Developments in Transduction, Connectivity and AI/Machine Learning for Point-of-Care Testing
Abstract
1. Introduction
2. Patient Need for POCT
2.1. Infectious Diseases
2.2. Non-Communicable Diseases
3. Developments Towards Portable Diagnostics
4. Novel Photonic Systems
4.1. Cavity Enhanced Absorption Spectrometry (CEAS)
4.2. Plasmonics
4.3. Digital Microarrays—Interferometric Reflectance Imaging Sensor (IRIS)
5. Mobile Phone Reader and Device Connectivity for POCT
5.1. Components of Mobile Phones Used in POCT
5.2. Auxiliary Hardware for Mobile Phone-Based POCT
6. Data Analytics for POCT
6.1. Data Management
- Store and allow access to comprehensive health data including the medical history of the patient;
- Harmonize with the workflow of health organizations and provide efficient interaction experience;
- Assist in administrative tasks such as billing, insurance claim filing, and scheduling;
- Allow efficient access and assist in statistical analysis of data.
6.2. Data-Driven Decision-Making Using Machine Learning Techniques
6.3. Application Scenarios
6.4. Case Studies
7. POCT for Clinical Diagnostics within Lower Middle Income Countries (LMICs) and Least Developed Countries (LDCs)
7.1. Overall Framework
7.2. Open Data Platforms for Infectious Diseases
7.3. Nucleic Acid Amplification Techniques
7.4. Rapid DNA/RNA Sequencing for Outbreak Response
7.5. Antimicrobial Resistance
8. Final Considerations and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Engel, N.; Ganesh, G.; Patil, M.; Yellappa, V.; Vadnais, C.; Pai, N.P.; Pai, M. Point-of-care testing in India: Missed opportunities to realize the true potential of point-of-care testing programs. BMC Health Serv. Res. 2015, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Baca, J.T.; Severns, V.; Lovato, D.; Branch, D.W.; Larson, R.S. Rapid detection of Ebola virus with a reagent-free, point-of-care biosensor. Sensors 2015, 15, 8605–8614. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 43, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Antibiotic/Antimicrobial Resistance (AR/AMR); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Chambers, H.; Bartlett, J.; Bonomo, R.; Chiou, C.; Cosgrove, S.; Cross, H.; Daum, R.; Downing, M.; Evans, S.; Knisely, J.; et al. Antibacterial Resistance Leadership Group: Open for Business. Clin. Infect. Dis. 2014, 58, 1571–1576. [Google Scholar] [CrossRef]
- Zumla, A.; Al-Tawfiq, J.A.; Enne, V.I.; Kidd, M.; Drosten, C.; Breuer, J.; Muller, M.A.; Hui, D.; Maeurer, M.; Bates, M.; et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Cooke, J.; Butler, C.; Hopstaken, R.; Dryden, M.S.; McNulty, C.; Hurding, S.; Moore, M.; Livermore, D.M. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir. Res. 2015, 2, e000086. [Google Scholar] [CrossRef]
- Rittoo, D.; Jones, A.; Lecky, B.; Neithercut, D. Elevation of Cardiac Troponin T, But Not Cardiac Troponin I, in Patients With Neuromuscular Diseases: Implications for the Diagnosis of Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 2411–2420. [Google Scholar] [CrossRef]
- Jones, E.A.; Sajid, M.I.; Shenton, A.; Evans, D.G. Basal Cell Carcinomas in Gorlin Syndrome: A Review of 202 Patients. J. Skin Cancer 2011, 2011, 217–378. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Garrett, N.J. The arrival of a true point-of-care molecular assay—Ready for global implementation? Lancet Glob. Health 2015, 3, e663–e664. [Google Scholar] [CrossRef]
- Albery, W.J.; Bartlett, P.N. Amperometric enzyme electrodes: Part I. Theory. J. Electroanal. Chem. Interfacial Electrochem. 1985, 194, 211–222. [Google Scholar] [CrossRef]
- Bartlett, P.; Ali, Z.; Eastwick-Field, V. Electrochemical immobilisation of enzymes. Part 4.—Co-immobilisation of glucose oxidase and ferro/ferricyanide in poly (N-methylpyrrole) films. J. Chem. Soc. Faraday Trans. 1992, 88, 2677–2683. [Google Scholar] [CrossRef]
- Chebil, S.; Hafaiedh, I.; Sauriat-Dorizon, H.; Jaffrezic-Renault, N.; Errachid, A.; Ali, Z.; Korri-Youssoufi, H. Electrochemical detection of D-dimer as deep vein thrombosis marker using single-chain D-dimer antibody immobilized on functionalized polypyrrole. Biosens. Bioelectron. 2010, 26, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Hafaid, I.; Chebil, S.; Korri-Youssoufi, H.; Bessueille, F.; Errachid, A.; Sassi, Z.; Ali, Z.; Abdelghani, A.; Jaffrezic-Renault, N. Effect of electrical conditions on an impedimetric immunosensor based on a modified conducting polypyrrole. Sens. Actuators B Chem. 2010, 144, 323–331. [Google Scholar] [CrossRef]
- Van Gerwen, P.; Laureyn, W.; Laureys, W.; Huyberechts, G.; De Beeck, M.O.; Baert, K.; Suls, J.; Sansen, W.; Jacobs, P.; Hermans, L.; et al. Nanoscaled interdigitated electrode arrays for biochemical sensors. Sens. Actuators B Chem. 1998, 49, 73–80. [Google Scholar] [CrossRef]
- Lakey, A.; Ali, Z.; Scott, S.M.; Chebil, S.; Korri-Youssoufi, H.; Hunor, S.; Ohlander, A.; Kuphal, M.; Marti, J.S. Impedimetric array in polymer microfluidic cartridge for low cost point-of-care diagnostics. Biosens. Bioelectron. 2019, 129, 147–154. [Google Scholar] [CrossRef]
- Manz, A.; Graber, N.; Widmer, H.A. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Pasirayi, G.; Auger, V.; M Scott, S.; KSM Rahman, P.; Islam, M.; O’Hare, L.; Ali, Z. Microfluidic bioreactors for cell culturing: A review. Micro Nanosyst. 2011, 3, 137–160. [Google Scholar] [CrossRef]
- Pasirayi, G.; Scott, S.M.; Islam, M.; Bateson, S.; Ali, Z. Low cost microfluidic cell culture array using normally closed valves for cytotoxicity assay. Talanta 2014, 129, 491–498. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Reis, N.M. A critical insight into the development pipeline of microfluidic immunoassay devices for the sensitive quantitation of protein biomarkers at the point of care. Analyst 2017, 142, 858–882. [Google Scholar] [CrossRef]
- Chan, H.N.; Shu, Y.; Xiong, B.; Chen, Y.; Chen, Y.; Tian, Q.; Michael, S.A.; Shen, B.; Wu, H. Simple, cost-effective 3D printed microfluidic components for disposable, point-of-care colorimetric analysis. ACS Sens. 2015, 1, 227–234. [Google Scholar] [CrossRef]
- Chudobova, D.; Cihalova, K.; Skalickova, S.; Zitka, J.; Rodrigo, M.A.M.; Milosavljevic, V.; Hynek, D.; Kopel, P.; Vesely, R.; Adam, V.; et al. 3D-printed chip for detection of methicillin-resistant Staphylococcus aureus labeled with gold nanoparticles. Electrophoresis 2015, 36, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Mulberry, G.; White, K.A.; Vaidya, M.; Sugaya, K.; Kim, B.N. 3D printing and milling a real-time PCR device for infectious disease diagnostics. PLoS ONE 2017, 12, e0179133. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 016501. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, C.M.; Davies, J.; Cabral, J.T.; Dolan, P.R.; Smith, J.M.; Vallance, C. Cavity-enhanced optical methods for online microfluidic analysis. Chem. Phys. Lett. 2012, 554, 1–14. [Google Scholar] [CrossRef]
- Thompson, J.E. Cavity-Enhanced Spectroscopy in Condensed Phases: Recent Literature and Remaining Challenges. J. Spectrosc. 2017, 2017. [Google Scholar] [CrossRef]
- Islam, M.; Seetohul, L.N.; Ali, Z. Liquid-phase broadband cavity-enhanced absorption spectroscopy measurements in a 2 mm cuvette. Appl. Spectrosc. 2007, 61, 649–658. [Google Scholar] [CrossRef]
- Seetohul, L.N.; Ali, Z.; Islam, M. Broadband cavity enhanced absorption spectroscopy as a detector for HPLC. Anal. Chem. 2009, 81, 4106–4112. [Google Scholar] [CrossRef]
- Bajuszova, Z.; Ali, Z.; Scott, S.; Seetohul, L.N.; Islam, M. Cavity-enhanced immunoassay measurements in microtiter plates using BBCEAS. Anal. Chem. 2016, 88, 5264–5270. [Google Scholar] [CrossRef]
- Bajuszova, Z.; Naif, H.; Ali, Z.; McGinnis, J.; Islam, M. Cavity enhanced liquid-phase stopped-flow kinetics. Analyst 2018, 143, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R.; Umviligihozo, G.; et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Patnaik, R.; Kuhlmann, K.; Rai, A.J.; Sia, S.K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015, 15, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
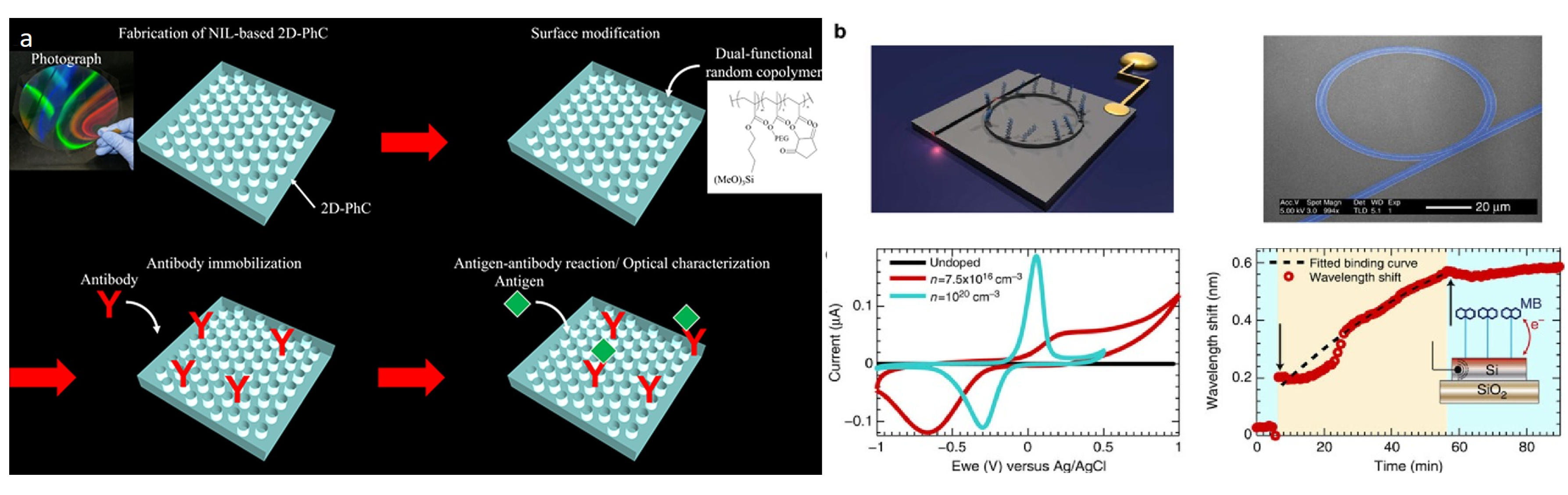
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef]
- Endo, T.; Kajita, H.; Kawaguchi, Y.; Kosaka, T.; Himi, T. Label-free optical detection of C-reactive protein by nanoimprint lithography-based 2D-photonic crystal film. Biotechnol. J. 2016, 11, 831–837. [Google Scholar] [CrossRef]
- Juan-Colás, J.; Parkin, A.; Dunn, K.E.; Scullion, M.G.; Krauss, T.F.; Johnson, S.D. The electrophotonic silicon biosensor. Nat. Commun. 2016, 7, 12769. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Zheng, W.; Cao, F.; Jiang, X. Inkjet-printed barcodes for a rapid and multiplexed paper-based assay compatible with mobile devices. Lab Chip 2017, 17, 3874–3882. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Mirkin, C.A. Drivers of biodiagnostic development. Nature 2009, 462, 461. [Google Scholar] [CrossRef]
- Barletta, J.M.; Edelman, D.C.; Constantine, N.T. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am. J. Clin. Pathol. 2004, 122, 20–27. [Google Scholar] [CrossRef]
- Cretich, M.; Daaboul, G.G.; Sola, L.; Ünlü, M.S.; Chiari, M. Digital detection of biomarkers assisted by nanoparticles: application to diagnostics. Trends Biotechnol. 2015, 33, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Digital PCR hits its stride. Nat. Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
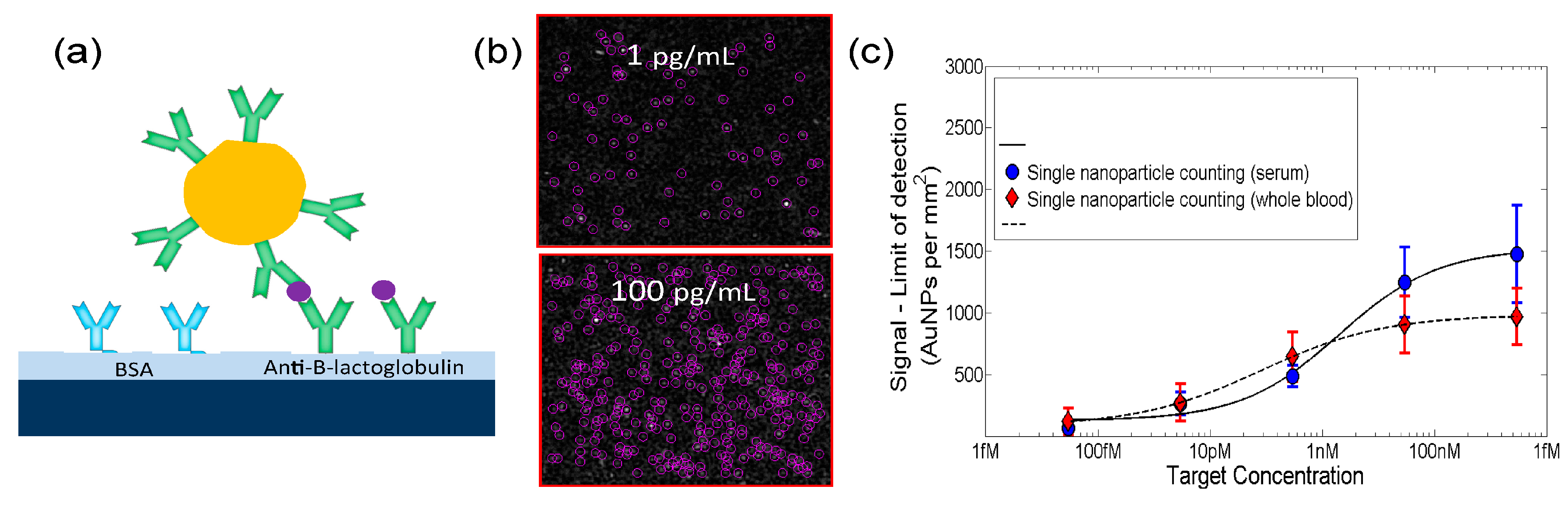
- Avci, O.; Ünlü, N.L.; Özkumur, A.Y.; Ünlü, M.S. Interferometric reflectance imaging sensor (IRIS)—A platform technology for multiplexed diagnostics and digital detection. Sensors 2015, 15, 17649–17665. [Google Scholar] [CrossRef]
- Daaboul, G.; Yurt, A.; Zhang, X.; Hwang, G.; Goldberg, B.; Ünlü, M.S. High-throughput detection and sizing of individual low-index nanoparticles and viruses for pathogen identification. Nano Lett. 2010, 10, 4727–4731. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, G.G.; Lopez, C.A.; Chinnala, J.; Goldberg, B.B.; Connor, J.H.; Ünlü, M.S. Digital sensing and sizing of vesicular stomatitis virus pseudotypes in complex media: A model for Ebola and Marburg detection. ACS Nano 2014, 8, 6047–6055. [Google Scholar] [CrossRef] [PubMed]
- Scherr, S.M.; Freedman, D.S.; Agans, K.N.; Rosca, A.; Carter, E.; Kuroda, M.; Fawcett, H.E.; Mire, C.E.; Geisbert, T.W.; Ünlü, M.S.; et al. Disposable cartridge platform for rapid detection of viral hemorrhagic fever viruses. Lab Chip 2017, 17, 917–925. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital detection of exosomes by interferometric imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef]
- Monroe, M.R.; Daaboul, G.G.; Tuysuzoglu, A.; Lopez, C.A.; Little, F.F.; Ünlü, M.S. Single nanoparticle detection for multiplexed protein diagnostics with attomolar sensitivity in serum and unprocessed whole blood. Anal. Chem. 2013, 85, 3698–3706. [Google Scholar] [CrossRef]
- Sevenler, D.; Daaboul, G.G.; Ekiz Kanik, F.; Ünlü, N.L.; Ünlü, M.S. Digital Microarrays: Single-Molecule Readout with Interferometric Detection of Plasmonic Nanorod Labels. ACS Nano 2018, 12, 5880–5887. [Google Scholar] [CrossRef]
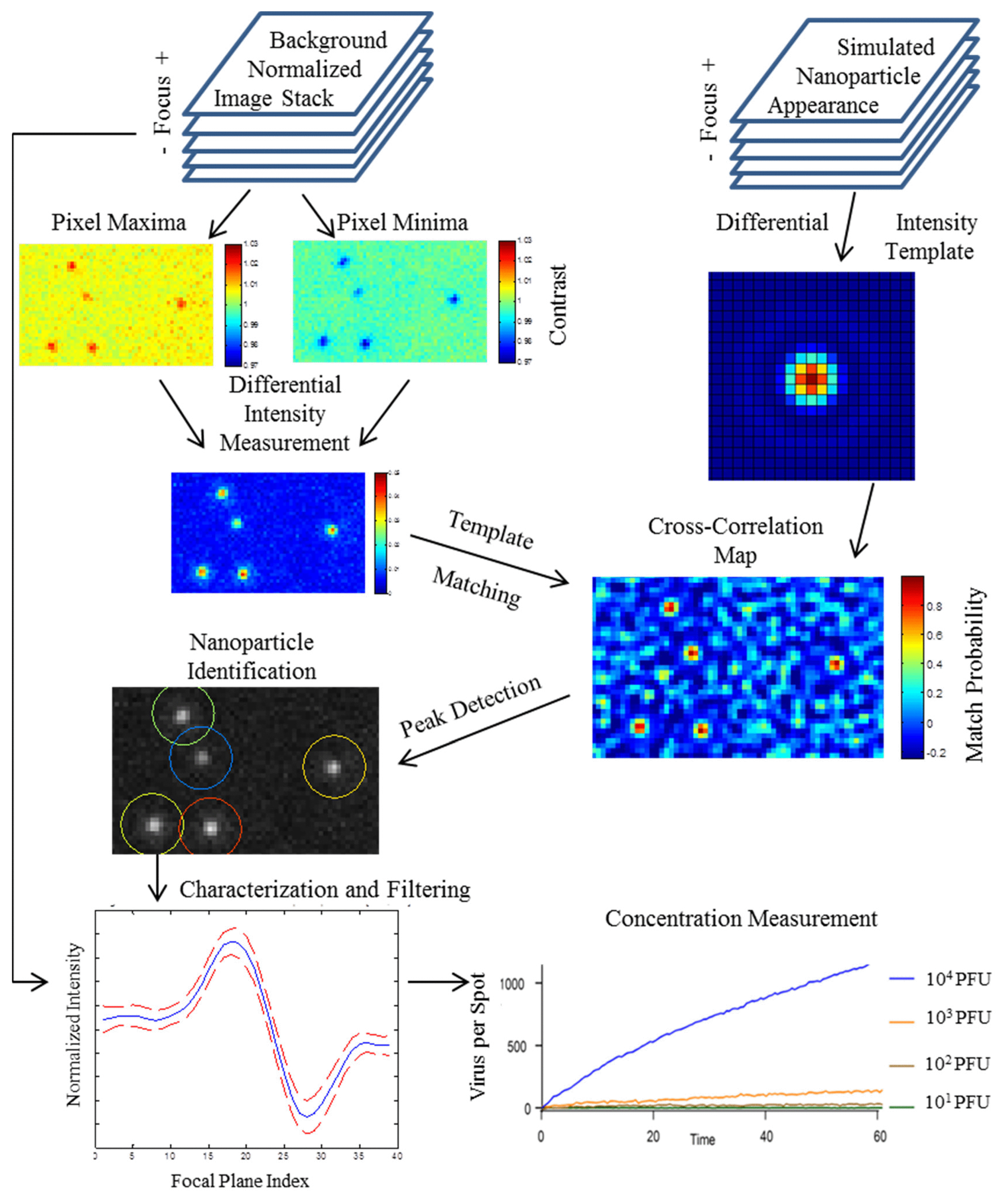
- Trueb, J.T.; Avci, O.; Sevenler, D.; Connor, J.H.; Ünlü, M.S. Robust visualization and discrimination of nanoparticles by interferometric imaging. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 394–403. [Google Scholar] [CrossRef]
- Avci, O.; Yurdakul, C.; Ünlü, M.S. Nanoparticle classification in wide-field interferometric microscopy by supervised learning from model. Appl. Opt. 2017, 56, 4238–4242. [Google Scholar] [CrossRef]
- Cakmak, O.; Ermek, E.; Kilinc, N.; Bulut, S.; Baris, I.; Kavakli, I.; Yaralioglu, G.; Urey, H. A cartridge based sensor array platform for multiple coagulation measurements from plasma. Lab Chip 2015, 15, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Ermek, E.; Kilinc, N.; Yaralioglu, G.; Urey, H. Precision density and viscosity measurement using two cantilevers with different widths. Sens. Actuators A Phys. 2015, 232, 141–147. [Google Scholar] [CrossRef]
- Chen, D.; Song, S.; Ma, J.; Zhang, Z.; Wang, P.; Liu, W.; Guo, Q. Micro-electromechanical film bulk acoustic sensor for plasma and whole blood coagulation monitoring. Biosens. Bioelectron. 2017, 91, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Abdolvand, R. Resonant micro-sensor platform for contact-free characterization of liquid properties. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 132–135. [Google Scholar]
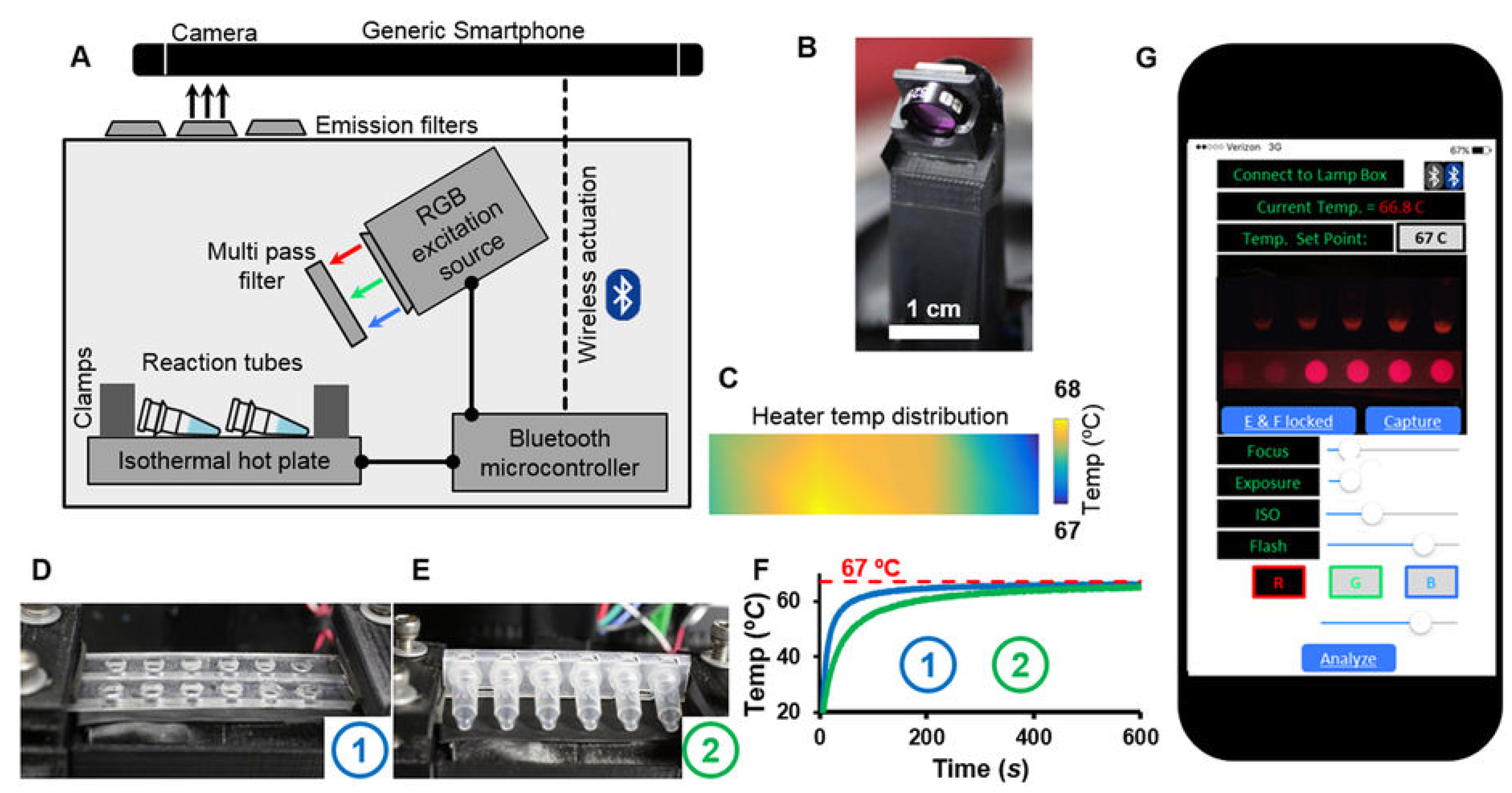
- Liao, S.C.; Peng, J.; Mauk, M.G.; Awasthi, S.; Song, J.; Friedman, H.; Bau, H.H.; Liu, C. Smart cup: A minimally-instrumented, smartphone-based point-of-care molecular diagnostic device. Sens. Actuators B Chem. 2016, 229, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Anderson, J.A.; Evans, R.; Woo, K.; Beland, B.; Sasseville, D.; Moreau, L. Point-of-care wound visioning technology: Reproducibility and accuracy of a wound measurement app. PLoS ONE 2017, 12, e0183139. [Google Scholar] [CrossRef]
- Larson, E.C.; Goel, M.; Boriello, G.; Heltshe, S.; Rosenfeld, M.; Patel, S.N. SpiroSmart: Using a microphone to measure lung function on a mobile phone. In Proceedings of the 2012 ACM Conference on Ubiquitous Computing, Raleigh, NC, USA, 16–18 October 2012; pp. 280–289. [Google Scholar]
- Goel, M.; Saba, E.; Stiber, M.; Whitmire, E.; Fromm, J.; Larson, E.C.; Borriello, G.; Patel, S.N. Spirocall: Measuring lung function over a phone call. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; pp. 5675–5685. [Google Scholar]
- Priye, A.; Bird, S.W.; Light, Y.K.; Ball, C.S.; Negrete, O.A.; Meagher, R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep. 2017, 7, 44778. [Google Scholar] [CrossRef]
- Chan, K.; Weaver, S.C.; Wong, P.Y.; Lie, S.; Wang, E.; Guerbois, M.; Vayugundla, S.P.; Wong, S. Rapid, affordable and portable medium-throughput molecular device for Zika virus. Sci. Rep. 2016, 6, 38223. [Google Scholar] [CrossRef]
- Priye, A.; Wong, S.; Bi, Y.; Carpio, M.; Chang, J.; Coen, M.; Cope, D.; Harris, J.; Johnson, J.; Keller, A.; et al. Lab-on-a-drone: Toward pinpoint deployment of smartphone-enabled nucleic acid-based diagnostics for mobile health care. Anal. Chem. 2016, 88, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Laksanasopin, T.; Guo, T.W.; Nayak, S.; Sridhara, A.A.; Xie, S.; Olowookere, O.O.; Cadinu, P.; Meng, F.; Chee, N.H.; Kim, J.; et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7, 273re1. [Google Scholar] [CrossRef]
- Guan, L.; Tian, J.; Cao, R.; Li, M.; Cai, Z.; Shen, W. Barcode-like paper sensor for smartphone diagnostics: An application of blood typing. Anal. Chem. 2014, 86, 11362–11367. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Su, Y.; Chen, H. Recent Progress in Optical Biosensors Based on Smartphone Platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef] [PubMed]
- Pilavaki, E.; Demosthenous, A. Optimized Lateral Flow Immunoassay Reader for the Detection of Infectious Diseases in Developing Countries. Sensors 2017, 17, 2673. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Jing, W.; Liu, S.; Zhang, D.; Sui, G. First airborne pathogen direct analysis system. Analyst 2016, 141, 1637–1640. [Google Scholar] [CrossRef]
- Girod, R.; Guidez, A.; Carinci, R.; Issaly, J.; Gaborit, P.; Ferrero, E.; Ardillon, V.; Fontaine, A.; Dusfour, I.; Briolant, S. Detection of Chikungunya virus circulation using sugar-baited traps during a major outbreak in French Guiana. PLoS Negl. Trop. Dis. 2016, 10, e0004876. [Google Scholar] [CrossRef][Green Version]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Srinivasan, B.; O’Dell, D.; Finkelstein, J.L.; Lee, S.; Erickson, D.; Mehta, S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef]
- Long, K.D.; Woodburn, E.V.; Le, H.M.; Shah, U.K.; Lumetta, S.S.; Cunningham, B.T. Multimode smartphone biosensing: The transmission, reflection, and intensity spectral (TRI)-analyzer. Lab Chip 2017, 17, 3246–3257. [Google Scholar] [CrossRef]
- Punter-Villagrasa, J.; Colomer-Farrarons, J.; del Campo, F.J.; Miribel, P. Amperometric and Impedance Monitoring Systems for Biomedical Applications; Springer: Berlin, Germany, 2017; Volume 4. [Google Scholar]
- Xia, Y. Soft Lithography: Micro-and Nanofabrication Based on Microcontact Printing and Replica Molding; Harvard University: Cambridge, MA, USA, 1997. [Google Scholar]
- Sharma, S.; El-Laboudi, A.; Reddy, M.; Jugnee, N.; Sivasubramanyam, S.; el Sharkawy, M.; Georgiou, P.; Johnston, D.; Oliver, N.; Cass, A.E. A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring. Anal. Methods 2018, 10, 2088–2095. [Google Scholar] [CrossRef]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.F.; Castro, C.M.; Swirski, F.K.; Weissleder, R.; et al. Integrated Biosensor for Rapid and Point-Of-Care Sepsis Diagnosis. ACS Nano 2018, 12, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Erguri, A.; Huang, Y.; Zhuang, X.; Oralkan, O.; Yarahoglu, G.G.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers: Fabrication technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2242–2258. [Google Scholar] [CrossRef]
- Friedman, B.; Jiang, H.J.; Elixhauser, A.; Segal, A. Hospital inpatient costs for adults with multiple chronic conditions. Med. Care Res. Rev. 2006, 63, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Amatayakul, M.K.; Lazarus, S.S. Electronic Health Records: Transforming Your Medical Practice; Medical Group Management Assn: Washington, DC, USA, 2005. [Google Scholar]
- Sinha, P.K.; Sunder, G.; Bendale, P.; Mantri, M.; Dande, A. Electronic Health Record: Standards, Coding Systems, Frameworks, and Infrastructures; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bishop, C.M. Pattern Recognition and Machine Learning, 5th ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Delgado, M.F.; Cernadas, E.; Barro, S.; Amorim, D.G. Do we need hundreds of classifiers to solve real world classification problems? J. Mach. Learn. Res. 2014, 15, 3133–3181. [Google Scholar]
- Zhou, Z.H. Ensemble Methods: Foundations and Algorithms; CRC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kuncheva, L.I. Combining Pattern Classifiers: Methods and Algorithms, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Criminisi, A.; Shotton, J. (Eds.) Decision Forests for Computer Vision and Medical Image Analysis; Springer: Berlin, Germany, 2013. [Google Scholar]
- Shotton, J.; Sharp, T.; Kipman, A.; Fitzgibbon, A.W.; Finocchio, M.; Blake, A.; Cook, M.; Moore, R. Real-time human pose recognition in parts from single depth images. Commun. ACM 2013, 56, 116–124. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed]
- Bengio, Y.L.Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; The MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Gu, J.; Wang, Z.; Kuen, J.; Ma, L.; Shahroudy, A.; Shuai, B.; Liu, T.; Wang, X.; Wang, G.; Cai, J.; Chen, T. Recent advances in convolutional neural networks. Pattern Recognit. 2018, 77, 354–377. [Google Scholar] [CrossRef]
- Aghdam, H.H.; Heravi, E.J. Guide to Convolutional Neural Networks: A Practical Application to Traffic-Sign Detection and Classification; Springer: Berlin, Germany, 2017. [Google Scholar]
- Lu, L.; Zheng, Y.; Carneiro, G.; Yang, L. (Eds.) Deep Learning and Convolutional Neural Networks for Medical Image Computing: Precision Medicine, High Performance and Large-Scale Datasets; Springer: Berlin, Germany, 2017. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, J. Deep forest: Towards an alternative to deep neural networks. In Proceedings of the 26th International Joint Conference on Artificial Intelligence, Melbourne, Australia, 19–25 August 2017; pp. 3553–3559. [Google Scholar]
- Bloice, M.D.; Holzinger, A. A Tutorial on Machine Learning and Data Science Tools with Python. In Machine Learning for Health Informatics; Lecture Notes in Artificial Intelligence LNAI 9605; Holzinger, A., Ed.; Springer: Berlin, Germany, 2016; pp. 437–483. [Google Scholar]
- Bengio, Y. Practical recommendations for gradient-based training of deep architectures. In Neural Networks: Tricks of the Trade, 2nd ed.; Montavon, G., Orr, G.B., Müller, K., Eds.; Springer: Berlin, Germany, 2012; pp. 437–478. [Google Scholar]
- Klemm, S.; Scherzinger, A.; Drees, D.; Jiang, X. Barista—A graphical tool for designing and training deep neural networks. arXiv preprint 2018, arXiv:1802.04626. [Google Scholar]
- O’Sullivan, S.; Holzinger, A.; Zatloukal, K.; Saldiva, P.; Sajid, M.I.; Wichmann, D. Machine learning enhanced virtual autopsy. Autopsy Case Rep. 2017, 7, 3. [Google Scholar] [CrossRef]
- Manguvo, A.; Mafuvadze, B. The impact of traditional and religious practices on the spread of Ebola in West Africa: Time for a strategic shift. Pan Afr. Med. J. 2015, 22, 9. [Google Scholar]
- Marshall, K.; Smith, S. Religion and Ebola: Learning from experience. Lancet 2015, 386, e24–e25. [Google Scholar] [CrossRef]
- Holzinger, A.; Kieseberg, P.; Weippl, E.; Tjoa, A.M. Current Advances, Trends and Challenges of Machine Learning and Knowledge Extraction: From Machine Learning to Explainable AI. In Springer Lecture Notes in Computer Science LNCS 11015; Springer: Berlin, Germany, 2018; pp. 1–8. [Google Scholar]
- Lim, S.; Tucker, C.S.; Kumara, S. An unsupervised machine learning model for discovering latent infectious diseases using social media data. J. Biomed. Inform. 2017, 66, 82–94. [Google Scholar] [CrossRef]
- Petz, G.; Karpowicz, M.; Fürschuß, H.; Auinger, A.; Stříteskỳ, V.; Holzinger, A. Reprint of: Computational approaches for mining user’s opinions on the Web 2.0. Inf. Process. Manag. 2015, 51, 510–519. [Google Scholar] [CrossRef]
- Gawehn, E.; Hiss, J.A.; Schneider, G. Deep learning in drug discovery. Mol. Inform. 2016, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Plass, M.; Holzinger, K.; Crisan, G.C.; Pintea, C.M.; Palade, V. Towards interactive Machine Learning (iML): Applying Ant Colony Algorithms to solve the Traveling Salesman Problem with the Human-in-the-Loop approach. In Springer Lecture Notes in Computer Science LNCS 9817; Springer: Berlin, Germany, 2016; pp. 81–95. [Google Scholar]
- Holzinger, A.; Biemann, C.; Pattichis, C.S.; Kell, D.B. What do we need to build explainable AI systems for the medical domain? arXiv 2017, arXiv:1712.09923. [Google Scholar]
- Turkay, C.; Jeanquartier, F.; Holzinger, A.; Hauser, H. On Computationally-enhanced Visual Analysis of Heterogeneous Data and its Application in Biomedical Informatics. In Interactive Knowledge Discovery and Data Mining: State-of-the-Art and Future Challenges in Biomedical Informatics; Lecture Notes in Computer Science LNCS 8401; Holzinger, A., Jurisica, I., Eds.; Springer: Berlin, Germany, 2014; pp. 117–140. [Google Scholar]
- Brownstein, J.S.; Freifeld, C.C.; Madoff, L.C. Digital disease detection—harnessing the Web for public health surveillance. N. Engl. J. Med. 2009, 360, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Malle, B.; Giuliani, N.; Kieseberg, P.; Holzinger, A. The more the merrier: Federated learning from local sphere recommendations. In International Cross-Domain Conference for Machine Learning and Knowledge Extraction; Springer: Berlin, Germany, 2017; pp. 367–373. [Google Scholar]
- Schieppati, A.; Henter, J.I.; Daina, E.; Aperia, A. Why rare diseases are an important medical and social issue. Lancet 2008, 371, 2039–2041. [Google Scholar] [CrossRef]
- Japkowicz, N.; Stephen, S. The class imbalance problem: A systematic study. Intell. Data Anal. 2002, 6, 429–449. [Google Scholar] [CrossRef]
- Batista, G.E.; Prati, R.C.; Monard, M.C. A study of the behavior of several methods for balancing machine learning training data. ACM SIGKDD Explor. Newslett. 2004, 6, 20–29. [Google Scholar] [CrossRef]
- Bengio, Y. Deep Learning of Representations: Looking Forward. In Statistical Language and Speech Processing, Proceedings of the First International Conference, SLSP 2013, Tarragona, Spain, 29–31 July 2013; Dediu, A.H., Martín-Vide, C., Mitkov, R., Truthe, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–37. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115. [Google Scholar] [CrossRef]
- Bologna, G.; Hayashi, Y. Characterization of Symbolic Rules Embedded in Deep DIMLP Networks: A Challenge to Transparency of Deep Learning. J. Artif. Intell. Soft Comput. Res. 2017, 7, 265–286. [Google Scholar] [CrossRef]
- Holzinger, A. Interactive machine learning for health informatics: When do we need the human-in-the-loop? Brain Inform. 2016, 3, 119–131. [Google Scholar] [CrossRef]
- Holzinger, A.; Plass, M.; Kickmeier-Rust, M.; Holzinger, K.; Crişan, G.C.; Pintea, C.M.; Palade, V. Interactive machine learning: Experimental evidence for the human in the algorithmic loop. Appl. Intell. 2018, 1–14. [Google Scholar] [CrossRef]
- Chakoumakos, R. Predicting Outbreak Severity through Machine Learning on Disease Outbreak Reports; Stanford University: Stanford, CA, USA, 2010. [Google Scholar]
- Chen, M.; Hao, Y.; Hwang, K.; Wang, L.; Wang, L. Disease prediction by machine learning over big data from healthcare communities. IEEE Access 2017, 5, 8869–8879. [Google Scholar] [CrossRef]
- Pineda, A.L.; Ye, Y.; Visweswaran, S.; Cooper, G.F.; Wagner, M.M.; Tsui, F.R. Comparison of machine learning classifiers for influenza detection from emergency department free-text reports. J. Biomed. Inform. 2015, 58, 60–69. [Google Scholar] [CrossRef]
- Libbrecht, M.W.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.G.; Gabuzda, D. A machine learning approach for identifying amino acid signatures in the HIV env gene predictive of dementia. PLoS ONE 2012, 7, e49538. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, Y.; Liu, X.; Xu, H.; Han, Z.; Rong, H.; Yang, H.; Yan, M.; Yu, H. Machine learning based single-frame super-resolution processing for lensless blood cell counting. Sensors 2016, 16, 1836. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, N.; Kovalik, T.; Lorenzini, I.; Spangler, S.; Lacoste, A.; Sponaugle, K.; Ferrante, P.; Argentinis, E.; Sattler, R.; Bowser, R. Artificial intelligence in neurodegenerative disease research: Use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathol. 2018, 135, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Makkapati, V.V.; Rao, R.M. Segmentation of malaria parasites in peripheral blood smear images. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Taipei, Taiwan, 19–24 April 2009; pp. 1361–1364. [Google Scholar]
- Liao, Y.; Xu, B.; Wang, J.; Liu, X. A new method for assessing the risk of infectious disease outbreak. Sci. Rep. 2017, 7, 40084. [Google Scholar] [CrossRef] [PubMed]
- Yach, D.; Hawkes, C.; Gould, C.L.; Hofman, K.J. The global burden of chronic diseases: Overcoming impediments to prevention and control. JAMA 2004, 291, 2616–2622. [Google Scholar] [CrossRef]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef]
- Romao, V.C.; Martins, S.A.; Germano, J.; Cardoso, F.A.; Cardoso, S.; Freitas, P.P. Lab-on-Chip Devices: Gaining Ground Losing Size. ACS Nano 2017, 11, 10659–10664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, Y.; Wang, C.; Liu, W.; Wang, Z.; Xia, Z.; Bu, Z.; Lu, H.; Sun, Y.; Zhang, X.; et al. Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic. PLoS Negl. Trop. Dis. 2017, 11, e0005622. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee on Effectiveness of National Biosurveillance Systems. BioWatch and Public Health Surveillance: Evaluating Systems for the Early Detection of Biological Threats: Abbreviated Version; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Coelho, B.; Veigas, B.; Fortunato, E.; Martins, R.; Águas, H.; Igreja, R.; Baptista, P.V. Digital Microfluidics for Nucleic Acid Amplification. Sensors 2017, 17, 1495. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.C.; Spoto, G. Integration of isothermal amplification methods in microfluidic devices: Recent advances. Biosens. Bioelectron. 2017, 90, 174–186. [Google Scholar] [CrossRef]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Son, R.; Chee, H.Y. Loop Mediated Isothermal Amplification (LAMP): A Versatile Technique for Detection of Microorganisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Rozand, C. Based analytical devices for point-of-care infectious disease testing. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafiah, K.; Arifin, N.; Bustami, Y.; Noordin, R.; Garcia, M.; Anderson, D. Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities. Diagnostics 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Hunt, W.D. Potential of surface acoustic wave biosensors for early sepsis diagnosis. J. Clin. Monit. Comput. 2013, 27, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Schoepp, N.G.; Schlappi, T.S.; Curtis, M.S.; Butkovich, S.S.; Miller, S.; Humphries, R.M.; Ismagilov, R.F. Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci. Transl. Med. 2017, 9, eaal3693. [Google Scholar] [CrossRef]
- Oehler, R.L.; Smith, K.; Toney, J.F. Infectious diseases resources for the iPhone. Clin. Infect. Dis. 2010, 50, 1268–1274. [Google Scholar] [CrossRef]
- Chan, T.C.; Teng, Y.C.; Kuo, C.l.; Yeh, Y.H.; Lin, B.C. Leveraging the Niche of Open Data for Disease Surveillance and Health Education. Online J. Public Health Inform. 2017, 9, e043. [Google Scholar] [CrossRef]
- Ahrberg, C.D.; Ilic, B.R.; Manz, A.; Neužil, P. Handheld real-time PCR device. Lab Chip 2016, 16, 586–592. [Google Scholar] [CrossRef]
- Raja, S.; Ching, J.; Xi, L.; Hughes, S.J.; Chang, R.; Wong, W.; McMillan, W.; Gooding, W.E.; McCarty, K.S.; Chestney, M.; et al. Technology for automated, rapid, and quantitative PCR or reverse transcription-PCR clinical testing. Clin. Chem. 2005, 51, 882–890. [Google Scholar] [CrossRef]
- Asiello, P.J.; Baeumner, A.J. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 2011, 11, 1420–1430. [Google Scholar] [CrossRef]
- Gilchrist, C.A.; Turner, S.D.; Riley, M.F.; Petri, W.A.; Hewlett, E.L. Whole-genome sequencing in outbreak analysis. Clin. Microbiol. Rev. 2015, 28, 541–563. [Google Scholar] [CrossRef]
- Rosenstein, J.K.; Wanunu, M.; Merchant, C.A.; Drndic, M.; Shepard, K.L. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat. Methods 2012, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.; Raangs, E.C.; Rosema, S.; Veloo, A.C.; et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Rosenstein, J.K.; Ramakrishnan, S.; Roseman, J.; Shepard, K.L. Single ion channel recordings with CMOS-anchored lipid membranes. Nano Lett. 2013, 13, 2682–2686. [Google Scholar] [CrossRef]
- Magierowski, S.; Huang, Y.; Wang, C.; Ghafar-Zadeh, E. Nanopore-CMOS interfaces for DNA sequencing. Biosensors 2016, 6, 42. [Google Scholar] [CrossRef]
- Quick, J.; Loman, N.J.; Duraffour, S.; Simpson, J.T.; Severi, E.; Cowley, L.; Bore, J.A.; Koundouno, R.; Dudas, G.; Mikhail, A.; et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 2016, 530, 228. [Google Scholar] [CrossRef]
- Schneider, C.; Babcock, K.; Harris, P.; Khera, K.; Strenn, S.; Markakis, S. Rapid Antibiotic Susceptibility Tests by Mass Measurement on a 96-Well Plate. In Proceedings of the 2016 ASM Microbe, Boston, MA, USA, 16–20 June 2016. [Google Scholar]
- Burg, T.; Godin, M.; Knudsen, S.; Shen, W.; Carlson, G.; Foster, J.; Babcock, K.; Manalis, S. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 2007, 446, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Susceptibility Testing; GeneFluidics, Inc.: Duarte, CA, USA, 2019.
- Andreyev, B.; Moravick, K.; Ciopyk, B.; Briones, V.; Loney, G.; Zerda, A.D.L.; Ching, J.; Kelly, C.; Chu, S.; Swenson, D.; et al. Devices and Methods for Molecular Diagnostic Testing. U.S. Pantent 9623415B2, 18 April 2017. [Google Scholar]
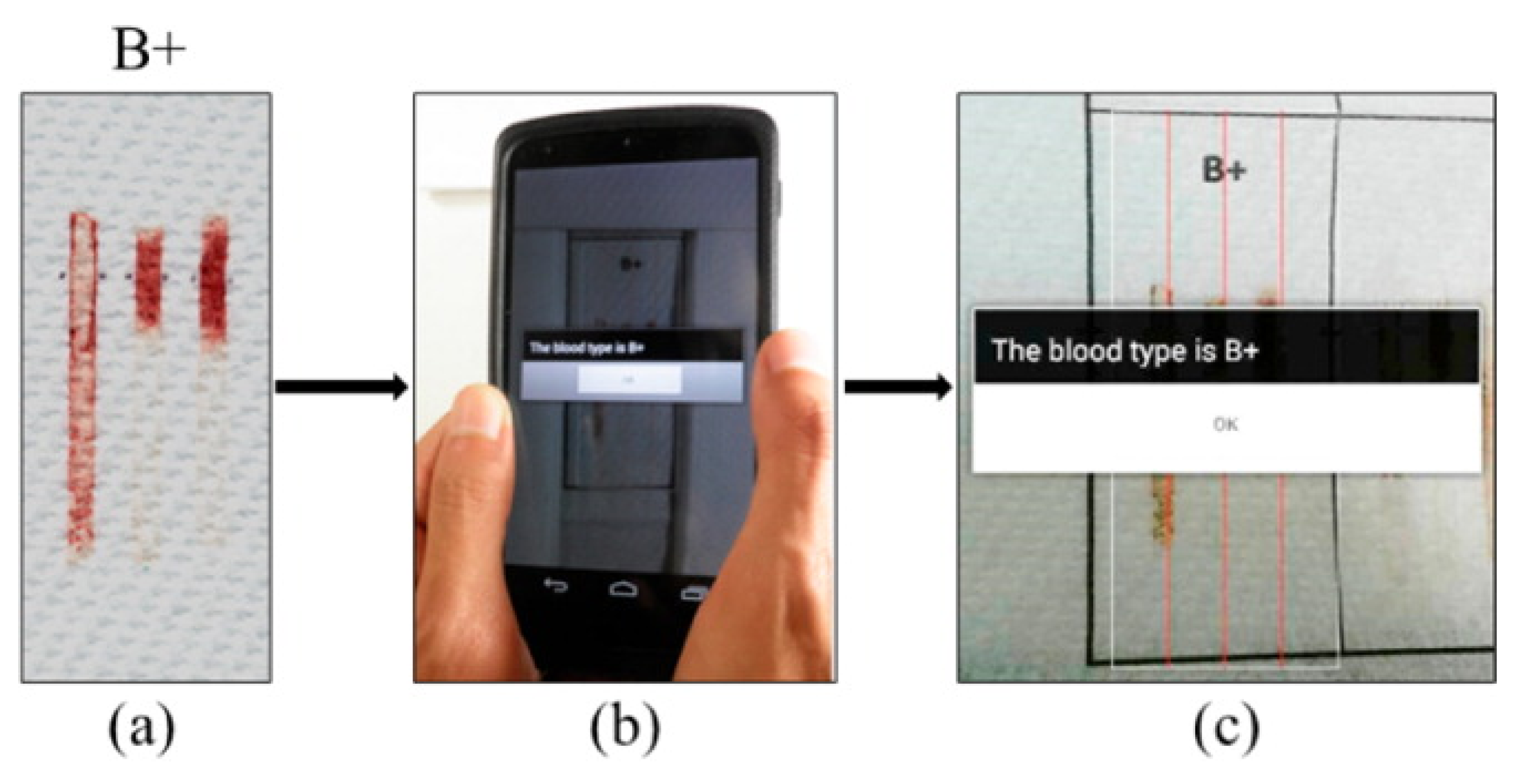
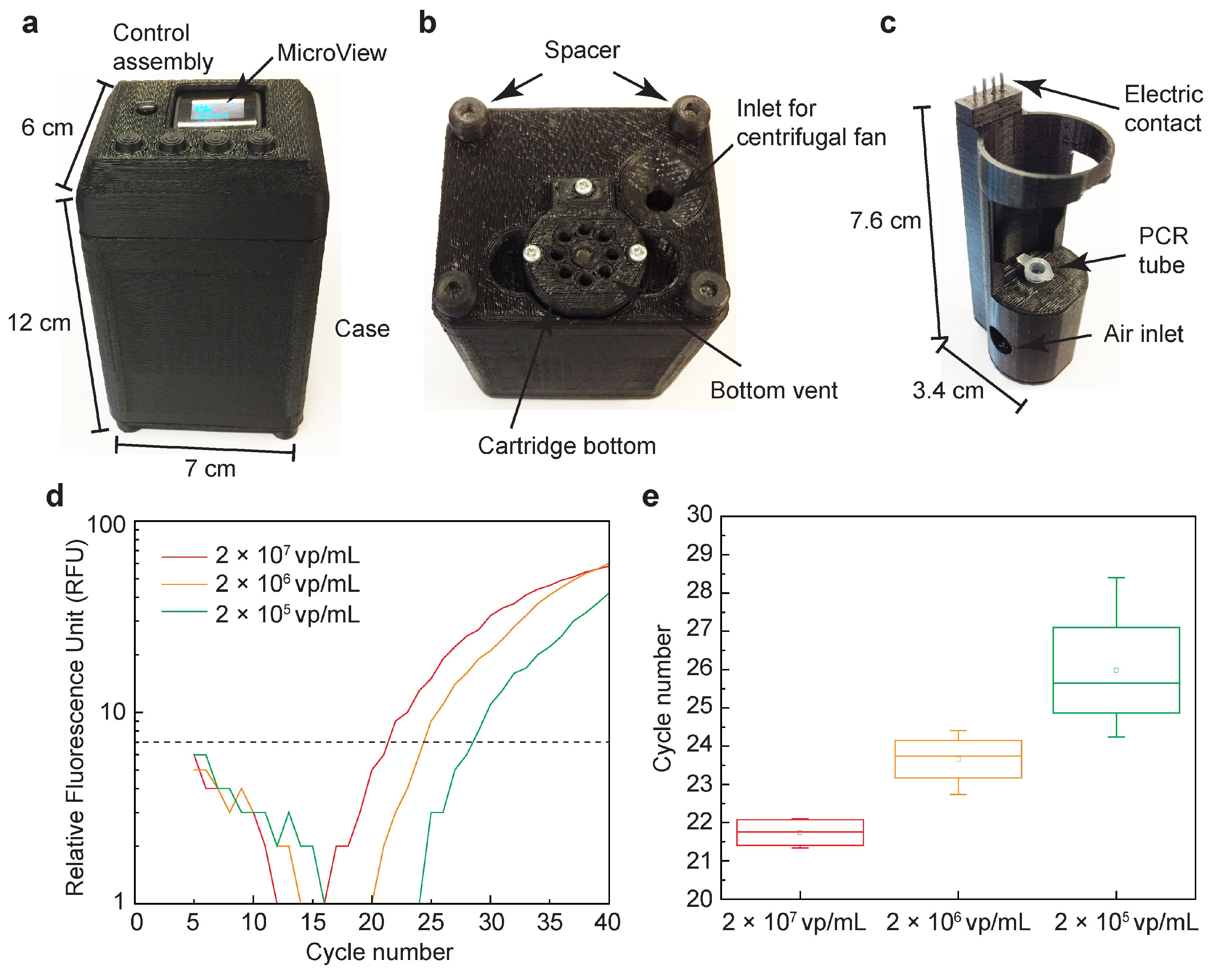

| Cancer Biomarkers | Biomarker | Normal Values in Blood |
|---|---|---|
| PSA | 4 ng/mL | |
| IL-6 | 6 pg/mL | |
| IL-8 | 13–20 pg/mL | |
| MMP-2 | 367–770 ng/mL | |
| MMP-3 | 15–72 ng/mL | |
| Alpha-fetoprotein | <20 ng/mL | |
| CEA | 5 ng/mL | |
| CA-125 | 35 U/mL | |
| Cardiac Biomarkers | CRP | 3 mg/mL |
| NT-proBNP | 1 ng/mL | |
| CTnT | 0.3 ng/mL | |
| CTnl | 0.01–0.1 ng/mL | |
| Myoglobin | 50–100 ng/mL |
| Title | Analytic Approach | Sample Type | Result and Time | Format |
|---|---|---|---|---|
| Ultra-Rapid Infection Confirmation and Phenotypical AST by Microbe Mass Measurement [http://www.lifescaleinstruments.com/ https://patents.google.com/patent/US20150072373A1/en] | Microfluidic, mass detection of bacterial growth | blood culture, urine culture demonstrated (extension to urine screen, cerebral spinal fluid, pleural fluid planned) | Phenotypic antibiotic resistance (minimum inhibitory concentration) Time to result: 3–3.5 h | desktop device |
| Single Cell Biometric Analysis for Rapid ID/AST [http://klarisdx.com/ https://patents.google.com/patent/US20180172675A1/en] | Microfluidic partitioning of single cells; detection of phenotypic antimicrobial susceptibility with redox-sensitive viability dye | not specified | Phenotypic antibiotic resistance, pathogen ID Time to result: 4 h | desktop device |
| Fully Automated Pathogen ID and AST Directly from Blood and Urine [http://www.genefluidics-lifescience.com/] | Electrochemical sandwich hybridization of 16S ribosomal RNA | unprocessed urine demonstrated, (whole blood in development) | Pathogen genus/species by 16S homology Time to result: 30 min for pathogen ID, 90 min for resistance profile (for urine) | desktop device with disposable sensor array chip |
| Patient-side, Disposable, Molecular PCR Diagnostic Device for Neisseria Gonorrhea and Drug Resistance Markers [https://www.sbir.gov/sbirsearch/detail/1323659 https://patents.google.com/patent/US9623415B2/en] | Miniaturised PCR | genital tract swab | Pathogen ID, (ciprofloxacin resistance in development) Time to result: 25 min | single-use, disposable POC device |
| Host Gene Expression to Classify Viral and Bacterial Infection Using Rapid Multiplex PCR [https://www.predigen.com/] Journal of Clinical Microbiology Jan 2010, 48 (1) 26-33; DOI: 10.1128/JCM.01447-09 | PCR of host gene expression patterns | blood | confirmation of viral-type host response pattern, determination of viral/bacterial co-infection Time to result: 45 min | desktop/multiplex PCR equipment |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, S.; Ali, Z.; Jiang, X.; Abdolvand, R.; Ünlü, M.S.; Plácido da Silva, H.; Baca, J.T.; Kim, B.; Scott, S.; Sajid, M.I.; et al. Developments in Transduction, Connectivity and AI/Machine Learning for Point-of-Care Testing. Sensors 2019, 19, 1917. https://doi.org/10.3390/s19081917
O’Sullivan S, Ali Z, Jiang X, Abdolvand R, Ünlü MS, Plácido da Silva H, Baca JT, Kim B, Scott S, Sajid MI, et al. Developments in Transduction, Connectivity and AI/Machine Learning for Point-of-Care Testing. Sensors. 2019; 19(8):1917. https://doi.org/10.3390/s19081917
Chicago/Turabian StyleO’Sullivan, Shane, Zulfiqur Ali, Xiaoyi Jiang, Reza Abdolvand, M Selim Ünlü, Hugo Plácido da Silva, Justin T. Baca, Brian Kim, Simon Scott, Mohammed Imran Sajid, and et al. 2019. "Developments in Transduction, Connectivity and AI/Machine Learning for Point-of-Care Testing" Sensors 19, no. 8: 1917. https://doi.org/10.3390/s19081917
APA StyleO’Sullivan, S., Ali, Z., Jiang, X., Abdolvand, R., Ünlü, M. S., Plácido da Silva, H., Baca, J. T., Kim, B., Scott, S., Sajid, M. I., Moradian, S., Mansoorzare, H., & Holzinger, A. (2019). Developments in Transduction, Connectivity and AI/Machine Learning for Point-of-Care Testing. Sensors, 19(8), 1917. https://doi.org/10.3390/s19081917










