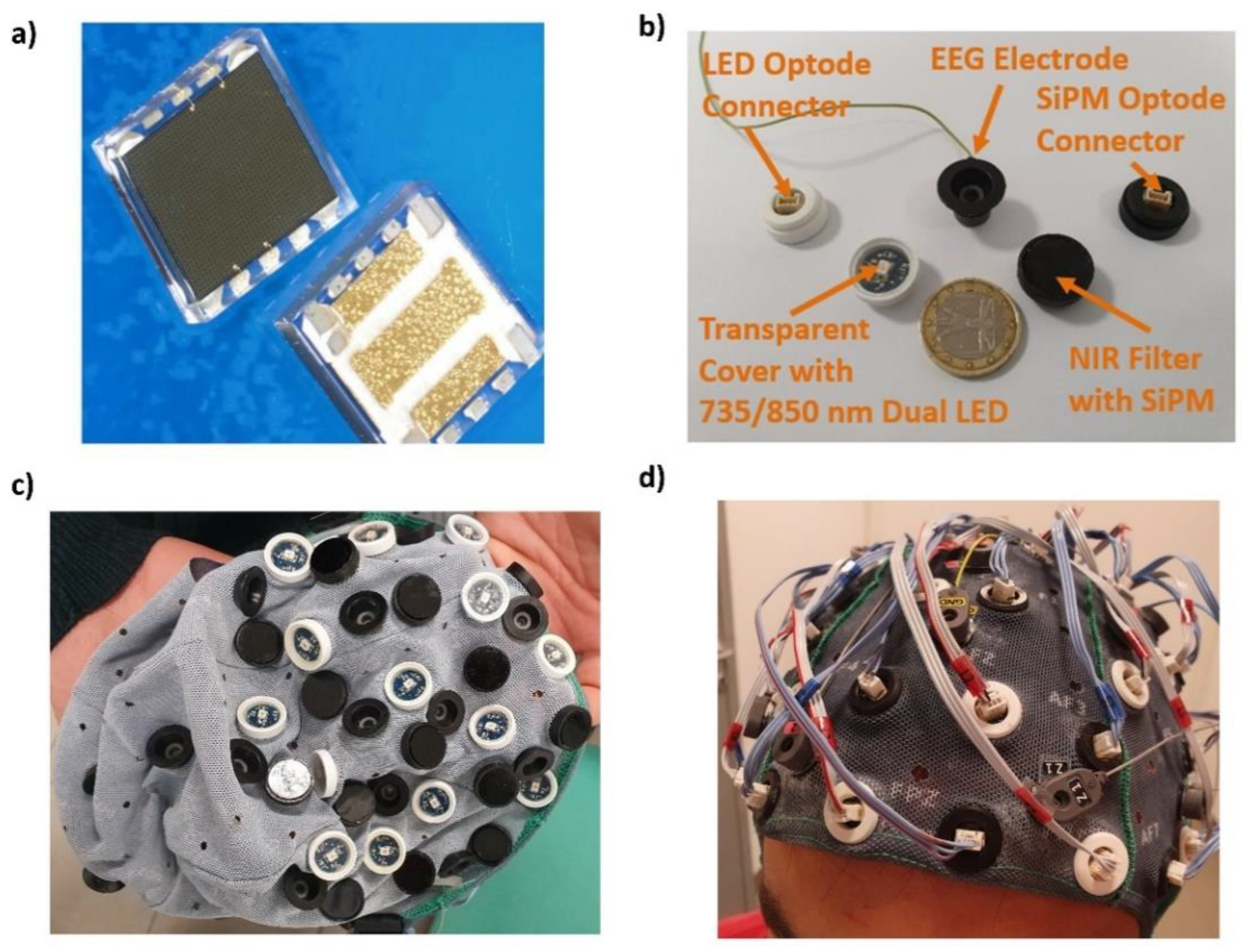
2.1. fNIRS Instrumentation: Silicon Photomultipliers and Light Emitting Diodes
2.2. fNIRS Instrumentation: Optical Probes
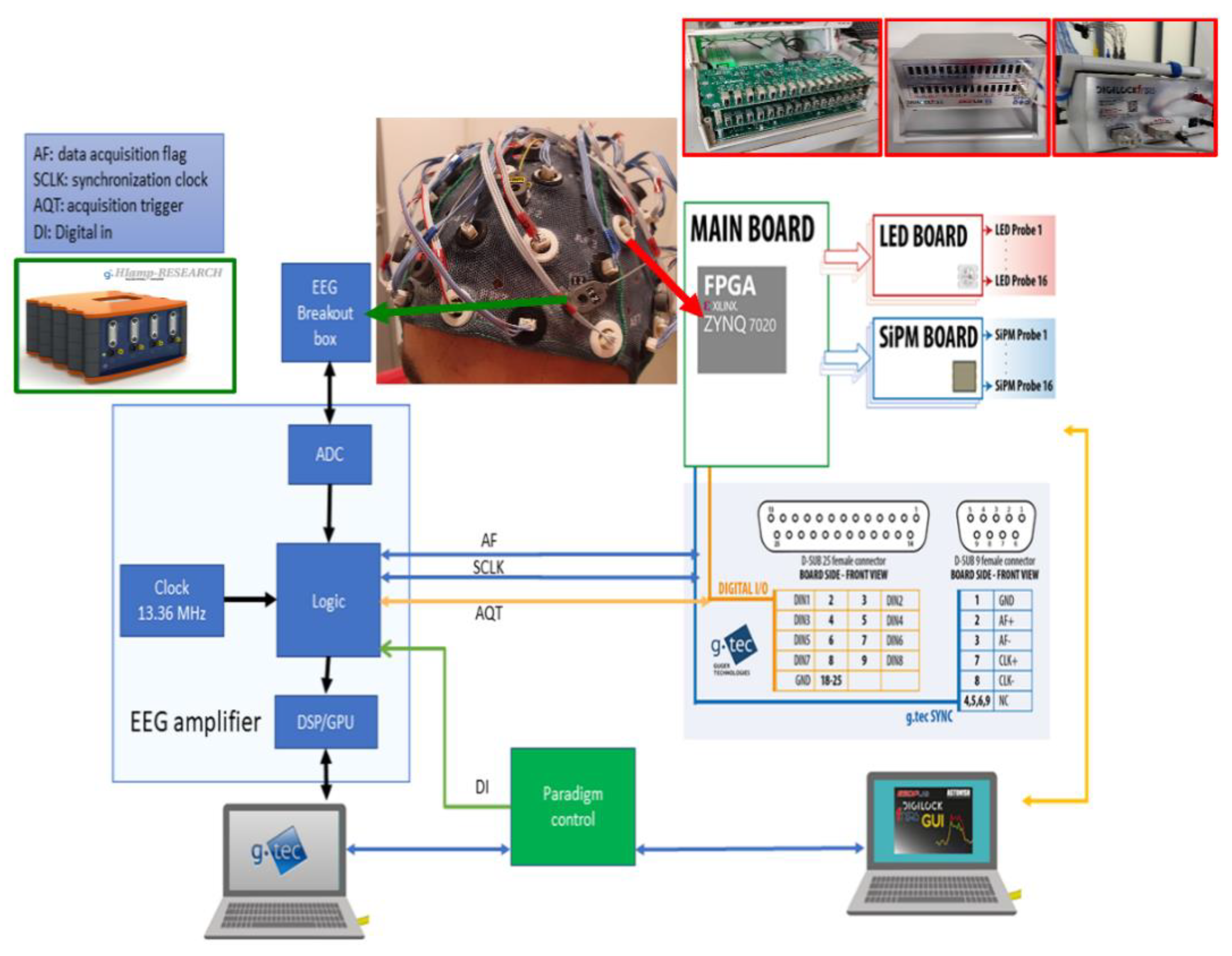
2.3. fNIRS Instrumentation: Architecture
2.4. EEG Instrumentation
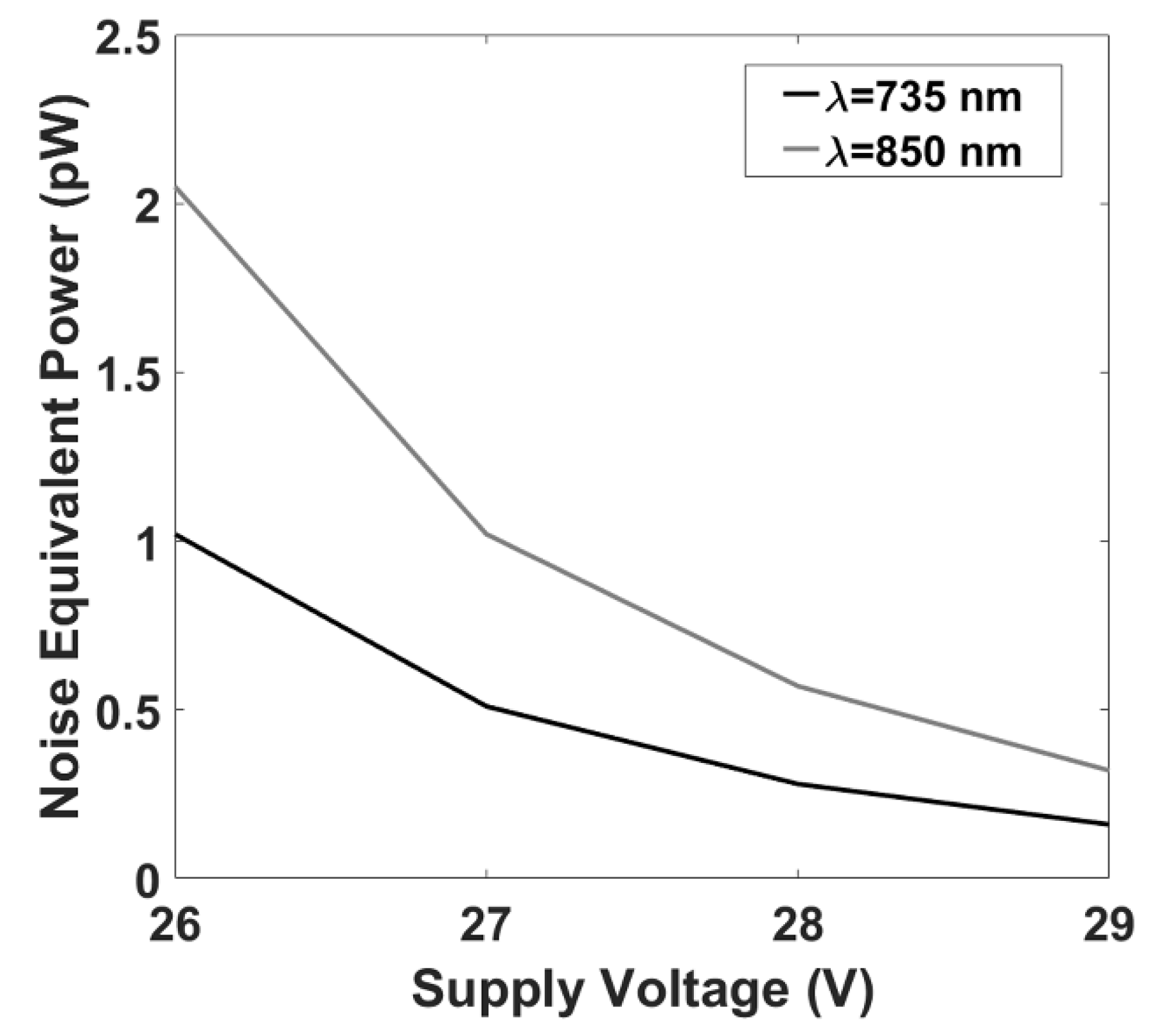
2.5. fNIRS Instrumentation: Noise Equivalent Power Evaluation
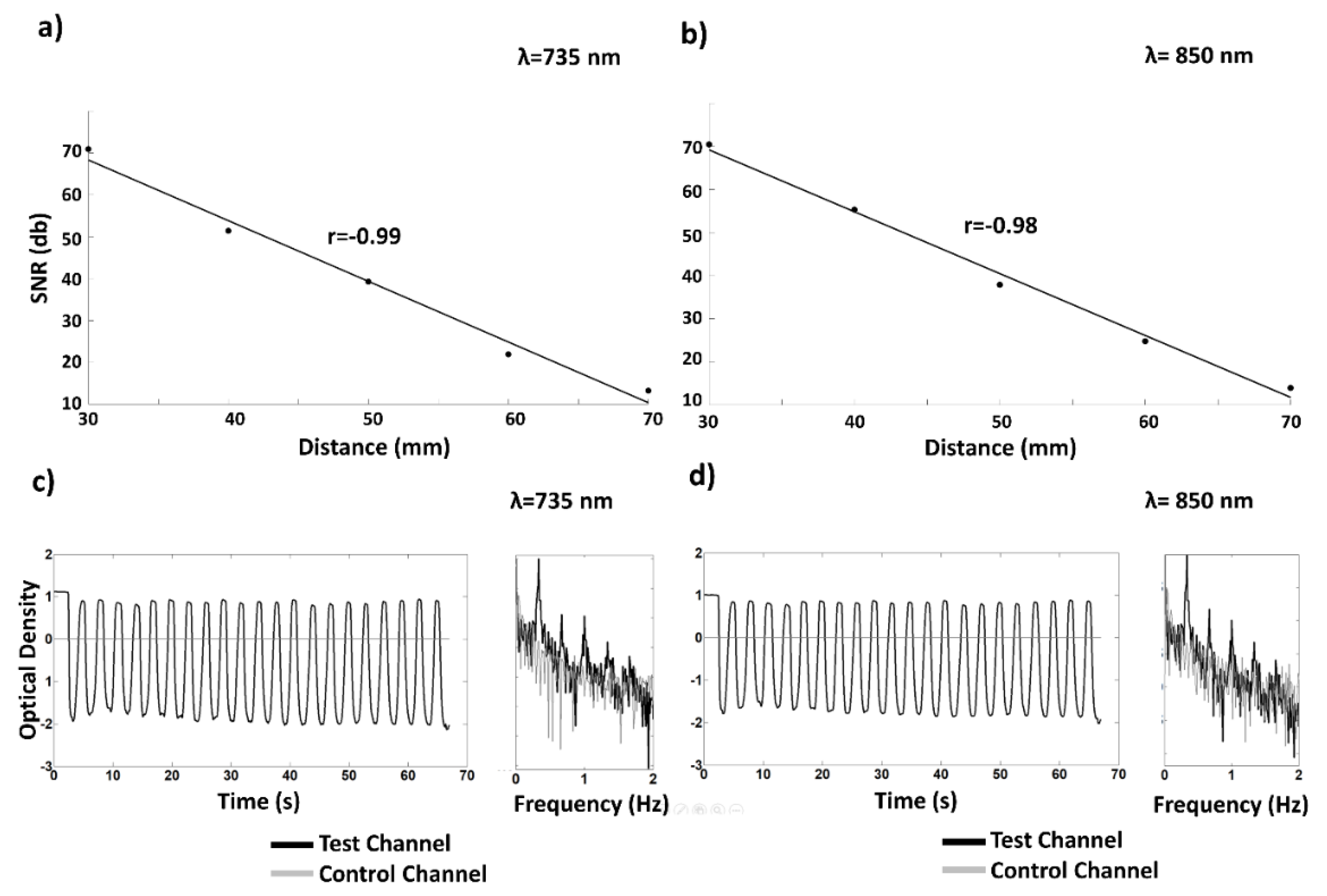
2.6. fNIRS Instrumentation: Phantom Validation
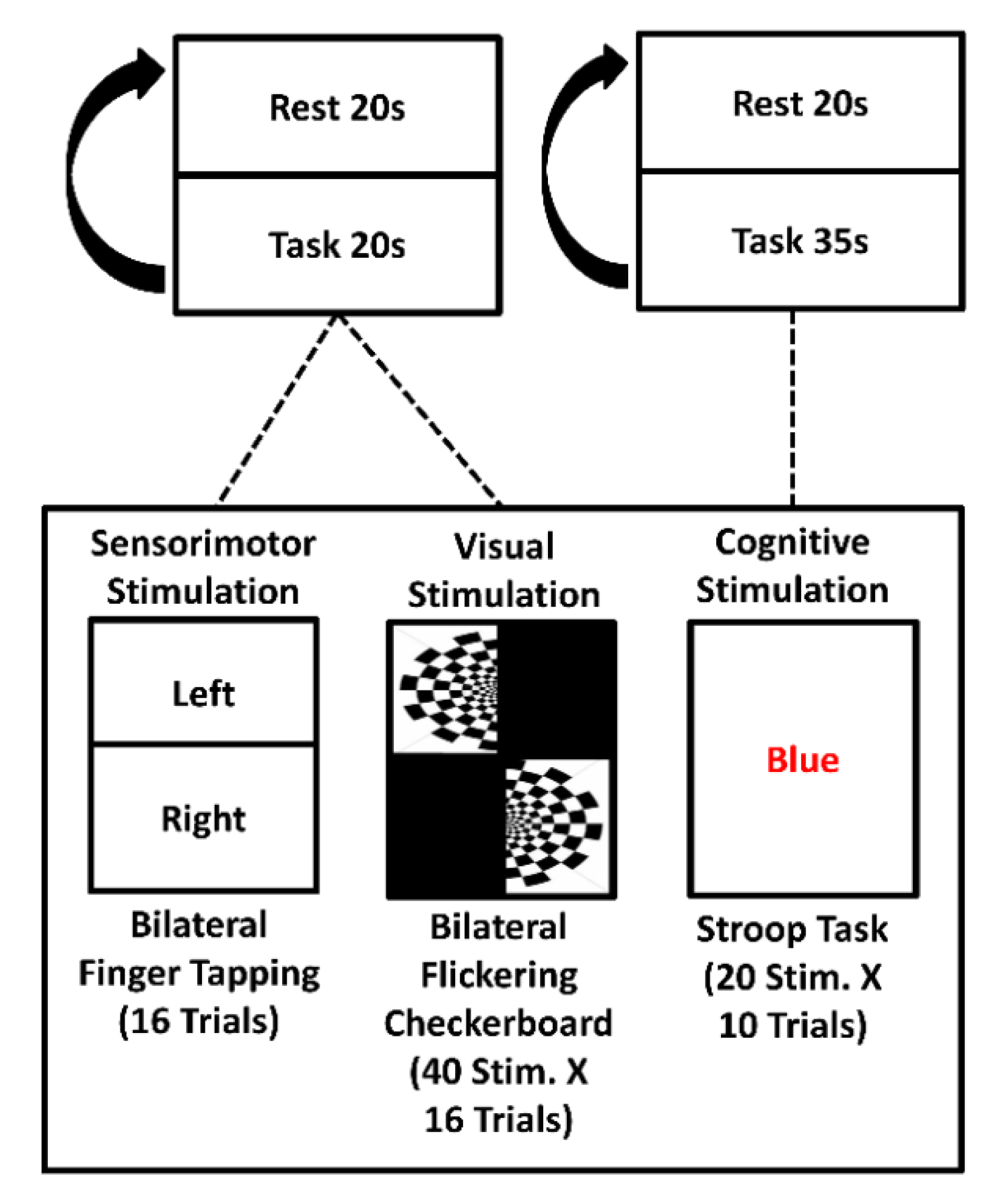
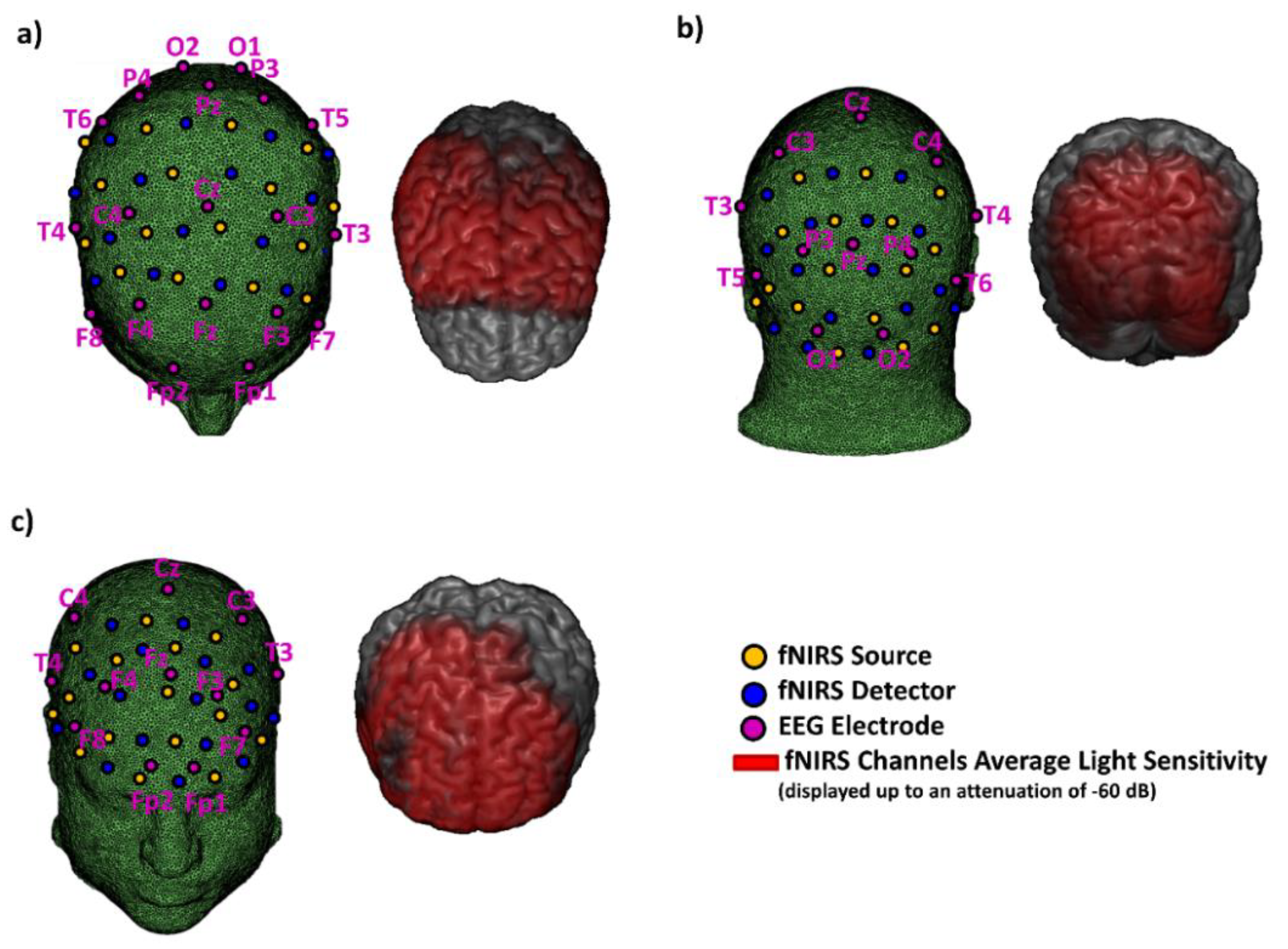
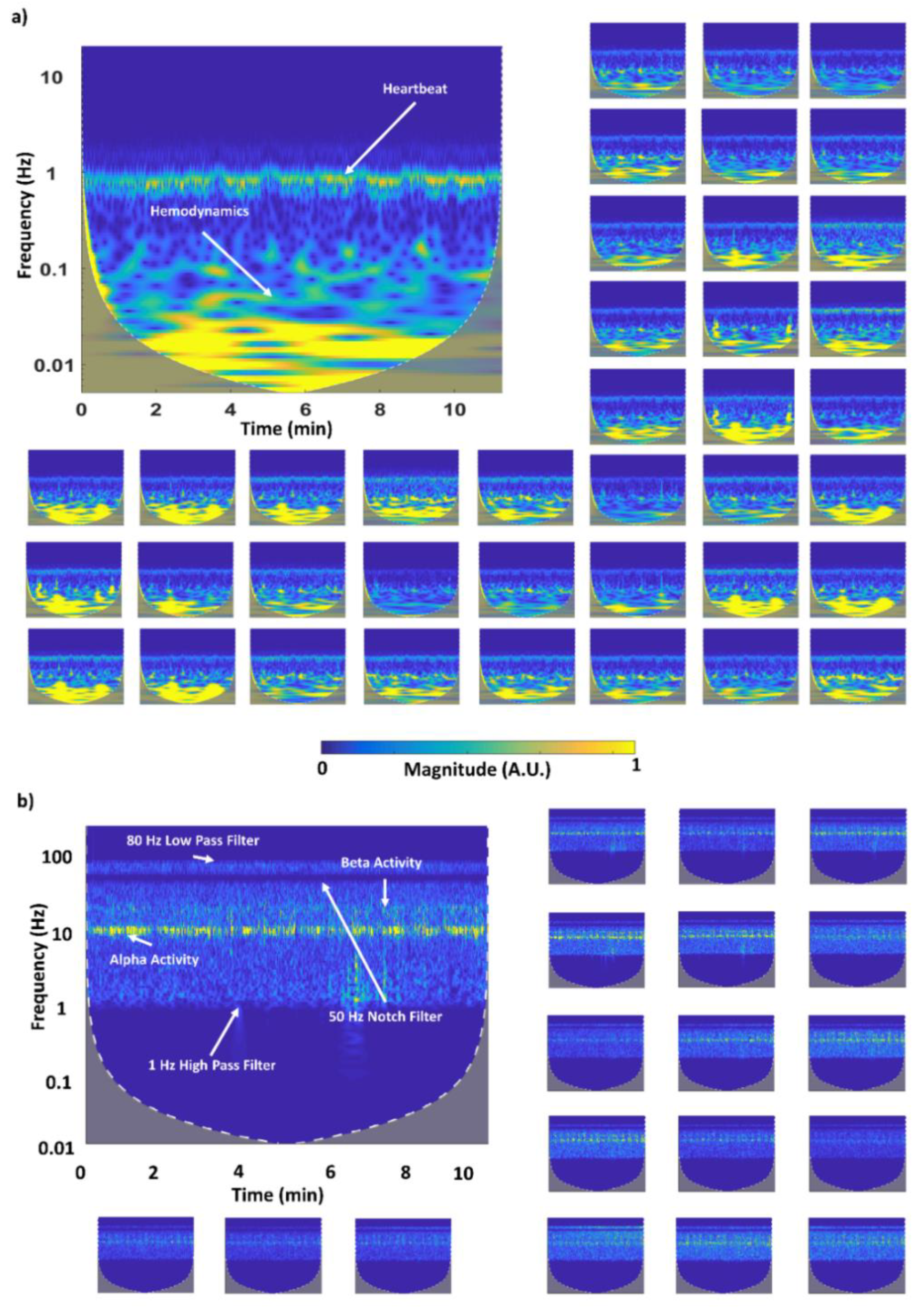
2.7. fNIRS-EEG System: In Vivo Validation
2.8. fNIRS-EEG Data Analysis
3. Results
3.1. fNIRS System: Noise Equivalent Power Evaluation
3.2. fNIRS System: Phantom Validation
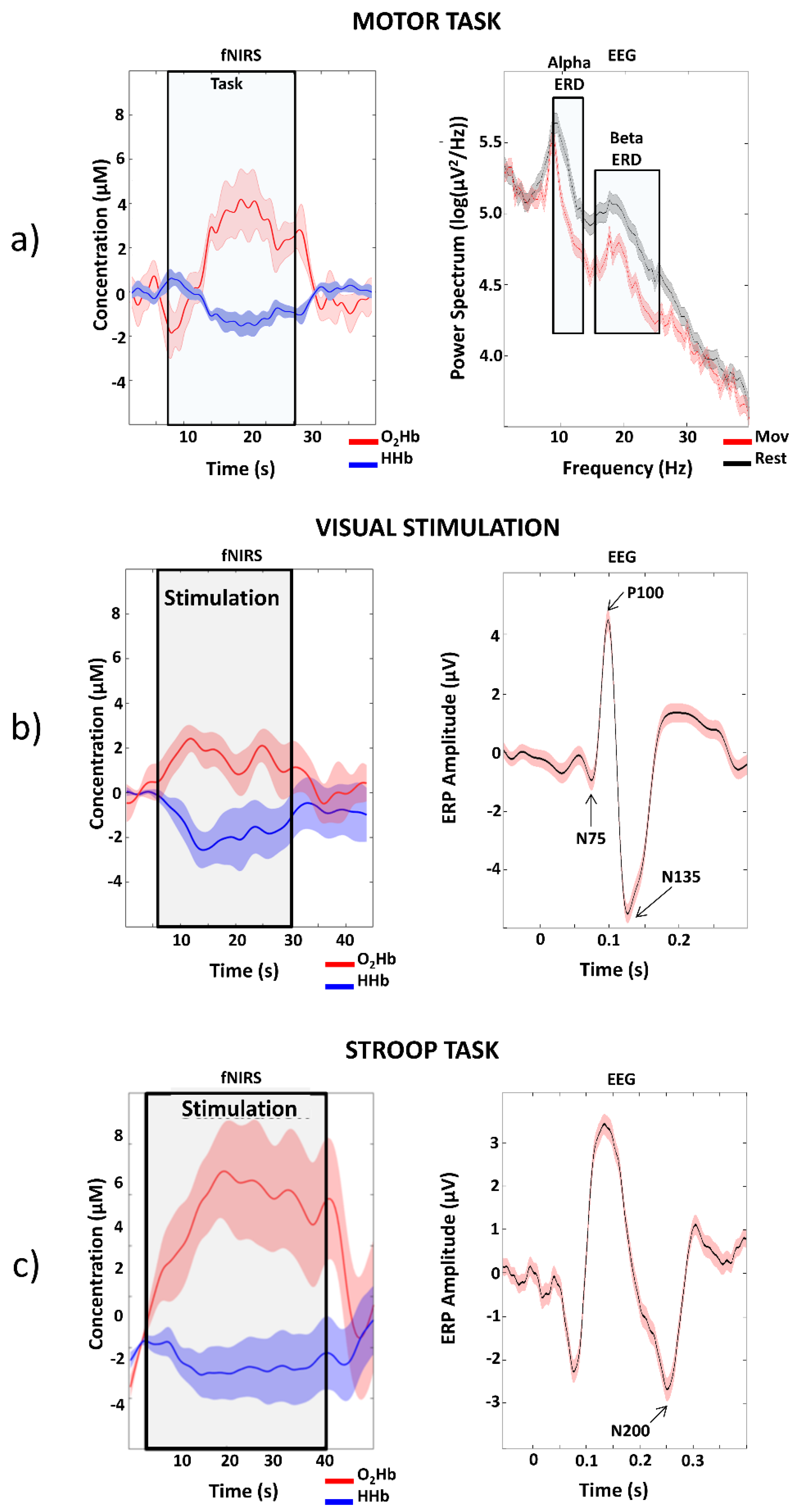
3.3. EEG-fNIRS System: In Vivo Validation
3.4. Proof of Concept: Ecological EEG-fNIRS in One Alzheimer Disease Patient during Cognitive Clinical Tests in an Ambulatory Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statement
References
- Chiarelli, A.M.; Zappasodi, F.; Pompeo, F.D.; Merla, A. Simultaneous functional near-infrared spectroscopy and electroencephalography for monitoring of human brain activity and oxygenation: A review. Neurophotonics 2017, 4, 041411. [Google Scholar] [CrossRef]
- Davidson, R.J.; Jackson, D.C.; Larson, C.L. Human electroencephalography. In Handbook of Psychophysiology, 2nd ed.; Cambridge University Press: New York, NY, USA, 2000; pp. 27–52. ISBN 978-0-521-62634-7. [Google Scholar]
- Lin, C.-T.; Ko, L.-W.; Chang, M.-H.; Duann, J.-R.; Chen, J.-Y.; Su, T.-P.; Jung, T.-P. Review of wireless and wearable electroencephalogram systems and brain-computer interfaces—A mini-review. Gerontology 2010, 56, 112–119. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Croce, P.; Zappasodi, F.; Merla, A.; Chiarelli, A.M. Exploiting neurovascular coupling: A Bayesian sequential Monte Carlo approach applied to simulated EEG fNIRS data. J. Neural Eng. 2017, 14, 046029. [Google Scholar] [CrossRef]
- Stephan, K.E.; Harrison, L.M.; Penny, W.D.; Friston, K.J. Biophysical models of fMRI responses. Curr. Opin. Neurobiol. 2004, 14, 629–635. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Perpetuini, D.; Chiarelli, A.M.; Cardone, D.; Filippini, C.; Bucco, R.; Zito, M.; Merla, A. Complexity of Frontal Cortex fNIRS Can Support Alzheimer Disease Diagnosis in Memory and Visuo-Spatial Tests. Entropy 2019, 21, 26. [Google Scholar] [CrossRef]
- Watanabe, E.; Nagahori, Y.; Mayanagi, Y. Focus Diagnosis of Epilepsy Using Near-Infrared Spectroscopy. Epilepsia 2002, 43, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Vacri, A.D.; Chiarelli, A.M.; Ferri, F.; Romani, G.L.; Merla, A. Studying social cognition using near-infrared spectroscopy: The case of social Simon effect. J. Biomed. Opt. 2013, 18, 025005. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Contini, D.; Pifferi, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional NIRS imaging for human brain mapping. NeuroImage 2014, 85, 28–50. [Google Scholar] [CrossRef]
- Chance, B.; Anday, E.; Nioka, S.; Zhou, S.; Hong, L.; Worden, K.; Li, C.; Murray, T.; Ovetsky, Y.; Pidikiti, D.; et al. A novel method for fast imaging of brain function, non-invasively, with light. Opt. Express 1998, 2, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Obrig, H. NIRS in clinical neurology—A “promising” tool? NeuroImage 2014, 85 Pt 1, 535–546. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Aichelburg, C.; Lind, F.; Power, S.; Swingler, E.; Merla, A.; Hamilton, A.; Gilbert, S.; Burgess, P.; Tachtsidis, I. Using Fiberless, Wearable fNIRS to Monitor Brain Activity in Real-world Cognitive Tasks. J. Vis. Exp. JoVE 2015. [Google Scholar] [CrossRef]
- Piper, S.K.; Krueger, A.; Koch, S.P.; Mehnert, J.; Habermehl, C.; Steinbrink, J.; Obrig, H.; Schmitz, C.H. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. NeuroImage 2014, 85 Pt 1, 64–71. [Google Scholar] [CrossRef]
- Muehlemann, T.; Haensse, D.; Wolf, M. Wireless miniaturized in-vivo near infrared imaging. Opt. Express 2008, 16, 10323–10330. [Google Scholar] [CrossRef]
- Von Lühmann, A.; Wabnitz, H.; Sander, T.; Müller, K.-R. M3BA: A Mobile, Modular, Multimodal Biosignal Acquisition Architecture for Miniaturized EEG-NIRS-Based Hybrid BCI and Monitoring. IEEE Trans. Biomed. Eng. 2017, 64, 1199–1210. [Google Scholar] [CrossRef]
- Sawan, M.; Salam, M.T.; Le Lan, J.; Kassab, A.; Gélinas, S.; Vannasing, P.; Lesage, F.; Lassonde, M.; Nguyen, D.K. Wireless Recording Systems: From Noninvasive EEG-NIRS to Invasive EEG Devices. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 186–195. [Google Scholar] [CrossRef]
- Safaie, J.; Grebe, R.; Moghaddam, H.A.; Wallois, F. Toward a fully integrated wireless wearable EEG-NIRS bimodal acquisition system. J. Neural Eng. 2013, 10, 056001. [Google Scholar] [CrossRef]
- Buzhan, P.; Dolgoshein, B.; Filatov, L.; Ilyin, A.; Kantzerov, V.; Kaplin, V.; Karakash, A.; Kayumov, F.; Klemin, S.; Popova, E.; et al. Silicon photomultiplier and its possible applications. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2003, 504, 48–52. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Sciuto, E.L.; Lombardo, S.A.; Busacca, A.C.; Petralia, S.; Conoci, S.; Libertino, S. Si Photomultipliers for Bio-Sensing Applications. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 335–341. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Ambra, E.; Maddiona, L.; Romeo, M.; Mazzillo, M.; Rundo, F.; Fallica, G.; di Pompeo, F.; Chiarelli, A.M.; Zappasodi, F.; et al. PPG/ECG multisite combo system based on SiPM technology. Lect. Notes Electr. Eng. 2019, 539, 353–360. [Google Scholar] [CrossRef]
- Pagano, R.; Libertino, S.; Sanfilippo, D.; Fallica, G.; Lombardo, S. Improvement of sensitivity in continuous wave near infra-red spectroscopy systems by using silicon photomultipliers. Biomed. Opt. Express 2016, 7, 1183–1192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamo, G.; Parisi, A.; Stivala, S.; Tomasino, A.; Agrò, D.; Curcio, L.; Giaconia, G.C.; Busacca, A.; Fallica, G. Silicon Photomultipliers Signal-to-Noise Ratio in the Continuous Wave Regime. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 284–290. [Google Scholar] [CrossRef]
- Maira, G.; Chiarelli, A.M.; Brafa, S.; Libertino, S.; Fallica, G.; Merla, A.; Lombardo, S. Imaging System Based on Silicon Photomultipliers and Light Emitting Diodes for Functional Near-Infrared Spectroscopy. Appl. Sci. 2020, 1068. [Google Scholar] [CrossRef]
- Zimmermann, R.; Braun, F.; Achtnich, T.; Lambercy, O.; Gassert, R.; Wolf, M. Silicon photomultipliers for improved detection of low light levels in miniature near-infrared spectroscopy instruments. Biomed. Opt. Express 2013, 4, 659–666. [Google Scholar] [CrossRef]
- Adamo, G.; Agró, D.; Stivala, S.; Parisi, A.; Tomasino, A.; Curcio, L.; Pernice, R.; Giaconia, C.; Busacca, A.C.; Fallica, G. Signal to Noise Ratio of silicon photomultipliers measured in the continuous wave regime. In Proceedings of the 2014 Third Mediterranean Photonics Conference, Trani, Italy, 7–9 May 2014; pp. 1–3. [Google Scholar]
- Sanfilippo, D.; Valvo, G.; Mazzillo, M.; Piana, A.; Carbone, B.; Renna, L.; Fallica, P.G.; Agrò, D.; Morsellino, G.; Pinto, M.; et al. Design and development of a fNIRS system prototype based on SiPM detectors. In Proceedings of the Silicon Photonics IX.; International Society for Optics and Photonics: San Francisco, CA, USA, 2014; Volume 8990, p. 899016. [Google Scholar]
- Sciacca, E.; Lombardo, S.; Mazzillo, M.; Condorelli, G.; Sanfilippo, D.; Contissa, A.; Belluso, M.; Torrisi, F.; Billotta, S.; Campisi, A.; et al. Arrays of Geiger mode avalanche photodiodes. IEEE Photonics Technol. Lett. 2006, 18, 1633–1635. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Libertino, S.; Zappasodi, F.; Mazzillo, M.C.; Pompeo, F.D.; Merla, A.; Lombardo, S.A.; Fallica, G.P. Characterization of a fiber-less, multichannel optical probe for continuous wave functional near-infrared spectroscopy based on silicon photomultipliers detectors: In-vivo assessment of primary sensorimotor response. Neurophotonics 2017, 4, 035002. [Google Scholar] [CrossRef]
- Adamo, G.; Agrò, D.; Stivala, S.; Parisi, A.; Giaconia, C.; Busacca, A.C.; Fallica, G. SNR measurements of silicon photomultipliers in the continuous wave regime. In Proceedings of the Silicon Photonics IX.; International Society for Optics and Photonics: San Francisco, CA, USA, 2014; Volume 8990, p. 899019. [Google Scholar]
- Zhao, H.; Cooper, R.J. Review of recent progress toward a fiberless, whole-scalp diffuse optical tomography system. Neurophotonics 2017, 5, 011012. [Google Scholar] [CrossRef]
- Chitnis, D.; Cooper, R.J.; Dempsey, L.; Powell, S.; Quaggia, S.; Highton, D.; Elwell, C.; Hebden, J.C.; Everdell, N.L. Functional imaging of the human brain using a modular, fibre-less, high-density diffuse optical tomography system. Biomed. Opt. Express 2016, 7, 4275–4288. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Perpetuini, D.; Greco, G.; Mistretta, L.; Rizzo, R.; Vinciguerra, V.; Romeo, M.F.; Merla, A.; Fallica, P.G.; Giaconia, G.C. Wearable, Fiber-less, Multi-Channel System for Continuous Wave Functional Near Infrared Spectroscopy Based on Silicon Photomultipliers Detectors and Lock-In Amplification. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 60–66. [Google Scholar]
- Mazzillo, M.; Ronzhin, A.; Los, S.; Abbisso, S.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Piana, A.; Fallica, G.; Albrow, M.; et al. Electro-Optical Performances of p-on-n and n-on-p Silicon Photomultipliers. IEEE Trans. Electron Devices 2012, 59, 3419–3425. [Google Scholar] [CrossRef]
- Giaconia, G.C.; Greco, G.; Mistretta, L.; Rizzo, R. FPGA Based Digital Lock-in Amplifier for fNIRS Systems. In Proceedings of the Applications in Electronics Pervading Industry, Environment and Society; De Gloria, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 33–39. [Google Scholar]
- Pollonini, L.; Bortfeld, H.; Oghalai, J.S. PHOEBE: A method for real time mapping of optodes-scalp coupling in functional near-infrared spectroscopy. Biomed. Opt. Express 2016, 7, 5104–5119. [Google Scholar] [CrossRef] [PubMed]
- Wyser, D.G.; Lambercy, O.; Scholkmann, F.; Wolf, M.; Gassert, R. Wearable and modular functional near-infrared spectroscopy instrument with multidistance measurements at four wavelengths. Neurophotonics 2017, 4, 041413. [Google Scholar] [CrossRef]
- Adamo, G.; Agrò, D.; Stivala, S.; Parisi, A.; Giaconia, C.; Busacca, A.; Mazzillo, M.C.; Sanfilippo, D.; Fallica, P.G. Responsivity measurements of N-on-P and P-on-N silicon photomultipliers in the continuous wave regime. In Proceedings of the Silicon Photonics VIII.; International Society for Optics and Photonics: San Francisco, CA, USA, 2013; Volume 8629, p. 86291A. [Google Scholar]
- Eggebrecht, A.T.; White, B.R.; Ferradal, S.L.; Chen, C.; Zhan, Y.; Snyder, A.Z.; Dehghani, H.; Culver, J.P. A quantitative spatial comparison of high-density diffuse optical tomography and fMRI cortical mapping. NeuroImage 2012, 61, 1120–1128. [Google Scholar] [CrossRef]
- Vendrell, P.; Junqué, C.; Pujol, J.; Jurado, M.A.; Molet, J.; Grafman, J. The role of prefrontal regions in the Stroop task. Neuropsychologia 1995, 33, 341–352. [Google Scholar] [CrossRef]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C. What’s new in psychtoolbox-3. Perception 2007, 36, 1–16. [Google Scholar]
- Brigadoi, S.; Cooper, R.J. How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2015, 2, 025005. [Google Scholar] [CrossRef]
- Dehghani, H.; Eames, M.E.; Yalavarthy, P.K.; Davis, S.C.; Srinivasan, S.; Carpenter, C.M.; Pogue, B.W.; Paulsen, K.D. Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction. Commun. Numer. Methods Eng. 2008, 25, 711–732. [Google Scholar] [CrossRef]
- Perpetuini, D.; Bucco, R.; Zito, M.; Merla, A. Study of memory deficit in Alzheimer’s disease by means of complexity analysis of fNIRS signal. Neurophotonics 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Maclin, E.L.; Fabiani, M.; Gratton, G. A kurtosis-based wavelet algorithm for motion artifact correction of fNIRS data. NeuroImage 2015, 112, 128–137. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. Differential pathlength factor in continuous wave functional near-infrared spectroscopy: Reducing hemoglobin’s cross talk in high-density recordings. Neurophotonics 2019, 6, 035005. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Academic Press: London, UK, 2011; ISBN 978-0-08-046650-7. [Google Scholar]
- Chiarelli, A.M.; Romani, G.L.; Merla, A. Fast optical signals in the sensorimotor cortex: General Linear Convolution Model applied to multiple source–detector distance-based data. NeuroImage 2014, 85, 245–254. [Google Scholar] [CrossRef]
- Barbati, G.; Porcaro, C.; Zappasodi, F.; Rossini, P.M.; Tecchio, F. Optimization of an independent component analysis approach for artifact identification and removal in magnetoencephalographic signals. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 1220–1232. [Google Scholar] [CrossRef]
- Croce, P.; Zappasodi, F.; Marzetti, L.; Merla, A.; Pizzela, V.; Chiarelli, A.M. Deep Convolutional Neural Networks for feature-less automatic classification of Independent Components in multi-channel electrophysiological brain recordings. IEEE Trans. Biomed. Eng. 2018, 1. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Barber, C.; Brigell, M.; Marmor, M.F.; Tormene, A.P.; Holder, G.E. Vaegan Visual evoked potentials standard (2004). Doc. Ophthalmol. 2004, 108, 115–123. [Google Scholar] [CrossRef]
- Badzakova-Trajkov, G.; Barnett, K.J.; Waldie, K.E.; Kirk, I.J. An ERP investigation of the Stroop task: The role of the cingulate in attentional allocation and conflict resolution. Brain Res. 2009, 1253, 139–148. [Google Scholar] [CrossRef]
- Yennu, A.; Tian, F.; Smith-Osborne, A.; Gatchel, R.J.; Woon, F.L.; Liu, H. Prefrontal responses to Stroop tasks in subjects with post-traumatic stress disorder assessed by functional near infrared spectroscopy. Sci. Rep. 2016, 6, 30157. [Google Scholar] [CrossRef] [PubMed]
- Chuderski, A.; Smolen, T. An integrated utility-based model of conflict evaluation and resolution in the Stroop task. Psychol. Rev. 2016, 123, 255–290. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Wilcox, R.R.; Rousselet, G.A. Robust Correlation Analyses: False Positive and Power Validation Using a New Open Source Matlab Toolbox. Front. Psychol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Fantini, S.; Fabiani, M.; Gratton, G. Low-resolution mapping of the effective attenuation coefficient of the human head: A multidistance approach applied to high-density optical recordings. Neurophotonics 2017, 4, 021103. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef]
- Wallois, F.; Patil, A.; Héberlé, C.; Grebe, R. EEG-NIRS in epilepsy in children and neonates. Neurophysiol. Clin. Neurophysiol. 2010, 40, 281–292. [Google Scholar] [CrossRef]
- Li, R.; Huang, W.; Lou, D.; Zhu, G.; Zhang, T.; Zhang, Y. The feasibility of utilizing EEG-fNIRS to characterize the cortical activation difference between healthy subjects and post-stroke patients. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 1–4. [Google Scholar]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS Based Assessment of Neurovascular Coupling During Anodal Transcranial Direct Current Stimulation—A Stroke Case Series. J. Med. Syst. 2015, 39, 36. [Google Scholar] [CrossRef]
- Maldonado, Y.; Singh, S.; Taylor, M.A. Cerebral near-infrared spectroscopy in perioperative management of left ventricular assist device and extracorporeal membrane oxygenation patients. Curr. Opin. Anaesthesiol. 2014, 27, 81–88. [Google Scholar] [CrossRef]
- Kassab, A.; Lan, J.L.; Tremblay, J.; Vannasing, P.; Dehbozorgi, M.; Pouliot, P.; Gallagher, A.; Lesage, F.; Sawan, M.; Nguyen, D.K. Multichannel wearable fNIRS-EEG system for long-term clinical monitoring. Hum. Brain Mapp. 2018, 39, 7–23. [Google Scholar] [CrossRef]
- Green, M.S.; Sehgal, S.; Tariq, R. Near-Infrared Spectroscopy: The New Must Have Tool in the Intensive Care Unit? Semin. Cardiothorac. Vasc. Anesth. 2016, 20, 213–224. [Google Scholar] [CrossRef]
- Keller, E.; Froehlich, J.; Baumann, D.; Böcklin, C.; Sikorski, C.; Oberle, M.; Muser, M. Detection of Delayed Cerebral Ischemia (DCI) in Subarachnoid Haemorrhage Applying Near-Infrared Spectroscopy: Elimination of the Extracerebral Signal by Transcutaneous and Intraparenchymatous Measurements in Parallel. In Neurovascular Events after Subarachnoid Hemorrhage: Towards Experimental and Clinical Standardisation; Acta Neurochirurgica Supplement; Fandino, J., Marbacher, S., Fathi, A.-R., Muroi, C., Keller, E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 243–247. ISBN 978-3-319-04981-6. [Google Scholar]
- Seule, M.; Keller, E.; Unterberg, A.; Sakowitz, O. The Hemodynamic Response of Spreading Depolarization Observed by Near Infrared Spectroscopy after Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2015, 23, 108–112. [Google Scholar] [CrossRef][Green Version]
- Forcione, M.; Chiarelli, A.M.; Davies, D.J.; Perpetuini, D.; Sawosz, P.; Merla, A.; Belli, A. Cerebral perfusion and blood–brain barrier assessment in brain trauma using contrast-enhanced near-infrared spectroscopy with indocyanine green: A review. J. Cerebral Blood Flow Metab. 2020, 0271678X20921973. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, P. Hybrid EEG-fNIRS Based BCI for Rehabilitation. In Proceedings of the International Conference on Photonics and Imaging in Biology and Medicine; Optical Society of America: Suzhou, China, 2017; p. W3A.134. [Google Scholar]
- Brain–Computer Interfaces for Communication and Rehabilitation, Nature Reviews Neurology. Available online: https://www.nature.com/articles/nrneurol.2016.113 (accessed on 28 October 2019).


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarelli, A.M.; Perpetuini, D.; Croce, P.; Greco, G.; Mistretta, L.; Rizzo, R.; Vinciguerra, V.; Romeo, M.F.; Zappasodi, F.; Merla, A.; et al. Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling. Sensors 2020, 20, 2831. https://doi.org/10.3390/s20102831
Chiarelli AM, Perpetuini D, Croce P, Greco G, Mistretta L, Rizzo R, Vinciguerra V, Romeo MF, Zappasodi F, Merla A, et al. Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling. Sensors. 2020; 20(10):2831. https://doi.org/10.3390/s20102831
Chicago/Turabian StyleChiarelli, Antonio Maria, David Perpetuini, Pierpaolo Croce, Giuseppe Greco, Leonardo Mistretta, Raimondo Rizzo, Vincenzo Vinciguerra, Mario Francesco Romeo, Filippo Zappasodi, Arcangelo Merla, and et al. 2020. "Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling" Sensors 20, no. 10: 2831. https://doi.org/10.3390/s20102831
APA StyleChiarelli, A. M., Perpetuini, D., Croce, P., Greco, G., Mistretta, L., Rizzo, R., Vinciguerra, V., Romeo, M. F., Zappasodi, F., Merla, A., Fallica, P. G., Edlinger, G., Ortner, R., & Giaconia, G. C. (2020). Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling. Sensors, 20(10), 2831. https://doi.org/10.3390/s20102831











