Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium
Abstract
1. Introduction
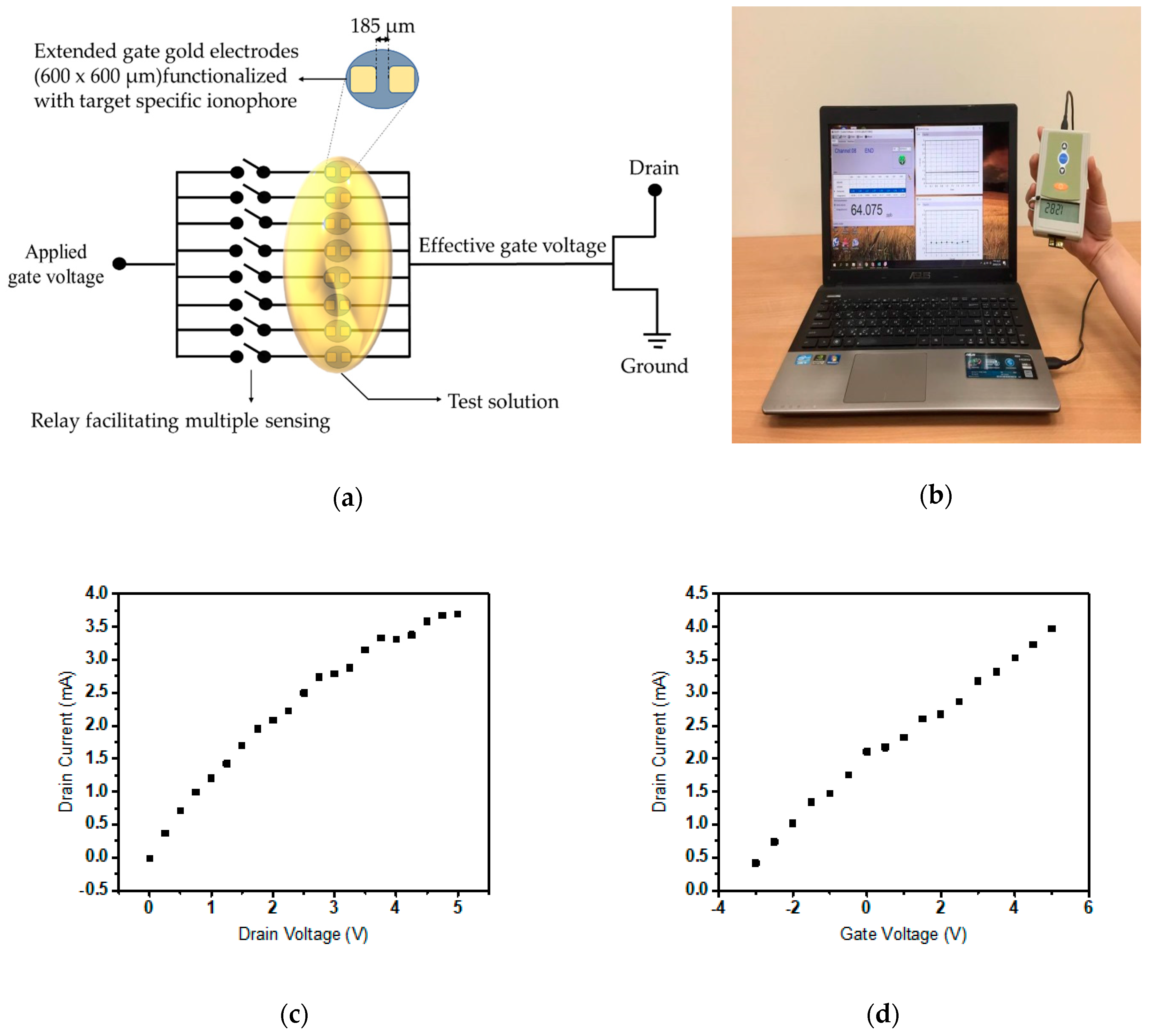
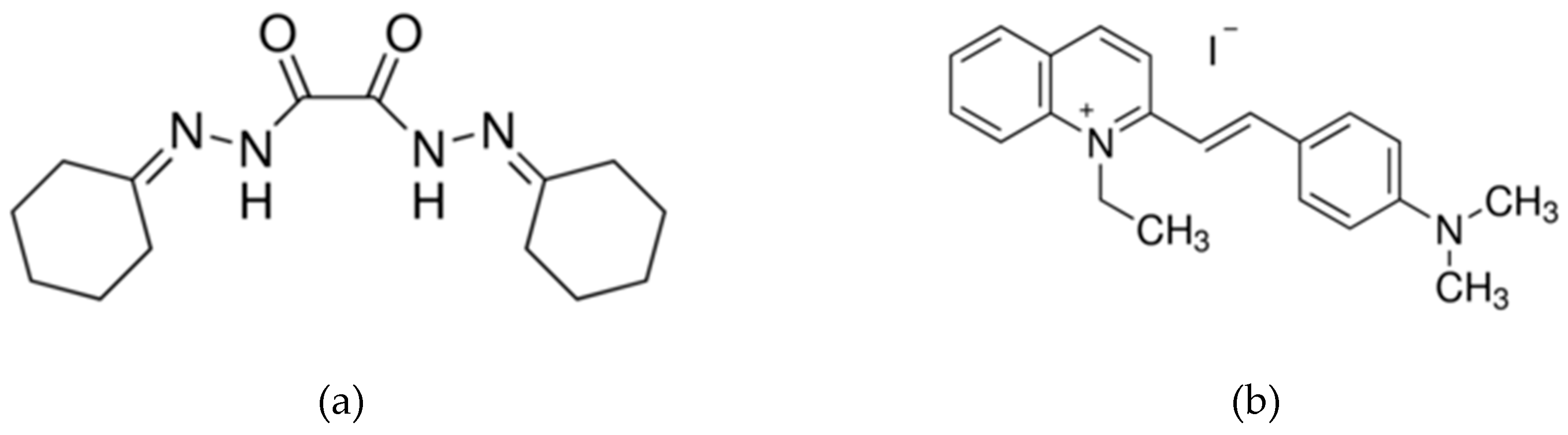
2. Materials and Methods
3. Results and Discussion
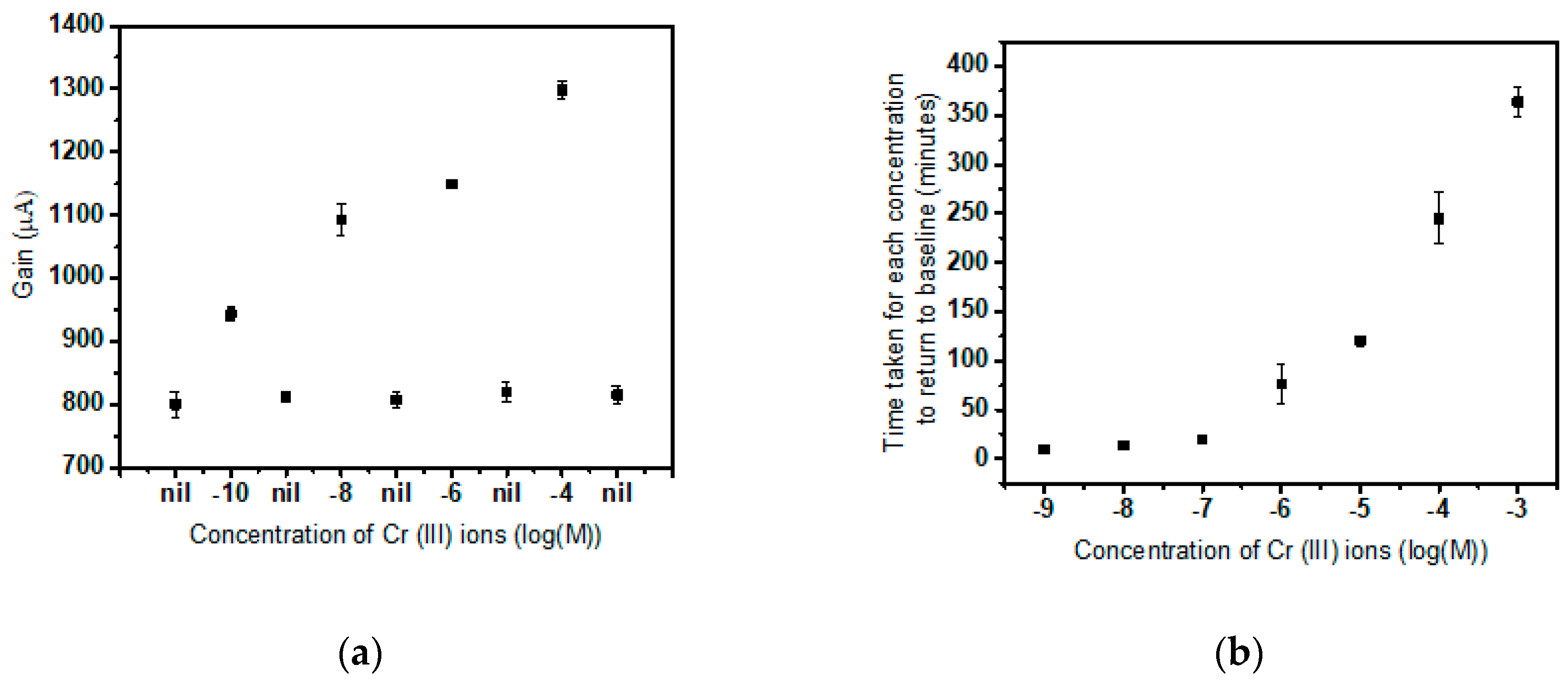
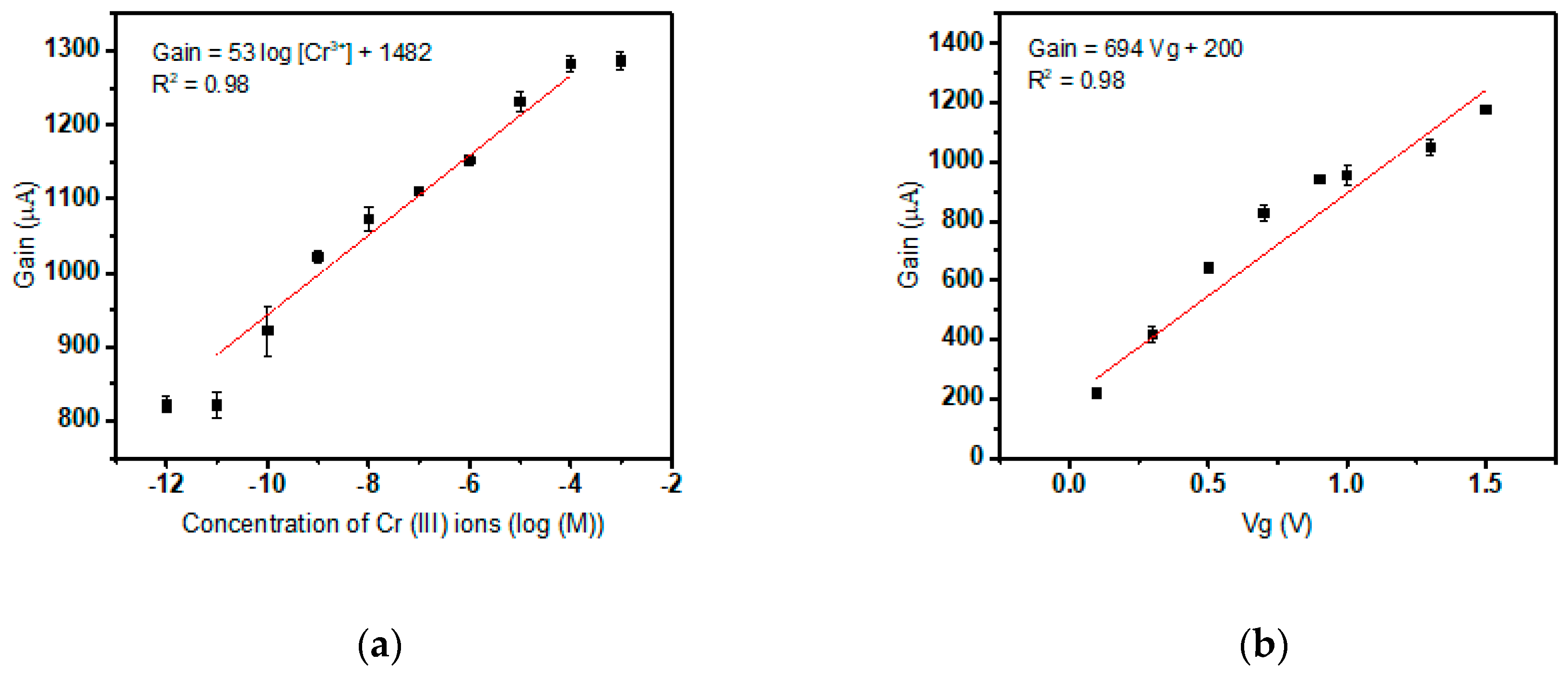
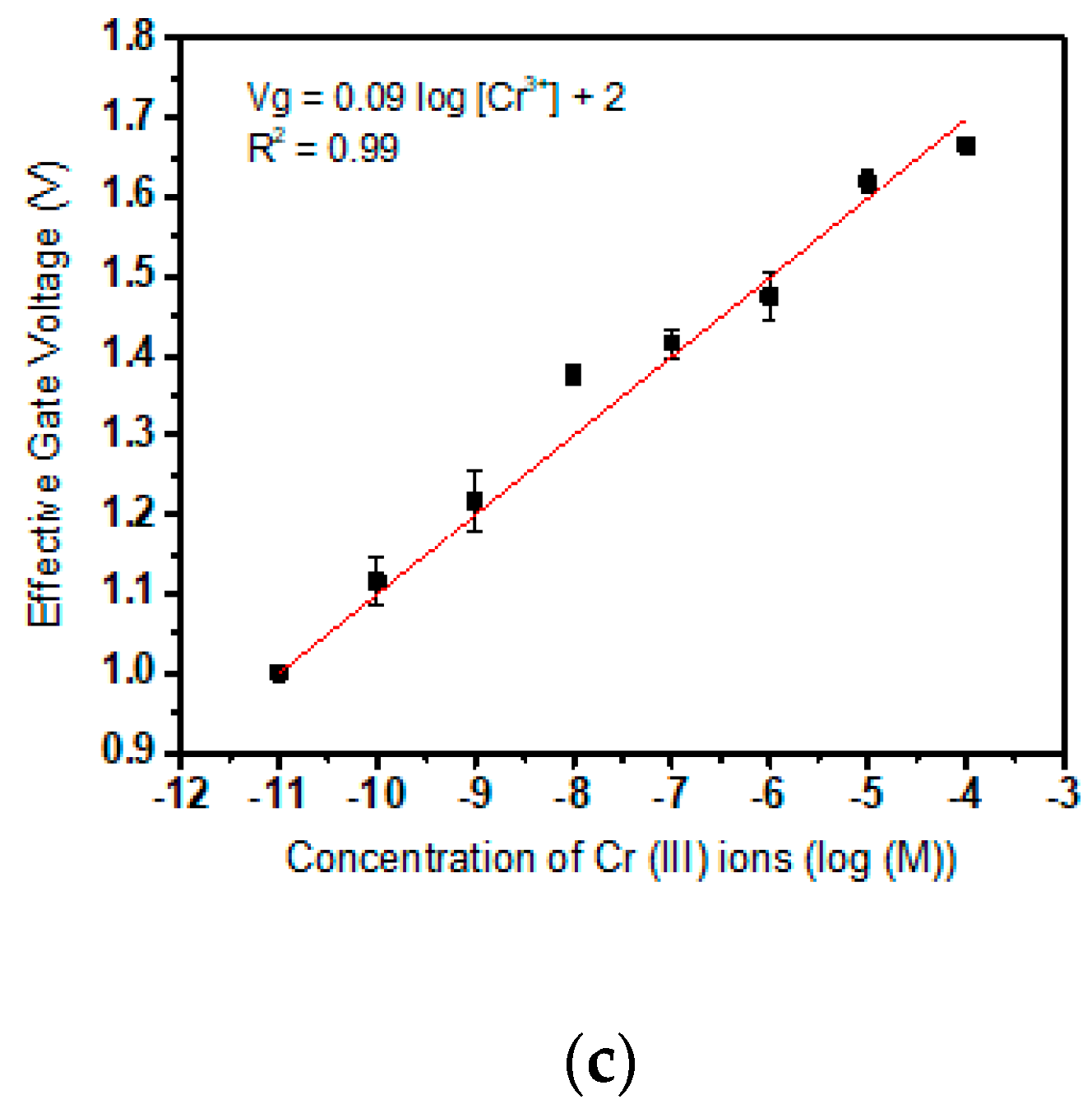
3.1. Calibration of Cr(III) Specific Sensor
3.2. Calibration of Cr(VI) Specific Sensor
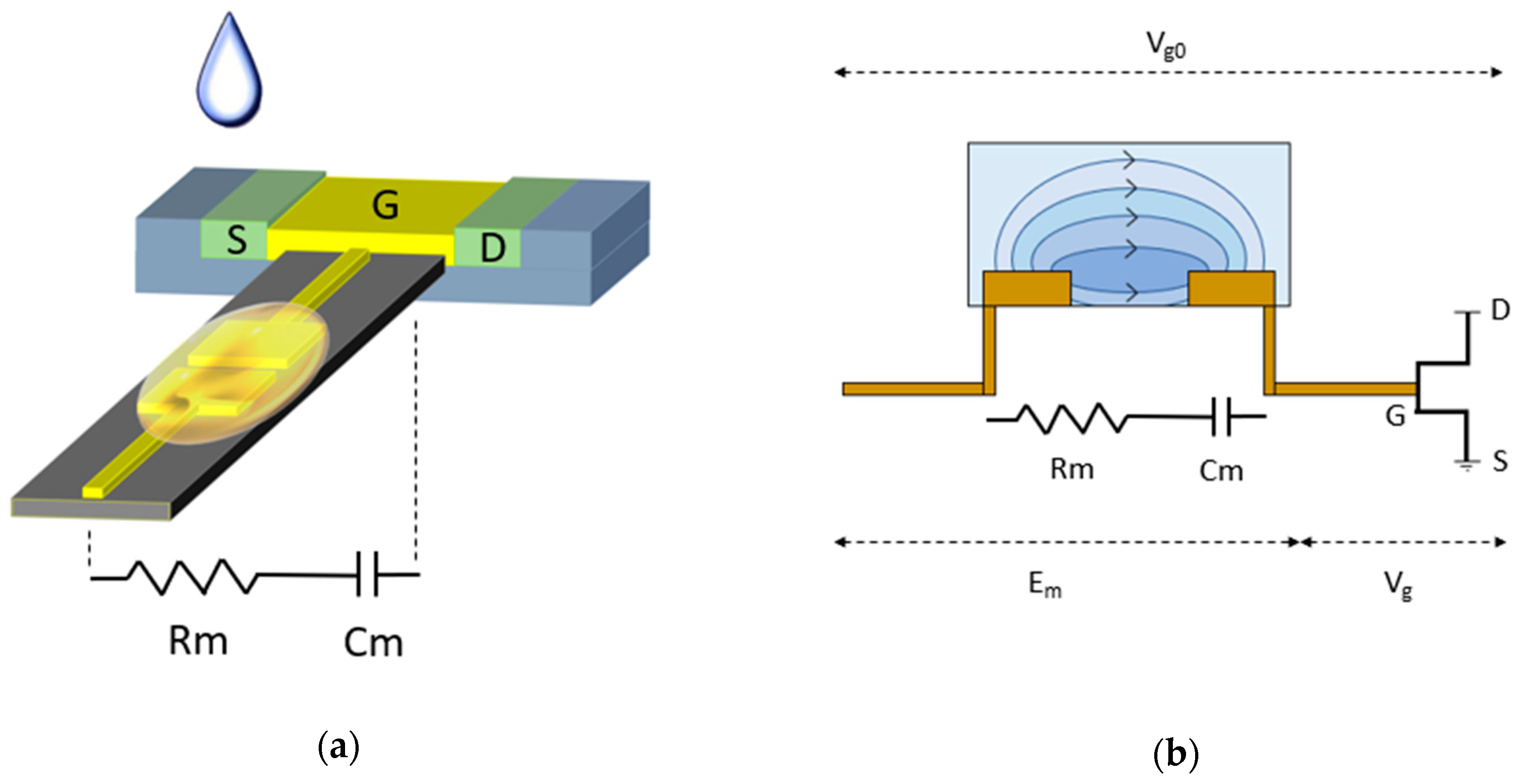
3.3. Two-Plate Capacitive Model of The Biosensor
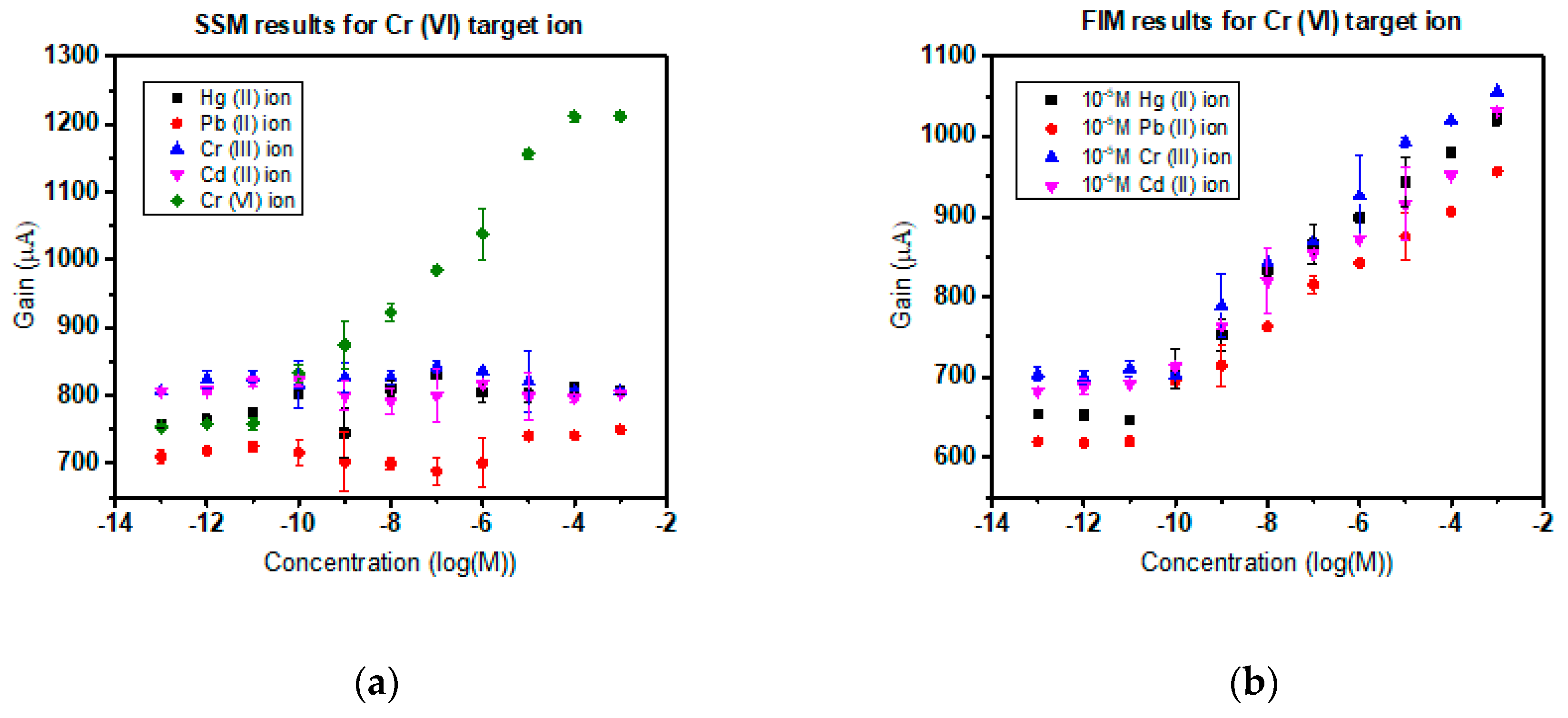
3.4. Selectivity in the Presence of Interfering Ions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hsu, L.-C.; Huang, C.-Y.; Chuang, Y.-H.; Chen, H.-W.; Chan, Y.-T.; Teah, H.Y.; Chen, T.-Y.; Chang, C.-F.; Liu, Y.-T.; Tzou, Y.-M. Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci. Rep. 2016, 6, 34250. [Google Scholar] [CrossRef]
- Norseth, T. The carcinogenicity of chromium. Environ. Health Perspect. 1981, 40, 121–130. [Google Scholar] [CrossRef]
- Russo, P.; Catassi, A.; Cesario, A.; Imperatori, A.; Rotolo, N.; Fini, M.; Granone, P.; Dominioni, L. Molecular mechanisms of hexavalent chromium-induced apoptosis in human bronchoalveolar cells. Am. J. Respir. Cell Mol. Biol. 2005, 33, 589–600. [Google Scholar] [CrossRef]
- Jia, Z.; Li, S.; Wang, L. Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Sci. Rep. 2018, 8, 3256. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer Basel: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Lennartson, A. The colours of chromium. Nat. Chem. 2014, 6, 942. [Google Scholar] [CrossRef]
- Junaid, M.; Hashmi, M.Z.; Tang, Y.-M.; Malik, R.N.; Pei, D.-S. Potential health risk of heavy metals in the leather manufacturing industries in Sialkot, Pakistan. Sci. Rep. 2017, 7, 8848. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Homa, D.; Haile, E.; Washe, A.P. Determination of Spatial Chromium Contamination of the Environment around Industrial Zones. Int. J. Anal. Chem. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Luch, A. Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Shupack, S.I. The chemistry of chromium and some resulting analytical problems. Environ. Health Perspect. 1991, 92, 7–11. [Google Scholar] [CrossRef]
- Izbicki, J.A.; Wright, M.T.; Seymour, W.A.; McCleskey, R.B.; Fram, M.S.; Belitz, K.; Esser, B.K. Cr(VI) occurrence and geochemistry in water from public-supply wells in California. Appl. Geochem. 2015, 63, 203–217. [Google Scholar] [CrossRef]
- McLean, J.E.; McNeill, L.S.; Edwards, M.A.; Parks, J.L. Hexavalent chromium review, part 1: Health effects, regulations, and analysis. J. Am. Water Works Assn. 2012, 104, E348–E357. [Google Scholar] [CrossRef]
- Gorchev, H.G.; Ozolins, G. WHO guidelines for drinking-water quality. WHO Chron. 1984, 38, 104–108. [Google Scholar]
- Registry, A.f.T.S.a.D. Chromium Toxicity. Case Stud. Environ. Med. 2011. WB 1466. Available online: https://www.atsdr.cdc.gov/csem/chromium/docs/chromium.pdf (accessed on 25 April 2019).
- Wang, L.-L.; Wang, J.-Q.; Zheng, Z.-X.; Xiao, P. Cloud point extraction combined with high-performance liquid chromatography for speciation of chromium(III) and chromium(VI) in environmental sediment samples. J. Hazard. Mater. 2010, 177, 114–118. [Google Scholar]
- Zhang, N.; Suleiman, J.S.; He, M.; Hu, B. Chromium(III)-imprinted silica gel for speciation analysis of chromium in environmental water samples with ICP-MS detection. Talanta 2008, 75, 536–543. [Google Scholar] [CrossRef]
- Yalcin, S.; Apak, R. Chromium(III, VI) speciation analysis with preconcentration on a maleic acid-functionalized XAD sorbent. Anal. Chim. Acta 2004, 505, 25–35. [Google Scholar] [CrossRef]
- Kiran, K.; Kumar, K.S.; Prasad, B.; Suvardhan, K.; Lekkala, R.B.; Janardhanam, K. Speciation determination of chromium(III) and (VI) using preconcentration cloud point extraction with flame atomic absorption spectrometry (FAAS). J. Hazard. Mater. 2008, 150, 582–586. [Google Scholar] [CrossRef]
- Shaffer, R.E.; Cross, J.O.; Rose-Pehrsson, S.L.; Elam, W.T. Speciation of chromium in simulated soil samples using X-ray absorption spectroscopy and multivariate calibration. Anal. Chim. Acta 2001, 442, 295–304. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shahawi, M.S.; Othman, A.M.; Mosaad, M.A. A potentiometric rhodamine-B based membrane sensor for the selective determination of chromium ions in wastewater. Anal. Sci. 2005, 21, 673–678. [Google Scholar] [CrossRef][Green Version]
- Yari, A.; Bagheri, H. Determination of Cr(VI) with Selective Sensing of Cr(VI) Anions by a PVC-Membrane Electrode Based on Quinaldine Red. J. Chin. Chem. Soc. 2009, 56, 289–295. [Google Scholar] [CrossRef]
- Choi, Y.W.; Minoura, N.; Moon, S.H. Potentiometric Cr(VI) selective electrode based on novel ionophore-immobilized PVC membranes. Talanta 2005, 66, 1254–1263. [Google Scholar] [CrossRef]
- Choi, Y.W.; Moon, S.H. Determination of Cr(VI) using an ion-selective electrode with SLMs containing Aliquat336. Environ. Monit. Assess. 2004, 92, 163–178. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Sarangadharan, I.; Sukesan, R.; Hseih, C.-Y.; Lee, G.-Y.; Chyi, J.-I.; Wang, Y.-L. High-field modulated ion-selective field-effect-transistor (FET) sensors with sensitivity higher than the ideal Nernst sensitivity. Sci. Rep. 2018, 8, 8300. [Google Scholar] [CrossRef]
- Biswas, P.; Karn, A.K.; Balasubramanian, P.; Kale, P.G. Biosensor for detection of dissolved chromium in potable water: A review. Biosens. Bioelectron. 2017, 94, 589–604. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.A.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Huang, Z.B.; Chang, S.H. Synthesis and characterization of novel ionophores of double-armed penta-crown ethers. Tetrahedron Lett. 2005, 46, 5351–5355. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahim, S.; Sukesan, R.; Sarangadharan, I.; Wang, Y.-L. Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium. Sensors 2019, 19, 1969. https://doi.org/10.3390/s19091969
Shahim S, Sukesan R, Sarangadharan I, Wang Y-L. Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium. Sensors. 2019; 19(9):1969. https://doi.org/10.3390/s19091969
Chicago/Turabian StyleShahim, Suman, Revathi Sukesan, Indu Sarangadharan, and Yu-Lin Wang. 2019. "Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium" Sensors 19, no. 9: 1969. https://doi.org/10.3390/s19091969
APA StyleShahim, S., Sukesan, R., Sarangadharan, I., & Wang, Y.-L. (2019). Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium. Sensors, 19(9), 1969. https://doi.org/10.3390/s19091969




