Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mouse Sperm
2.2. Electrical Measurement with ISFET Sensor
3. Results
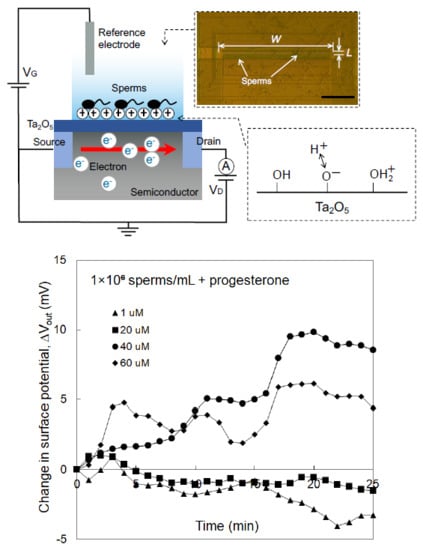
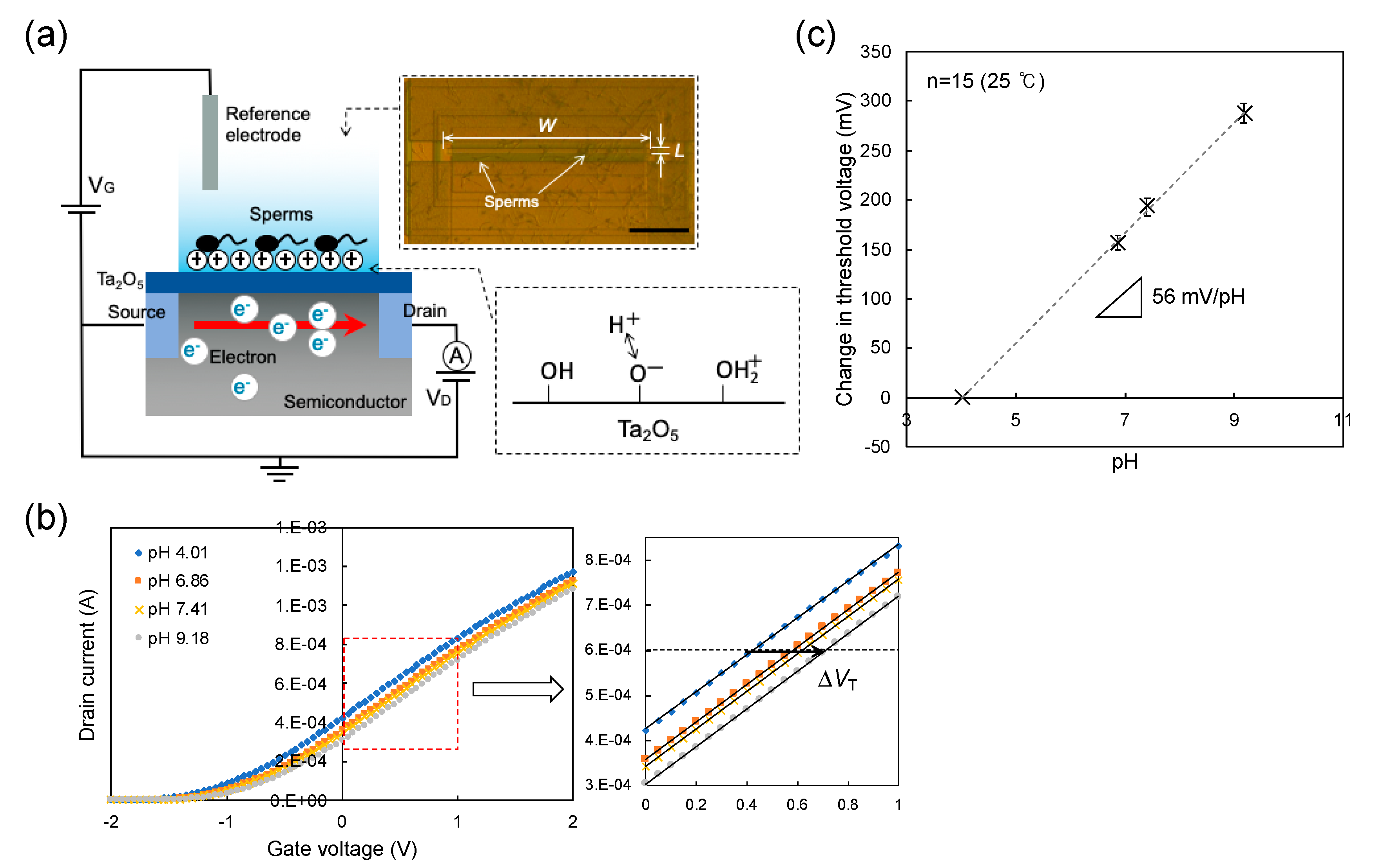
3.1. Concept of Sperm-Cultured Gate ISFET Sensor
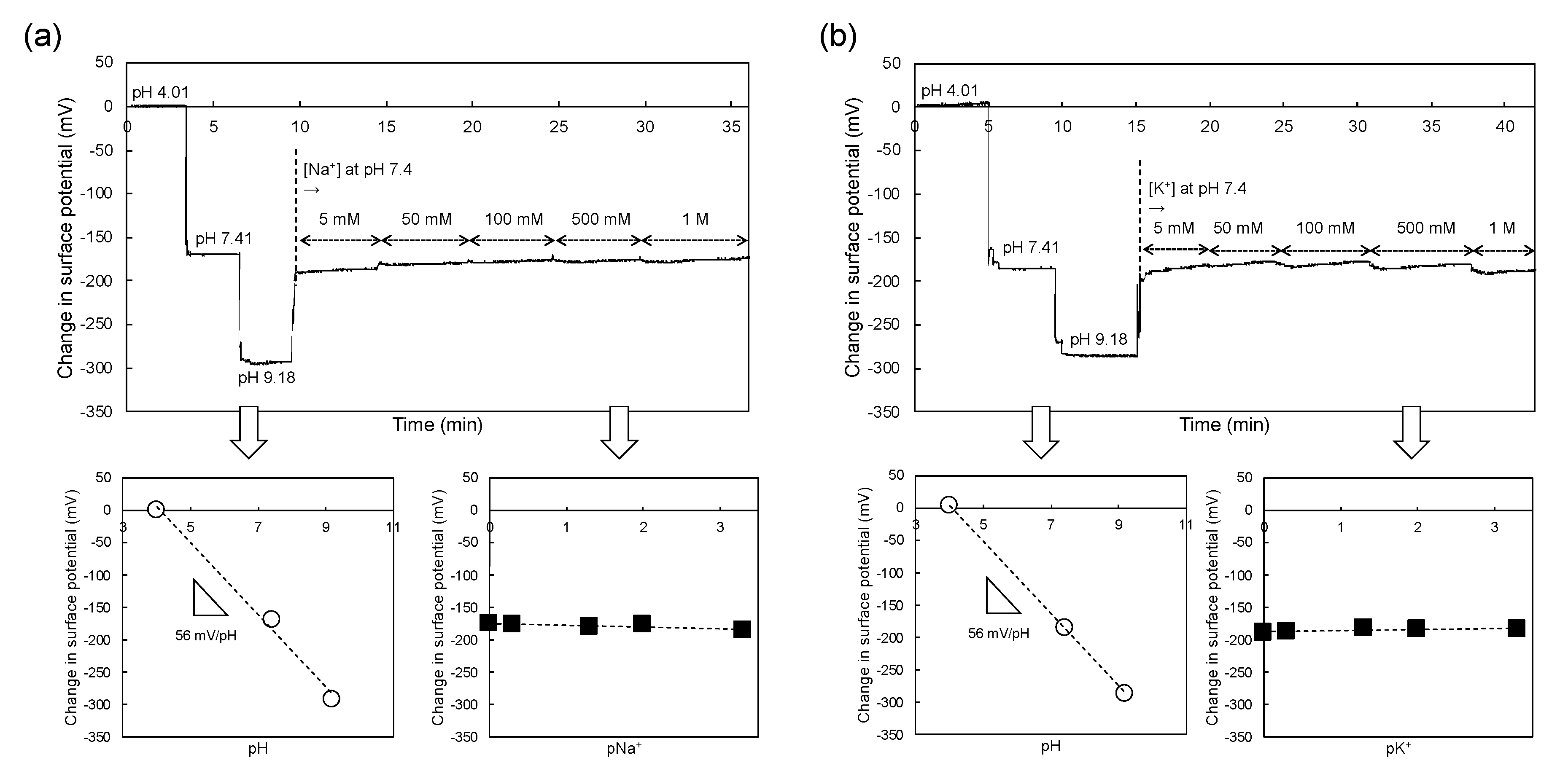
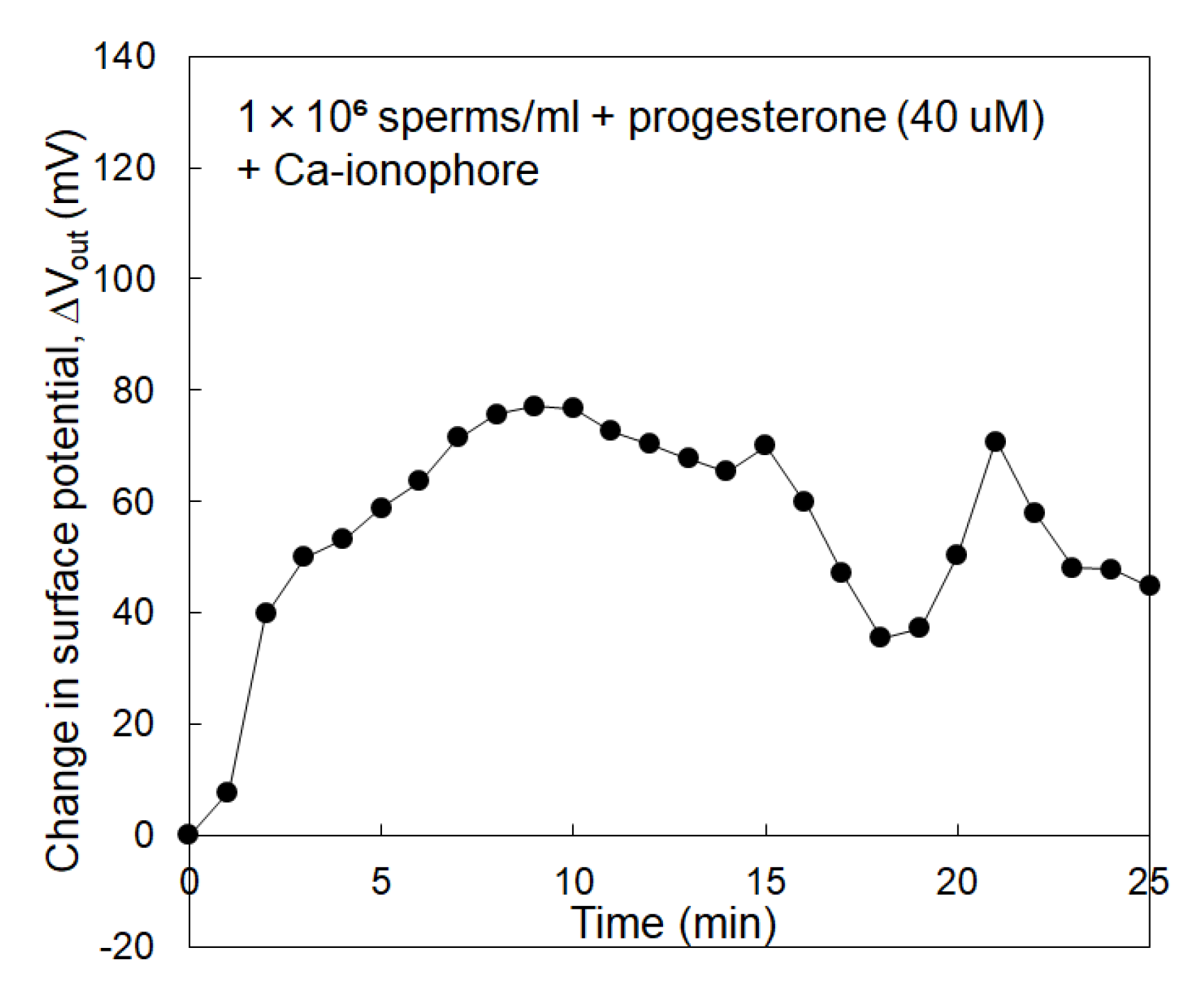
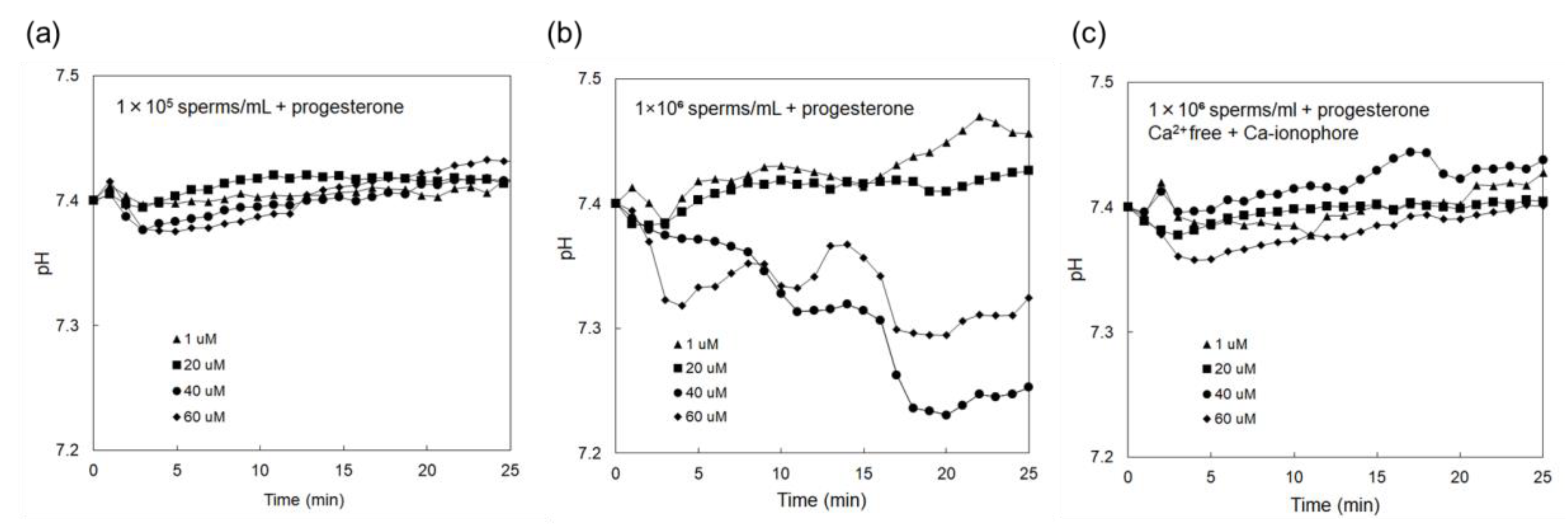
3.2. Electrical Monitoring of Sperm Respiration with Sperm-Cultured Gate ISFET Sensor: Effect of Progesterone and Ca2+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uhler, M.L.; Leung, A.; Chan, S.Y.; Wang, C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil. Steril. 1992, 58, 1191–1198. [Google Scholar] [CrossRef]
- Roldan, E.R.; Murase, T.; Shi, Q.X. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science 1994, 266, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Massobrio, M.; Tesarik, J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev. 1998, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals – an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef]
- Teves, M.E.; Barbano, F.; Guidobaldi, H.A.; Sanchez, R.; Miska, W.; Giojalas, L.C. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil. Steril. 2006, 86, 745–749. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Beebe, S.J.; Danforth, D.R.; Alexander, N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J. Biol. Chem. 1990, 265, 1376–1380. [Google Scholar] [PubMed]
- Publicover, S.; Harper, C.V.; Barratt, C. [Ca2+]i signalling in sperm 2 making the most of what you’ve got. Nat. Cell Biol. 2007, 9, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.; Van, Q.; Weyand, I.; Hagen, V.; Beyermann, M.; Matsumoto, M.; Hoshi, M.; Hildebrand, E.; Kaupp, U.B. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 2005, 24, 2741–2752. [Google Scholar] [CrossRef]
- Nosrati, R.; Graham, P.J.; Zhang, B.; Riordon, J.; Lagunov, A.; Hannam, T.G.; Escobedo, C.; Jarvi, K.; Sinton, D. Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 2017, 14, 707–730. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Saito, A.; Mizuno, J.; Sugimoto, H.; Noguchi, K.; Kikuchi, E.; Inui, H. Single embryo-coupled gate field effect transistor for elective single embryo transfer. Anal. Chem. 2013, 85, 6633–6638. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Honda, M.; Akiko, A.; Kajisa, T.; Yanase, Y.; Sakata, T. Non-optical detection of allergic response with a cell-coupled gate field-effect transistor. Anal. Chem. 2017, 89, 12918–12923. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Saito, A.; Sakata, T. Elucidation of interfacial pH behaviour at cell/substrate nanogap for in situ monitoring of cellular respiration. Nanoscale 2018, 10, 10130–10136. [Google Scholar] [CrossRef]
- Sakata, T.; Saito, A.; Sugimoto, H. In situ measurement of autophagy under nutrient starvation based on interfacial pH sensing. Sci. Rep. 2018, 8, 8282. [Google Scholar] [CrossRef]
- Bergveld, P. Development, Operation and Application of the Tool for Electrophysiology. IEEE Trans. Biomed. Eng. 1972, BME-19, 342–351. [Google Scholar] [CrossRef]
- Esashi, M.; Matsuo, T. Integrated micro-multi-ion sensor using field effect of semiconductor. IEEE Trans. Biomed. Eng. 1978, BME-25, 184–192. [Google Scholar] [CrossRef]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef]
- Copenhaver, J.H.; Lardy, H.A. Oxidative phosphorylations: Pathways and yield in mitochondrial preparations. J. Biol. Chem. 1952, 195, 225–238. [Google Scholar] [PubMed]
- Goldberg, E. Lactic and malic dehydrogenases in human spermatozoa. Science 1963, 139, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.T.; Kayne, F.J. Energy metabolism of spermatozoa. V. The Embden-Meyerhof pathway of glycolysis: Activities of the pathway enzymes in hypotonically treated rabbit epididymal spermatozoa. Fertil. Steril. 1975, 26, 1257–1265. [Google Scholar] [CrossRef]
- McBride, P.T.; Janata, J.; Comte, P.A.; Moss, S.D.; Johnson, C.C. Ion-selective field effect transistors with polymeric membranes. Anal. Chim. Acta 1979, 108, 239–245. [Google Scholar] [CrossRef]
- Sakata, T.; Kamahori, M.; Miyahara, Y. DNA analysis chip based on field effect transistors. Jpn. J. Appl. Phys. 2005, 44, 2854–2859. [Google Scholar] [CrossRef]
- Matsui, T.; Nishiyama, I.; Hino, A.; Hoshi, M. Induction of the Acrosome Reaction in Starfish. Dev. Growth Differ. 1986, 28, 339–348. [Google Scholar] [CrossRef]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1994; Volume 1, pp. 189–317. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, A.; Sakata, T. Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation. Sensors 2019, 19, 1784. https://doi.org/10.3390/s19081784
Saito A, Sakata T. Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation. Sensors. 2019; 19(8):1784. https://doi.org/10.3390/s19081784
Chicago/Turabian StyleSaito, Akiko, and Toshiya Sakata. 2019. "Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation" Sensors 19, no. 8: 1784. https://doi.org/10.3390/s19081784
APA StyleSaito, A., & Sakata, T. (2019). Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Non-Optical and Live Monitoring of Sperm Capacitation. Sensors, 19(8), 1784. https://doi.org/10.3390/s19081784