Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device
Abstract
1. Introduction
2. Experimental
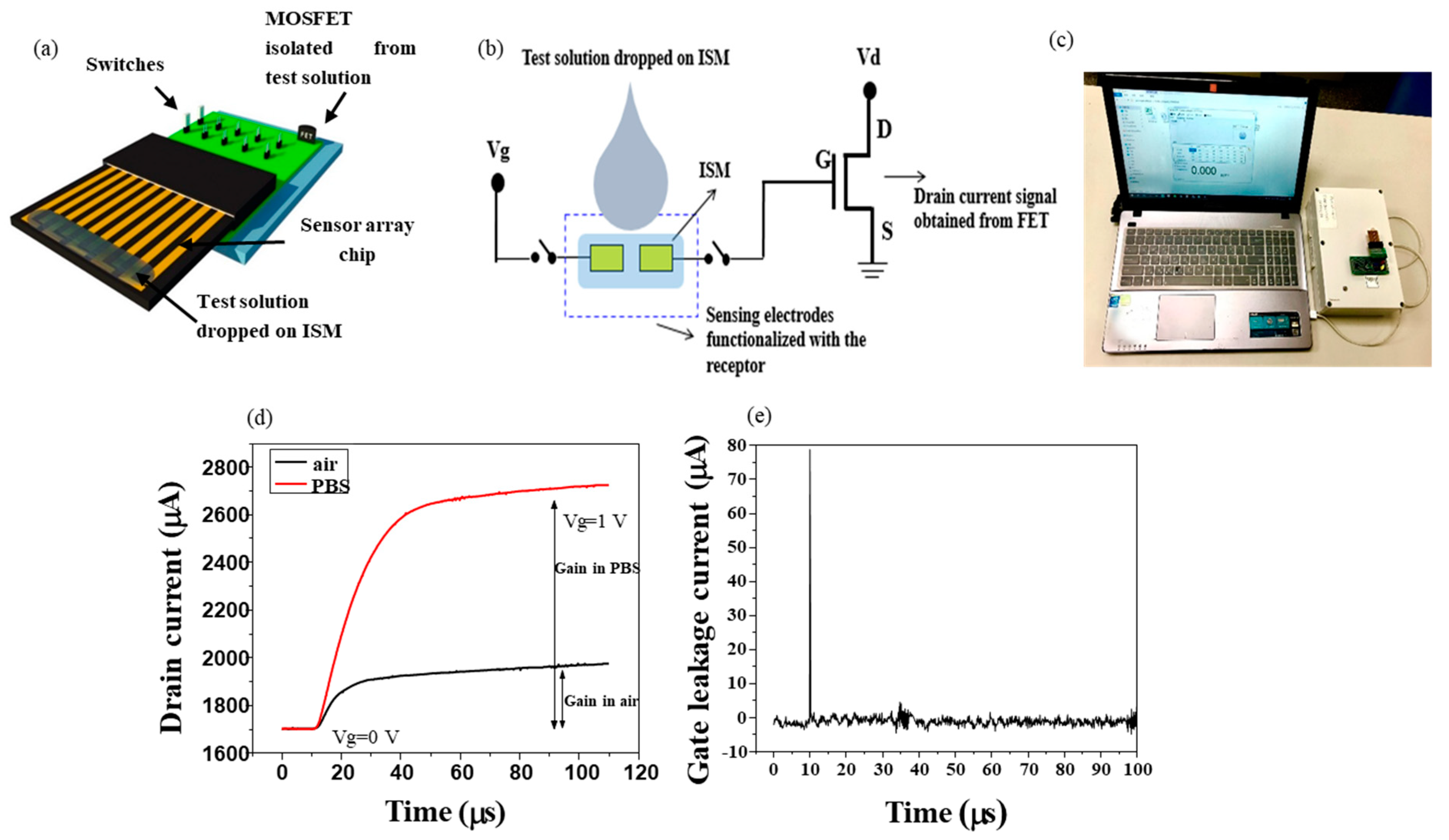
2.1. Structure of Extended Gate Hg-ISMFET
2.2. Sample Preparation and Regeneration
2.3. Sensor Measurement
3. Results and Discussion
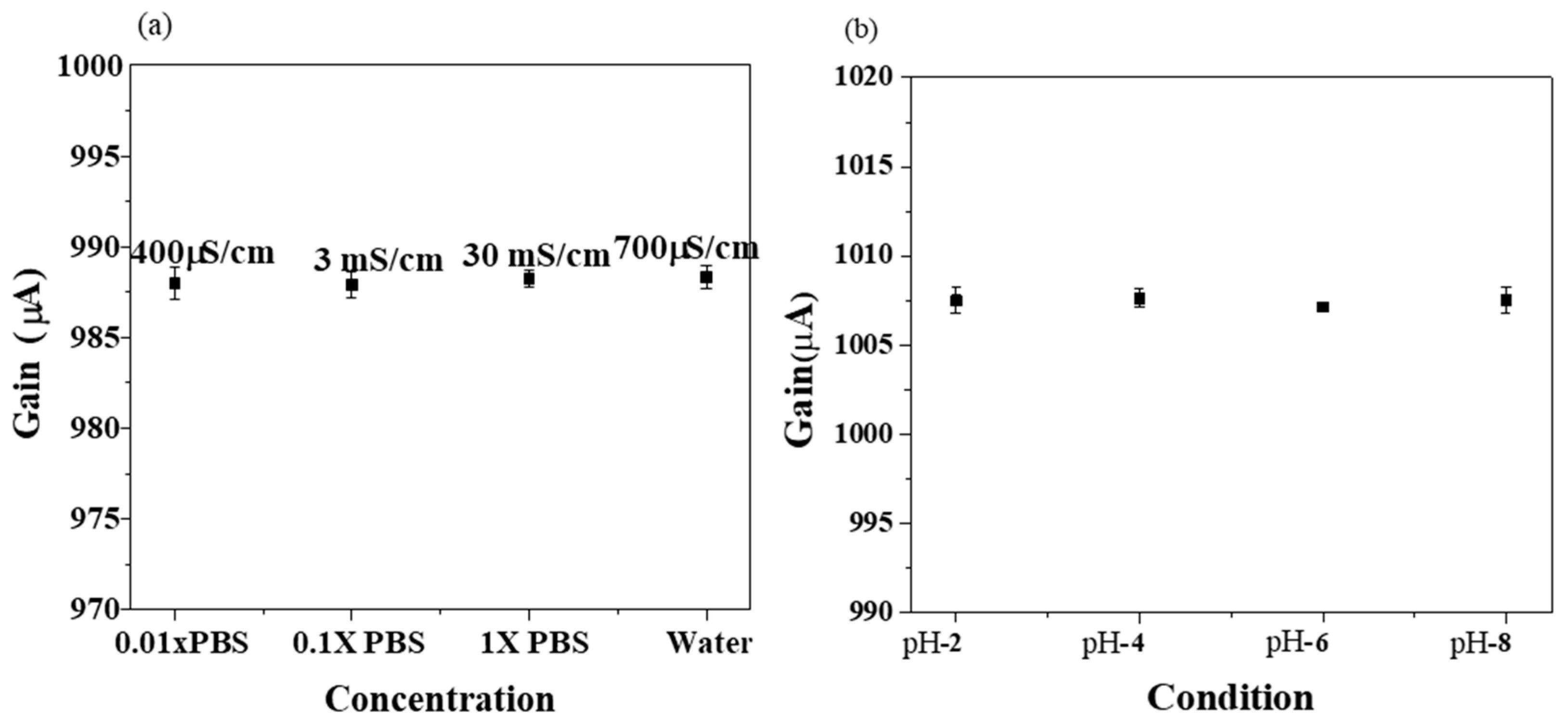
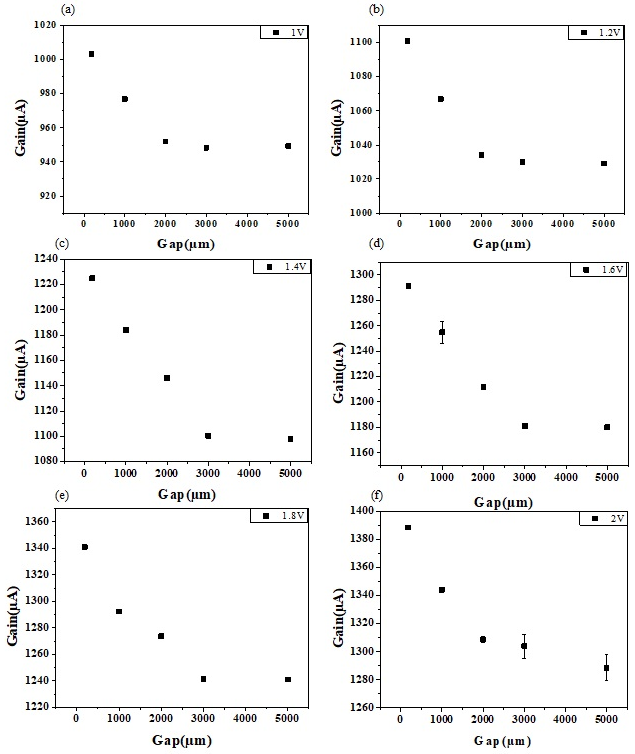
3.1. Sensing Characteristics of Extended Gate Hg-ISMFET Sensor
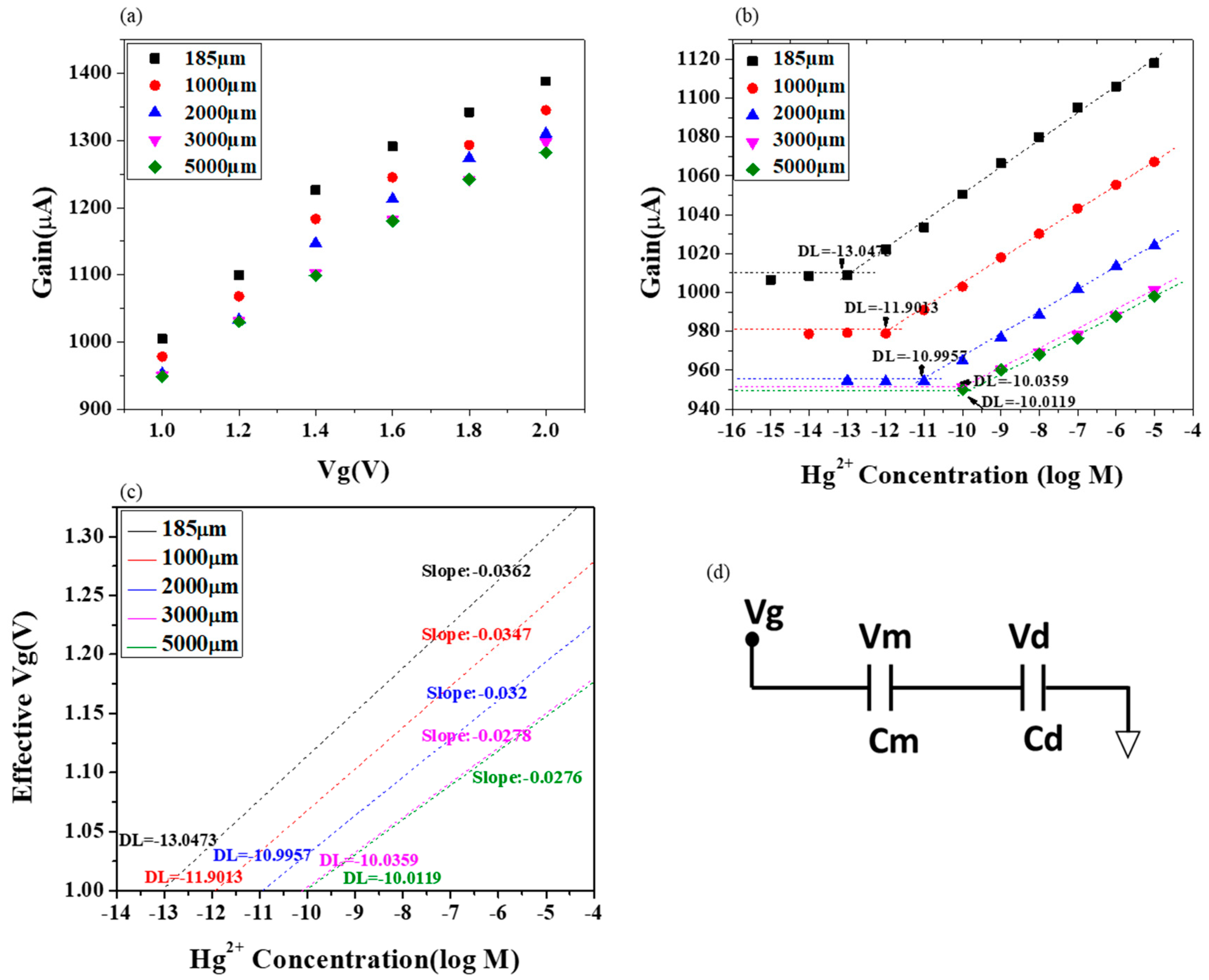
3.2. Sensor Model of Extended Gate Hg-ISMFET Sensor
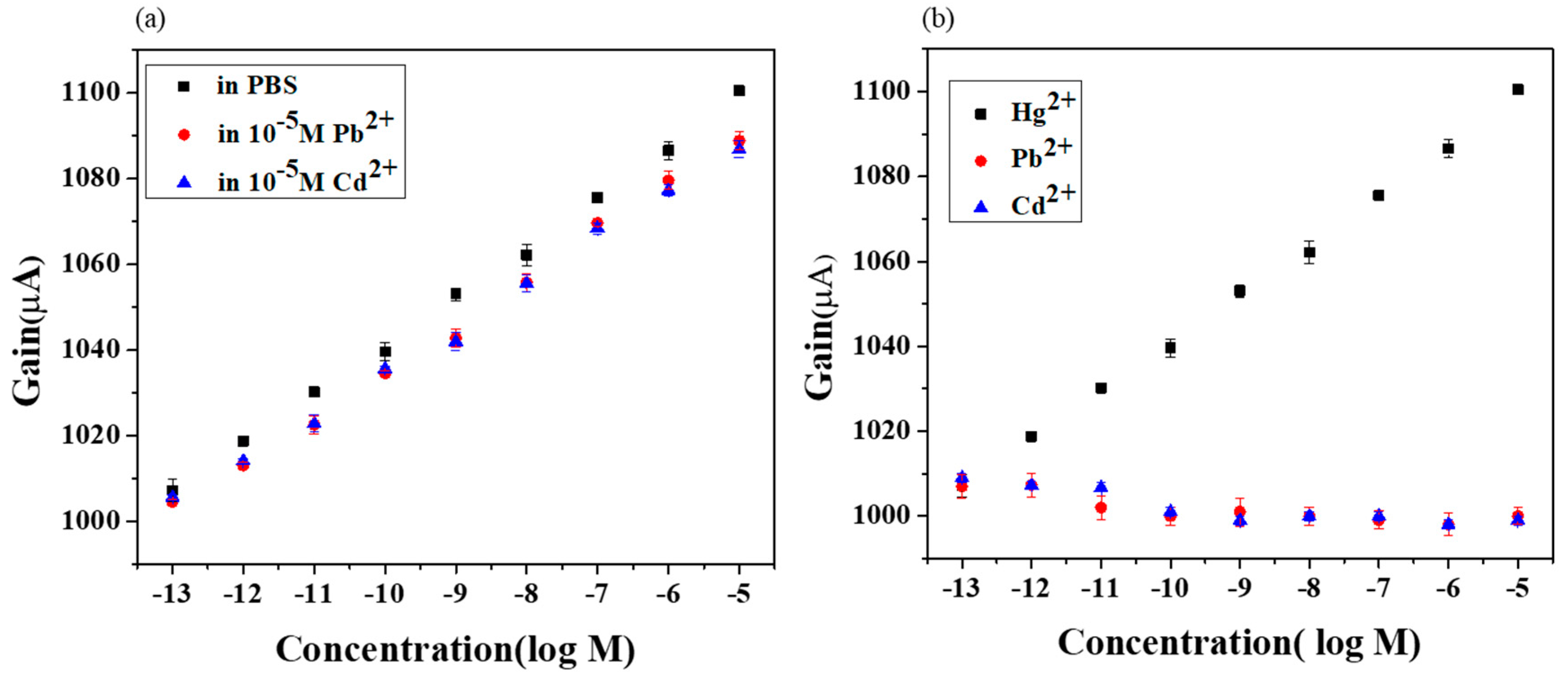
3.3. Selectivity Characteristics of Extended Gate Hg-ISMFET Sensor
Real Time Testing by Extended Gate Hg-ISMFET in Industrial Waste Water Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Blum, J.D. Mesmerized by mercury. Nat. Chem. 2013, 5, 1066. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 8034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Walker, S.J. The neuropathogenesis of mercury toxicity. Mol. Psychiatry 2002, 7, S40–S41. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Bengtsson, U.; Chirumbolo, S.; Kern, J.K. Concerns about environmental mercury toxicity: Do we forget something else? Environ. Res. 2017, 152, 514–516. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Public Health Impact of Chemicals: Knowns and Unknowns; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Pyle, S.M.; Nocerino, J.M.; Deming, S.N.; Palasota, J.A.; Palasota, J.M.; Miller, E.L.; Hillman, D.C.; Kuharic, C.A.; Cole, W.H.; Fitzpatrick, P.M.; et al. Comparison of AAS, ICP-AES, PSA, and XRF in Determining Lead and Cadmium in Soil. Environ. Sci. Technol. 1996, 30, 204–213. [Google Scholar] [CrossRef]
- Allibone, J.; Fatemian, E.; Walker, P.J. Determination of mercury in potable water by ICP-MS using gold as a stabilising agent. J. Anal. At. Spectrom. 1999, 14, 235–239. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Sarangadharan, I.; Sukesan, R.; Hseih, C.-Y.; Lee, G.-Y.; Chyi, J.-I.; Wang, Y.-L. High-field modulated ion-selective field-effect-transistor (FET) sensors with sensitivity higher than the ideal Nernst sensitivity. Sci. Rep. 2018, 8, 8300. [Google Scholar] [CrossRef]
- Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chen, P.-C.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Shiesh, S.-C.; Lee, G.-B.; et al. High sensitivity cardiac troponin I detection in physiological environment using AlGaN/GaN High Electron Mobility Transistor (HEMT) Biosensors. Biosens. Bioelectron. 2018, 100, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Chen, C.-C.; Shiesh, S.-C.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef] [PubMed]
- Sarangadharan, I.; Wang, S.-L.; Sukesan, R.; Chen, P.-C.; Dai, T.-Y.; Pulikkathodi, A.K.; Hsu, C.-P.; Chiang, H.-H.K.; Liu, L.Y.-M.; Wang, Y.-L. Single Drop Whole Blood Diagnostics: Portable Biomedical Sensor for Cardiac Troponin I Detection. Anal. Chem. 2018, 90, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Kuo, W.-C.; Tai, T.-Y.; Hsu, C.-P.; Sarangadharan, I.; Pulikkathodi, A.K.; Wang, S.-L.; Sukesan, R.; Lin, H.-Y.; Kao, K.-W.; et al. Highly sensitive and rapid MicroRNA detection for cardiovascular diseases with electrical double layer (EDL) gated AlGaN/GaN high electron mobility transistors. Sens. Actuators B Chem. 2018, 262, 365–370. [Google Scholar] [CrossRef]
- Edmonds, J.S.; Morita, M. The determination of iodine species in environmental and biological samples (Technical Report). Pure Appl. Chem. 1998, 70, 1567–1584. [Google Scholar] [CrossRef]
- Javanbakht, M.; Reza Ganjali, M.; Eshghi, H.; Sharghi, H.; Shamsipur, M. Mercury(II) Ion-Selective Electrode Based on Dibenzo-diazathia-18-crown-6-dione. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 1999, 11, 81–84. [Google Scholar] [CrossRef]
- Asadnia, M.; Myers, M.; Akhavan, N.; O’Donnell, K.; Umana-Membreno, G.A.; Mishra, U.; Nener, B.; Baker, M.; Parish, G. Mercury(II) selective sensors based on AlGaN/GaN transistors. Anal. Chim. Acta 2016, 943, 1–7. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.K.; Kim, M. Ion-Sensitive Field-Effect Transistor for Biological Sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Heal. 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kusaka, Y.; Sato, K.; Zhang, Q. The endocrine disruptive effects of mercury. Environ. Heal. Prev. Med. 2000, 4, 174–183. [Google Scholar] [CrossRef]
- Ambacher, O.; Smart, J.; Shealy, J.R.; Weimann, N.G.; Chu, K.; Murphy, M.; Schaff, W.J.; Eastman, L.F.; Dimitrov, R.; Wittmer, L.; et al. Two-dimensional electron gases induced by spontaneous and piezoelectric polarization charges in N- and Ga-face AlGaN/GaN heterostructures. J. Appl. Phys. 1999, 85, 3222–3233. [Google Scholar] [CrossRef]
- Lin, J.-L.; Hsu, H.-Y. Study of Sodium Ion Selective Electrodes and Differential Structures with Anodized Indium Tin Oxide. Sensors 2010, 10, 1798–1809. [Google Scholar] [CrossRef]
- Bergveld, P. Development, Operation, and Application of the Ion-Sensitive Field-Effect Transistor as a Tool for Electrophysiology. IEEE Trans. Biomed. Eng. 1972, 19, 342–351. [Google Scholar] [CrossRef]
- Go, J.; Alam, M. Effect of Fluid Gate on the Electrostatics of ISFET-Based pH Sensors. In Proceedings of the 2010 18th Biennial University/ Government/Industry Micro/Nano Symposium (UGIM 2010), West Lafayette, IN, USA, 28 June–1 July 2010; pp. 1–3. [Google Scholar]
- Li, P.; Liu, B.; Zhang, D.; Sun, Y.; Liu, J. Graphene field-effect transistors with tunable sensitivity for high performance Hg (II) sensing. Appl. Phys. Lett. 2016, 109, 153101. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Guerra, E.M.; Mulato, M. V2O5/WO3 Mixed Oxide Films as pH-EGFET Sensor: Sequential Re-Usage and Fabrication Volume Analysis. ECS J. Solid State Sci. Technol. 2012, 1, N39–N44. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Tsai, F.-S.; Wang, S.-J. Preparation of TiO2 nanowire arrays through hydrothermal growth method and their pH sensing characteristics. Jpn. J. Appl. Phys. 2014, 53, 6. [Google Scholar] [CrossRef]
- Das, A.; Ko, D.H.; Chen, C.-H.; Chang, L.-B.; Lai, C.-S.; Chu, F.-C.; Chow, L.; Lin, R.-M. Highly sensitive palladium oxide thin film extended gate FETs as pH sensor. Sens. Actuators B Chem. 2014, 205, 199–205. [Google Scholar] [CrossRef]
- Spijkman, M.; Smits, E.C.P.; Cillessen, J.F.M.; Biscarini, F.; Blom, P.W.M.; De Leeuw, D.M. Beyond the Nernst-limit with dual-gate ZnO ion-sensitive field-effect transistors. Appl. Phys. Lett. 2011, 98, 43502. [Google Scholar] [CrossRef]
- Liu, C.; Bocchicchio, D.; Overmyer, P.; Neuman, M. A Palladium-palladium oxide miniature pH electrode. Science 1980, 207, 188–189. [Google Scholar] [CrossRef]
- Yao, P.-C.; Chiang, J.-L.; Lee, M.-C. Application of sol–gel TiO2 film for an extended-gate H+ ion-sensitive field-effect transistor. Solid State Sci. 2013, 28, 47–54. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Huang, I.Y.; Wu, C.Y. A low-hysteresis and high-sensitivity extended gate FET-based chloride ion-selective sensor. In Proceedings of the 2010 IEEE Sensors, Kona, HI, USA, 1–4 November 2010; pp. 358–361. [Google Scholar]
- Li, B.; Fang, X.; Luo, H.; Petersen, E.; Seo, Y.-S.; Samuilov, V.; Rafailovich, M.; Sokolov, J.; Gersappe, D.; Chu, B. Influence of electric field intensity, ionic strength, and migration distance on the mobility and diffusion in DNA surface electrophoresis. Electrophoresis 2006, 27, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Jameson, E.; Alvarez-Tostado, C. A Study of Blood Serum Proteins by Electrophoresis. J. Phys. Chem. 1939, 43, 1165–1172. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. IUPAC Recommendations for Nomenclature of Ion-Selective Electrodes. Pure Appl. Chem. 1994, 66, 2527. [Google Scholar] [CrossRef]
- Tohda, K.; Dragoé, D.; Shibata, M.; Umezawa, Y. Studies on the Matched Potential Method for Determining the Selectivity Coefficients of Ion-Selective Electrodes Based on Neutral Ionophores: Experimental and Theoretical Verification. Anal. Sci. 2001, 17, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chang, J.; Pu, H.; Shi, K.; Mao, S.; Sui, X.; Ren, R.; Cui, S.; Chen, J. Ultrasensitive Mercury Ion Detection Using DNA-Functionalized Molybdenum Disulfide Nanosheet/Gold Nanoparticle Hybrid Field-Effect Transistor Device. ACS Sensors 2016, 1, 295–302. [Google Scholar] [CrossRef]
- Li, P.; Zhang, D.; Jiang, C.; Zong, X.; Cao, Y. Ultra-sensitive suspended atomically thin-layered black phosphorus mercury sensors. Biosens. Bioelectron. 2017, 98, 68–75. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Hammock, M.L.; Appleton, A.L.; Schwartz, G.; Mei, J.; Lei, T.; Pei, J.; Bao, Z. Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 2014, 5, 2954. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, G.; Gao, X.; Mao, S.; Cui, S.; Ocola, L.E.; Yuan, C.; Chen, J. Real-time detection of mercury ions in water using a reduced graphene oxide/DNA field-effect transistor with assistance of a passivation layer. Sens. Bio-Sens. Res. 2015, 5, 97–104. [Google Scholar] [CrossRef]
- Sudibya, H.G.; He, Q.; Zhang, H.; Chen, P. Electrical Detection of Metal Ions Using Field-Effect Transistors Based on Micropatterned Reduced Graphene Oxide Films. ACS Nano 2011, 5, 1990–1994. [Google Scholar] [CrossRef]
- Minamiki, T.; Anzenbacher, P.; Minami, T.; Sasaki, Y.; Koutnik, P.; Tokito, S. A Mercury(II) ion sensor device based on an organic field effect transistor with an extended-gate modified by dipicolylamine. Chem. Commun. 2015, 51, 17666–17668. [Google Scholar] [CrossRef] [PubMed]

| Sample No. | Extended Gate Hg-ISMFET | ICP-MS |
|---|---|---|
| A | 1.36 × 10−11 M | 1.9931 × 10−11 M |
| B | 7.72 × 10−11 M | 2.850 × 10−11 M |
| C | 4.10 × 10−11 M | 3.1327 × 10−11 M |
| D | 1.86 × 10−11 M | 1.7677 × 10−11 M |
| E | 2.55 × 10−11 M | 2.613 × 10−11 M |
| F | 1.583 × 10−13 M | ND |
| Methodology | Dynamic Range | Detection Limit | Response Time | Features |
|---|---|---|---|---|
| MoS2 nanosheet/gold nanoparticle hybrid field-effect transistor (FET) sensor [37] | 10−10–10−8 M | 10−10 M | 1–2 s | DNA as a ion selective agent; functionalization of FET with DNA required |
| Organic polymer field-effect transistor [39] | 10−6–10−3 M | 10−6 M | 200 s | Functionalization with DNA required; extended stability in marine environment demonstrated |
| Reduced graphene oxide field-effect transistor [40] | 10−9–10−6 M | 10−9 M | Several seconds | Functionalization with DNA required |
| Micropatterned reduced graphene oxide FET [41] | 10−9–10−10 M | 10−9 M | 50 s | Functionalization with protein required |
| Extended gate organic FET [42] | 10−11–10−5 M | 10−11 M | -- | Functionalization with dipicolylamine required |
| This work | 10−13–10−5 M | 10−13 M | 5 min | Ion selective polymer membrane is used as receptor |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukesan, R.; Chen, Y.-T.; Shahim, S.; Wang, S.-L.; Sarangadharan, I.; Wang, Y.-L. Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device. Sensors 2019, 19, 2209. https://doi.org/10.3390/s19092209
Sukesan R, Chen Y-T, Shahim S, Wang S-L, Sarangadharan I, Wang Y-L. Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device. Sensors. 2019; 19(9):2209. https://doi.org/10.3390/s19092209
Chicago/Turabian StyleSukesan, Revathi, Yi-Ting Chen, Suman Shahim, Shin-Li Wang, Indu Sarangadharan, and Yu-Lin Wang. 2019. "Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device" Sensors 19, no. 9: 2209. https://doi.org/10.3390/s19092209
APA StyleSukesan, R., Chen, Y.-T., Shahim, S., Wang, S.-L., Sarangadharan, I., & Wang, Y.-L. (2019). Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device. Sensors, 19(9), 2209. https://doi.org/10.3390/s19092209





