Current Advancements in Transdermal Biosensing and Targeted Drug Delivery
Abstract
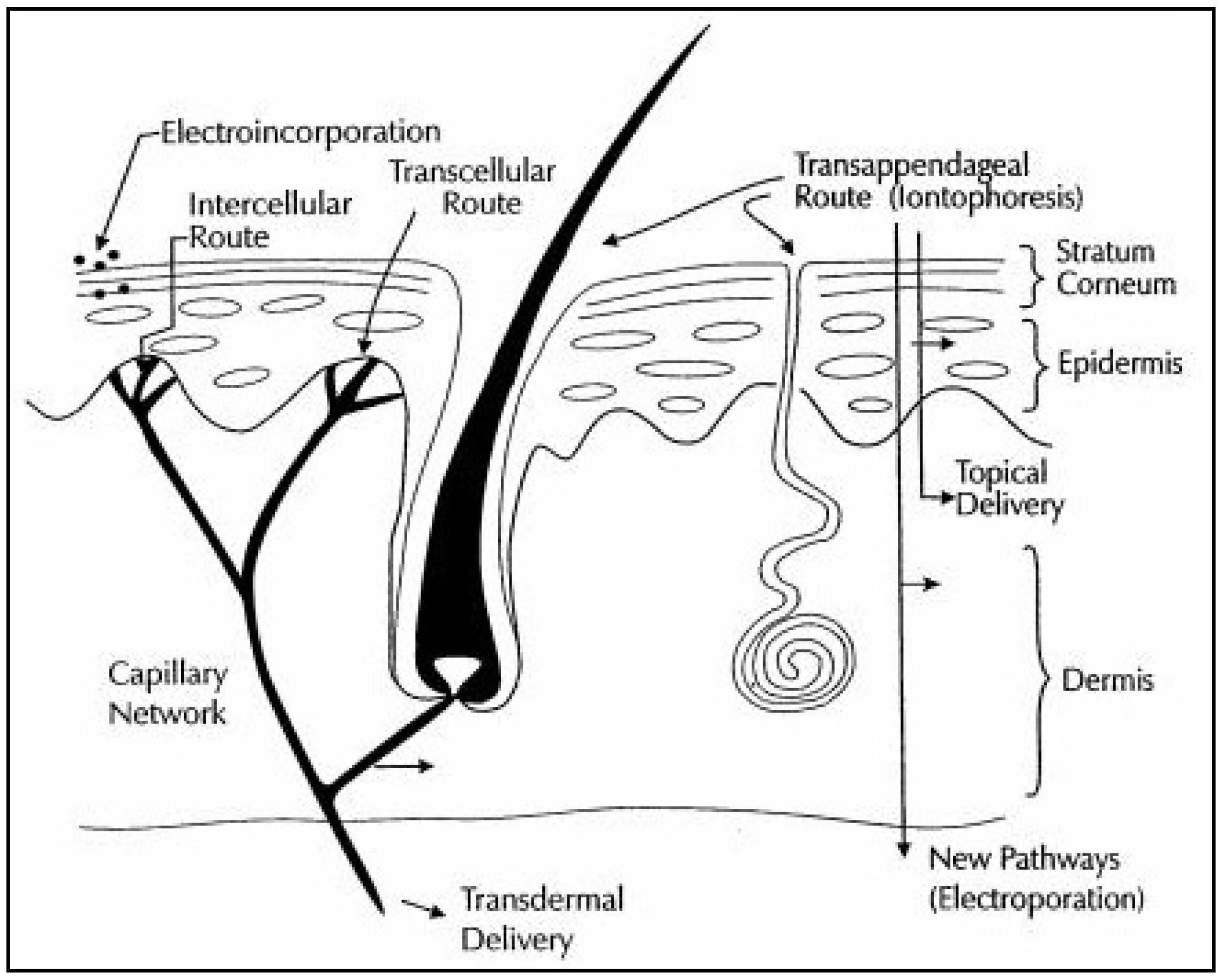
1. Introduction
2. Electrical Amplification
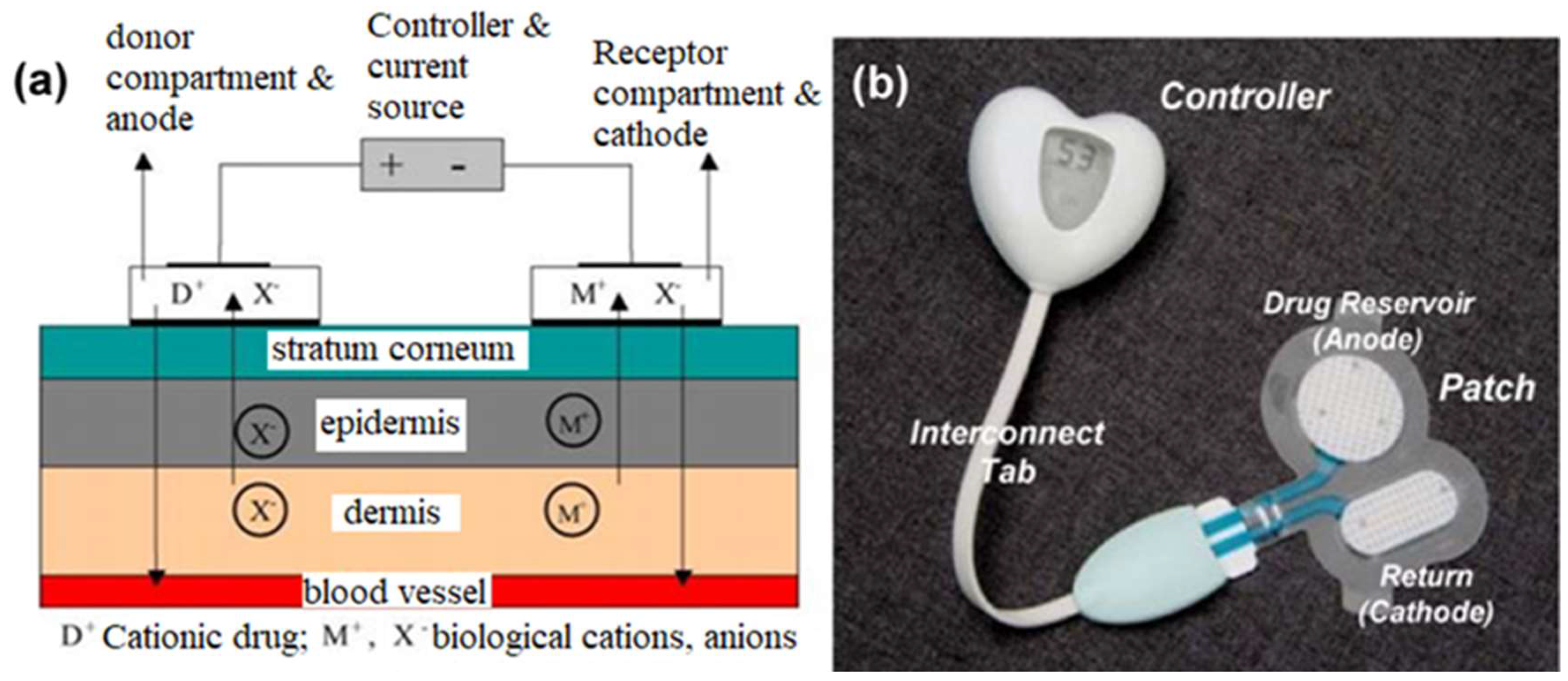
2.1. Iontophoresis
2.2. Iontophoresis in Combination with Electroporation
2.2.1. Particulate Delivery
2.2.2. Clinical Dermatological Applications
2.2.3. Protein Delivery
3. Applications of Iontophoresis
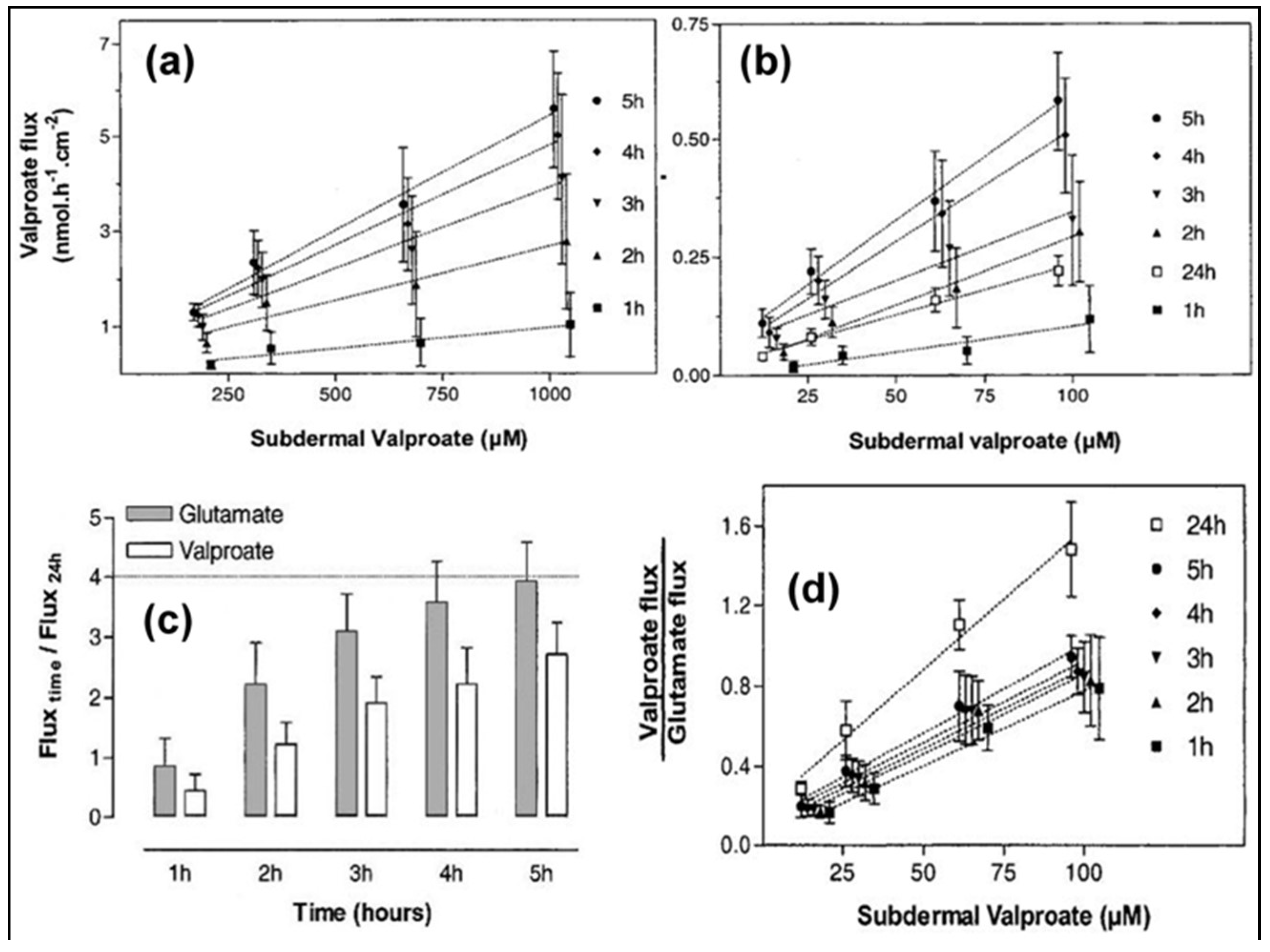
3.1. Iontophoresis in Drug Delivery
3.2. Iontophoresis in Transdermal Biosensing
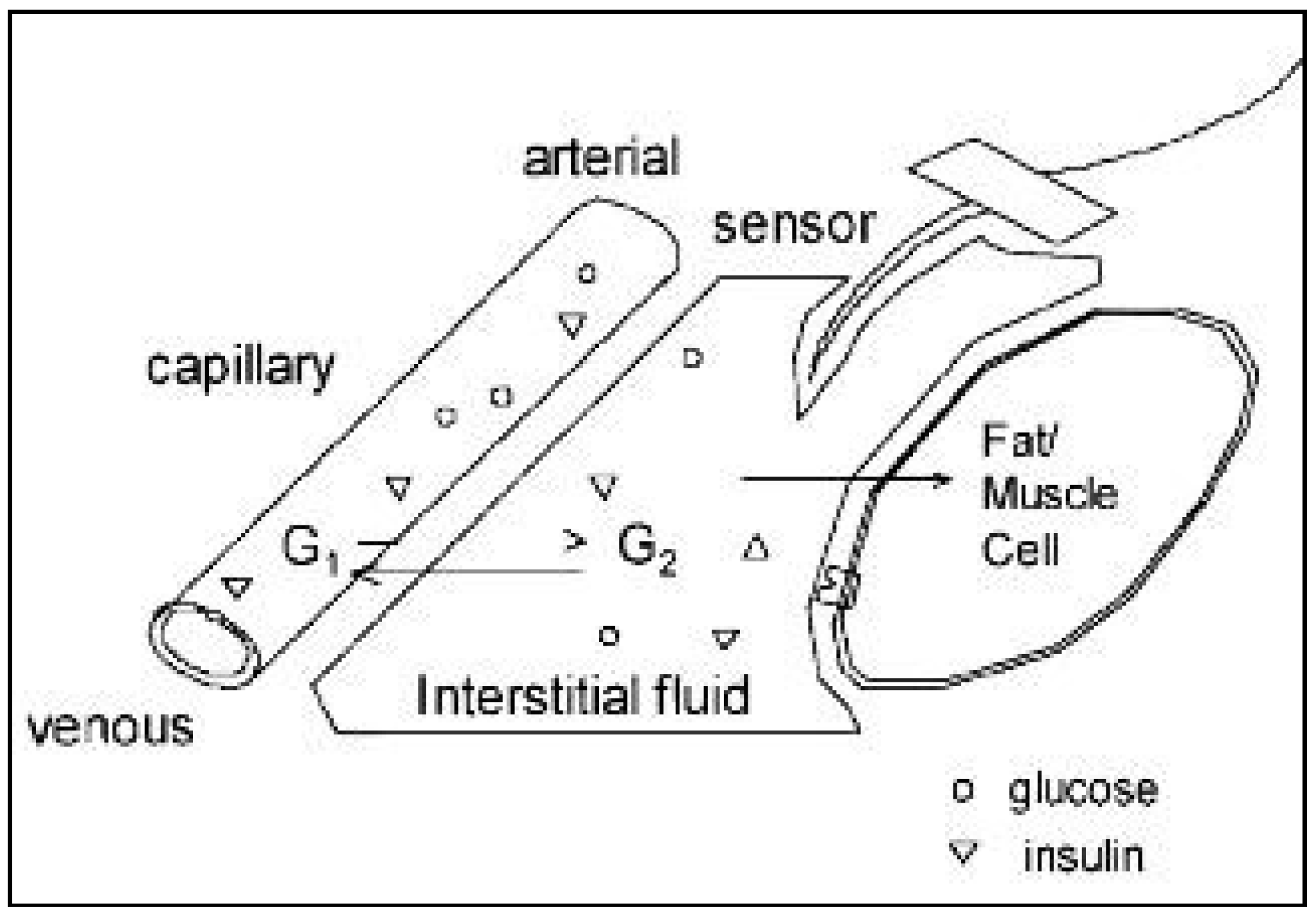
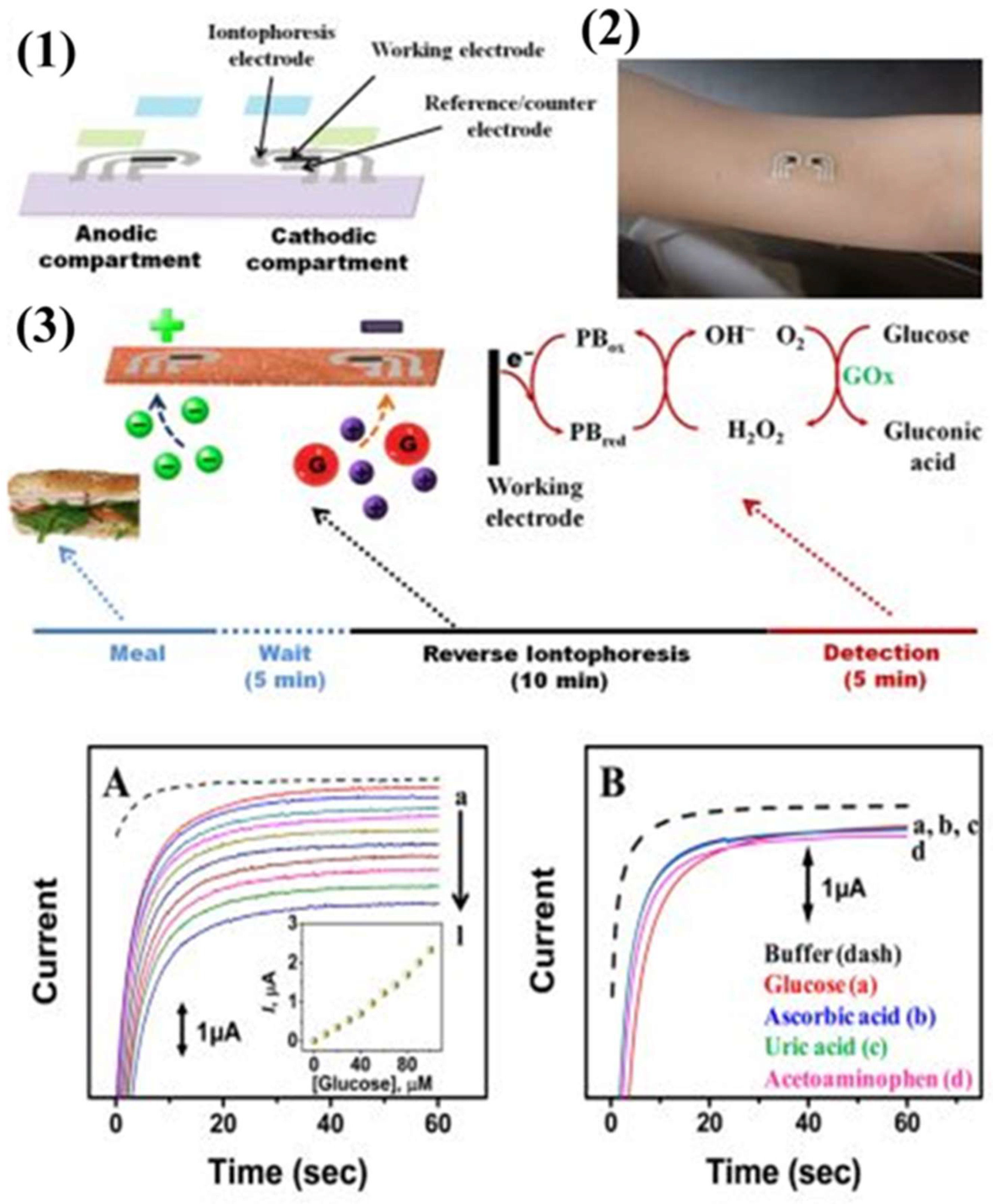
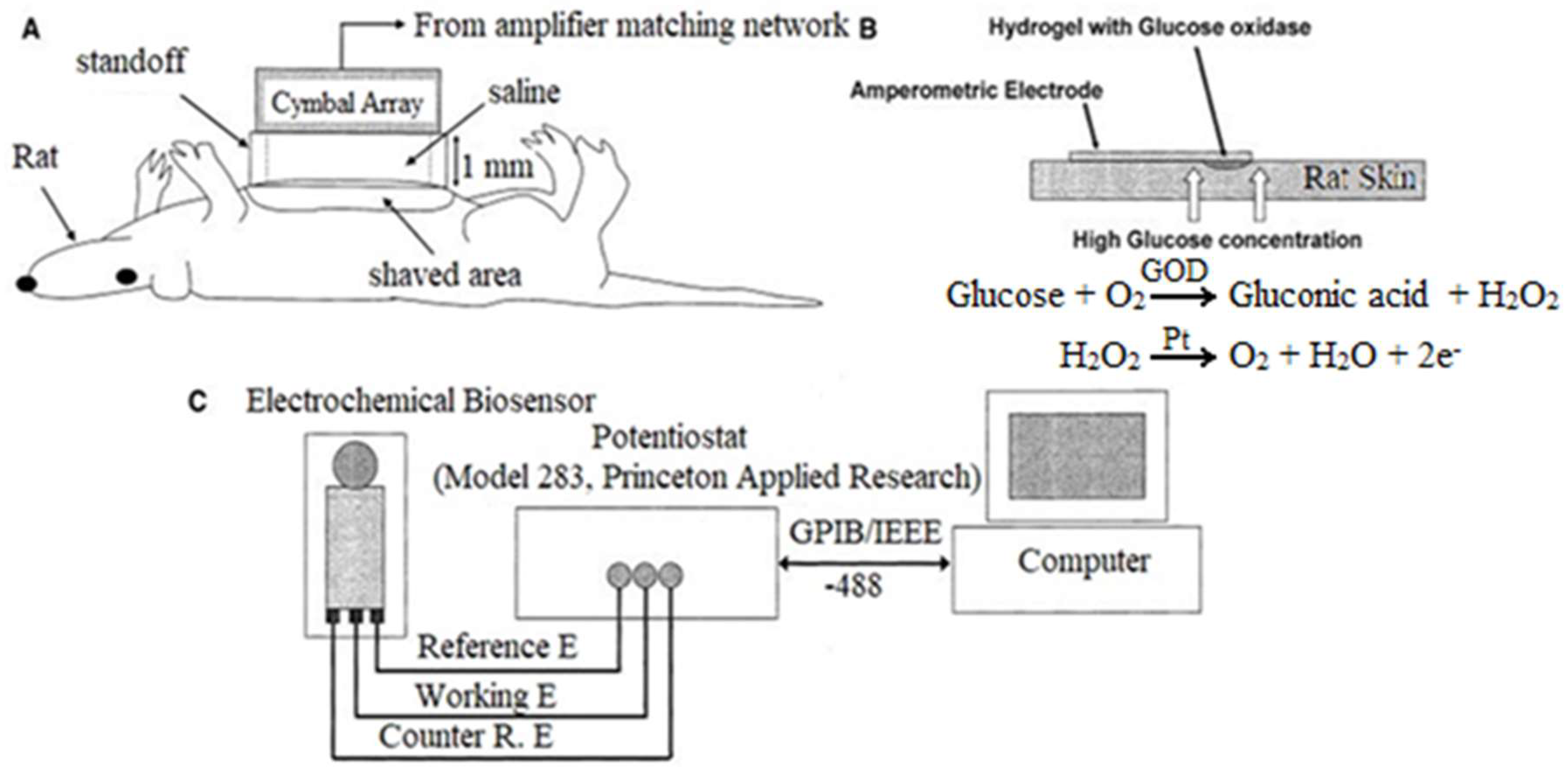
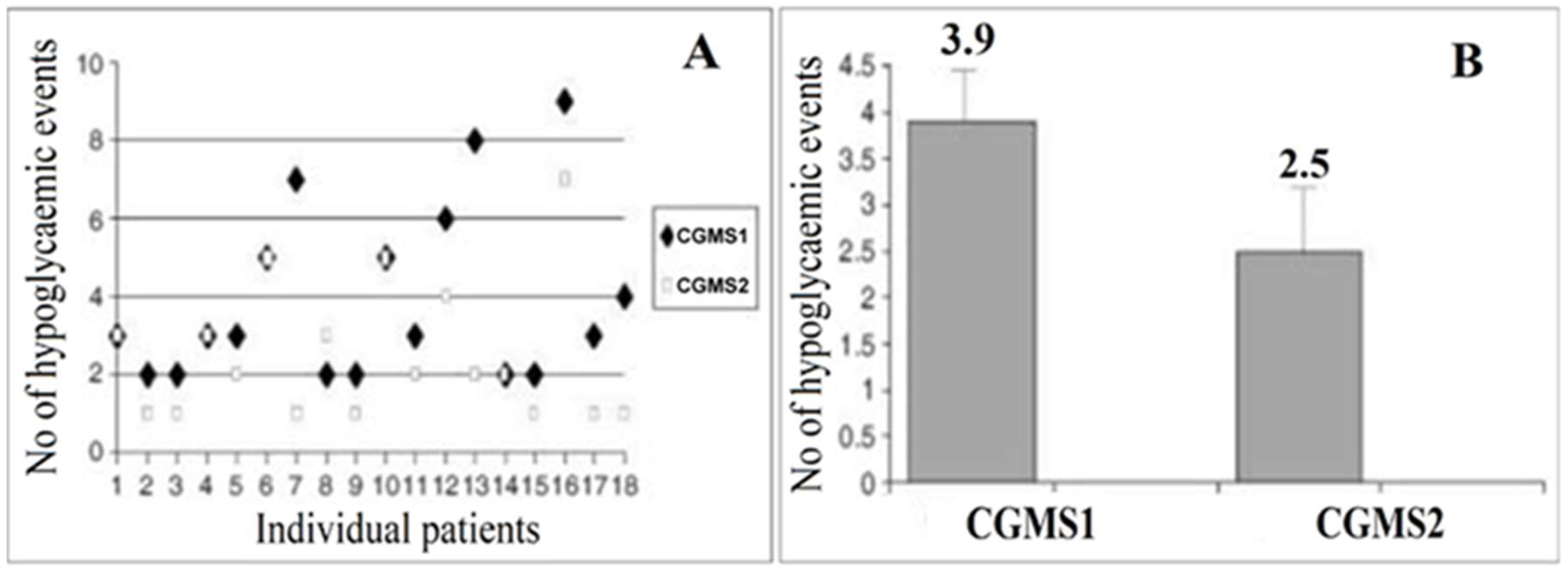
3.2.1. Transdermal Glucose Monitoring
3.2.2. Transdermal Sampling of Urea and Homocysteine
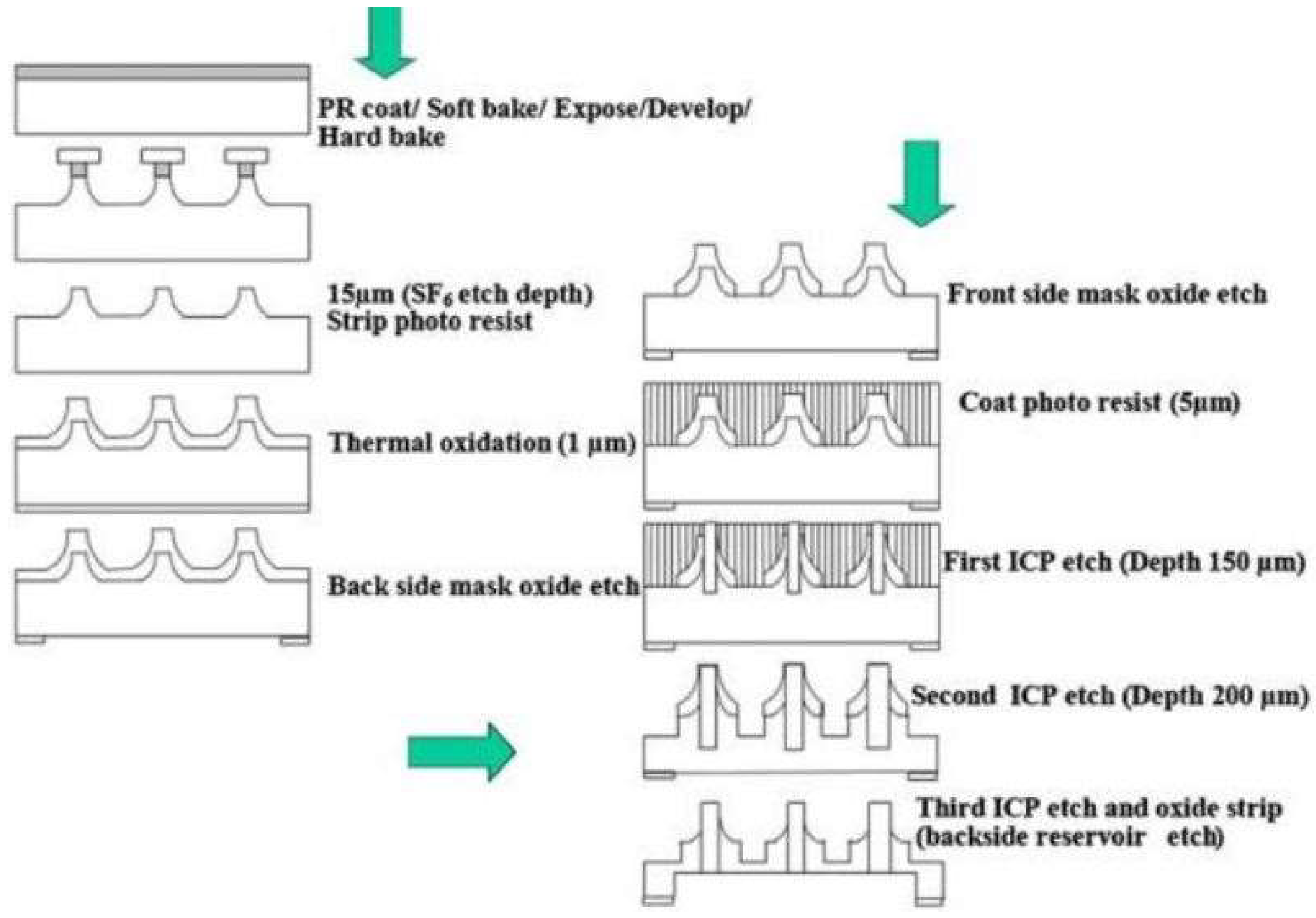
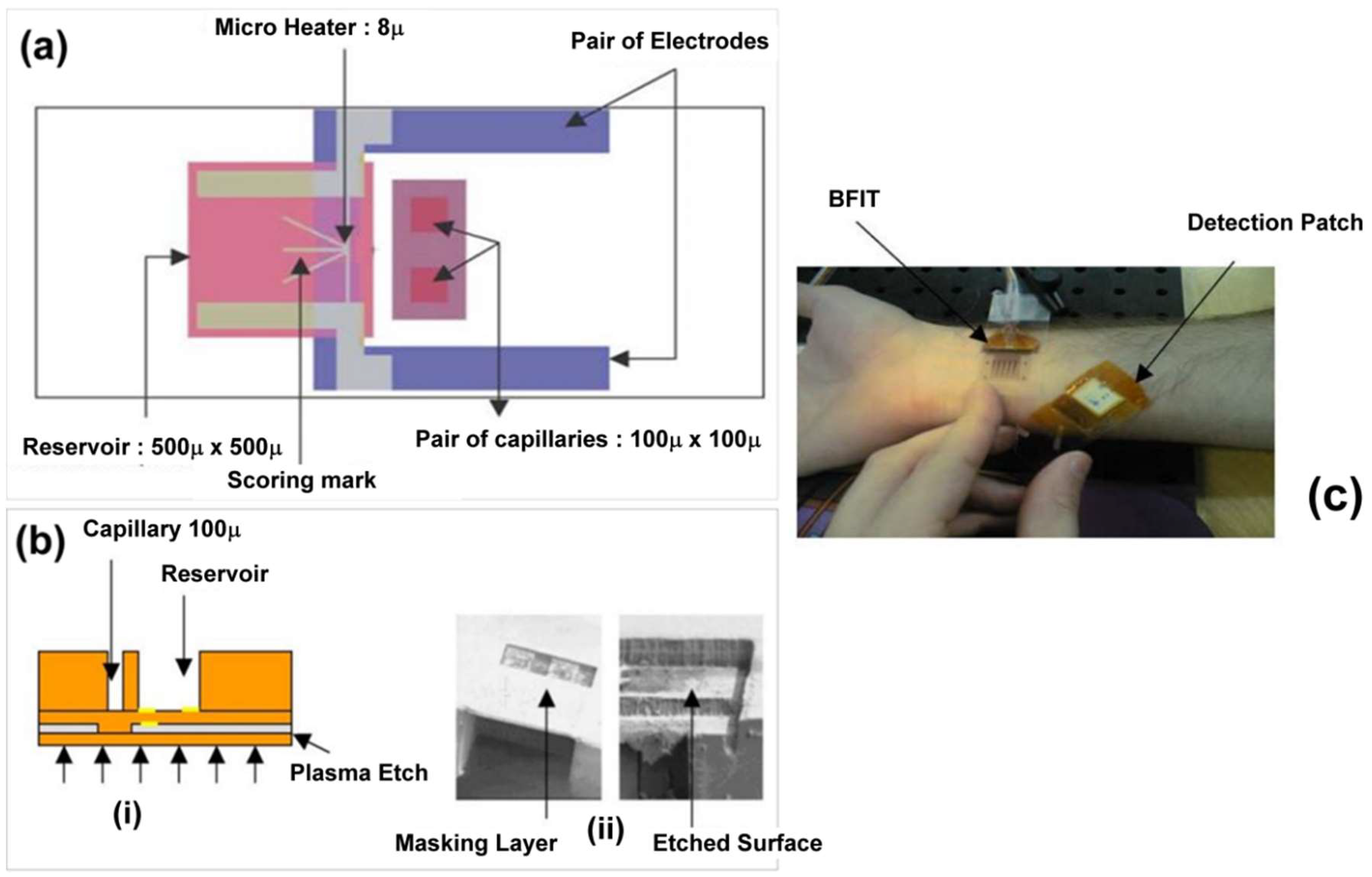
4. Microfabrication Technique for Transdermal Sensing and Drug Delivery
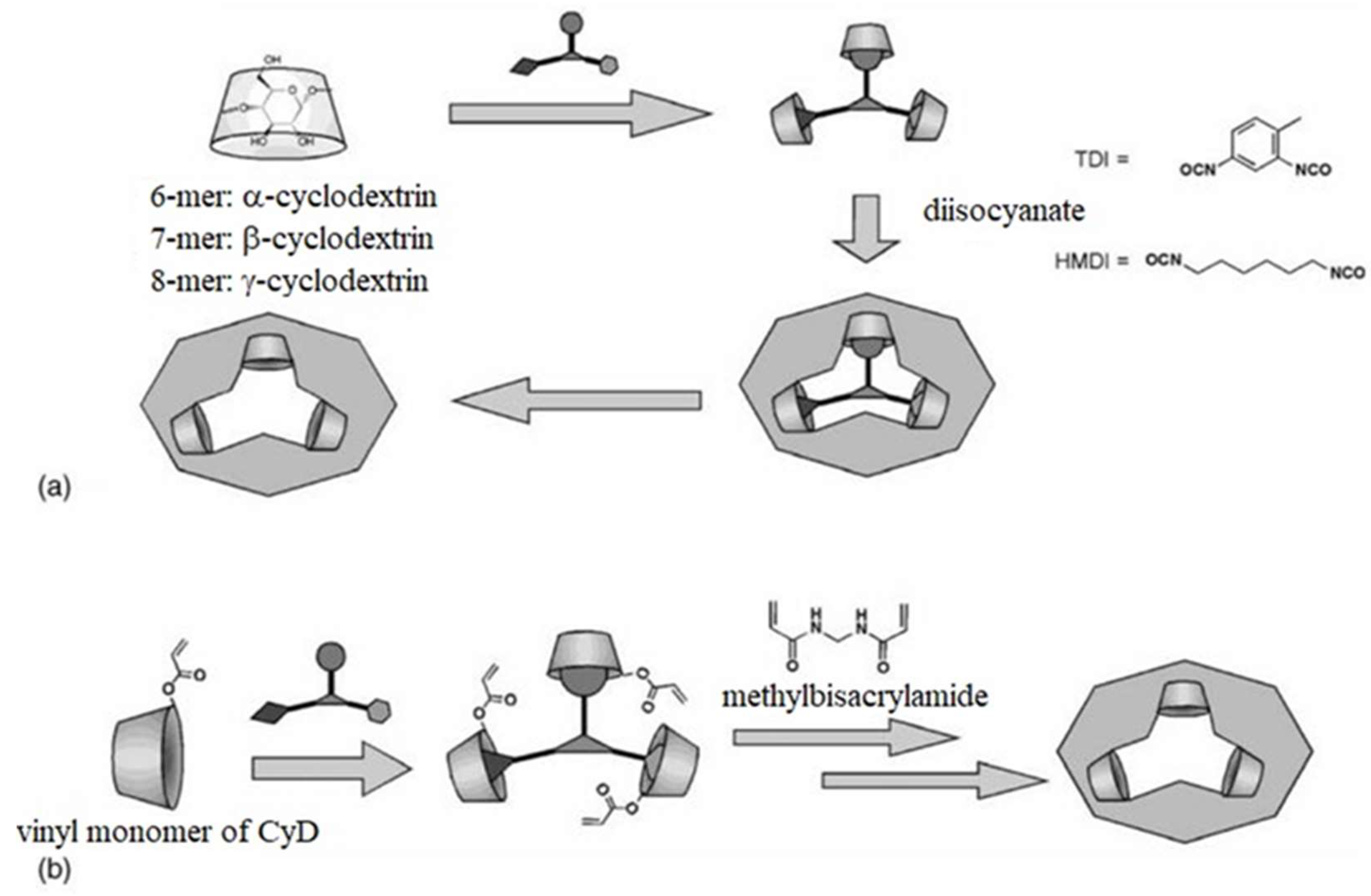
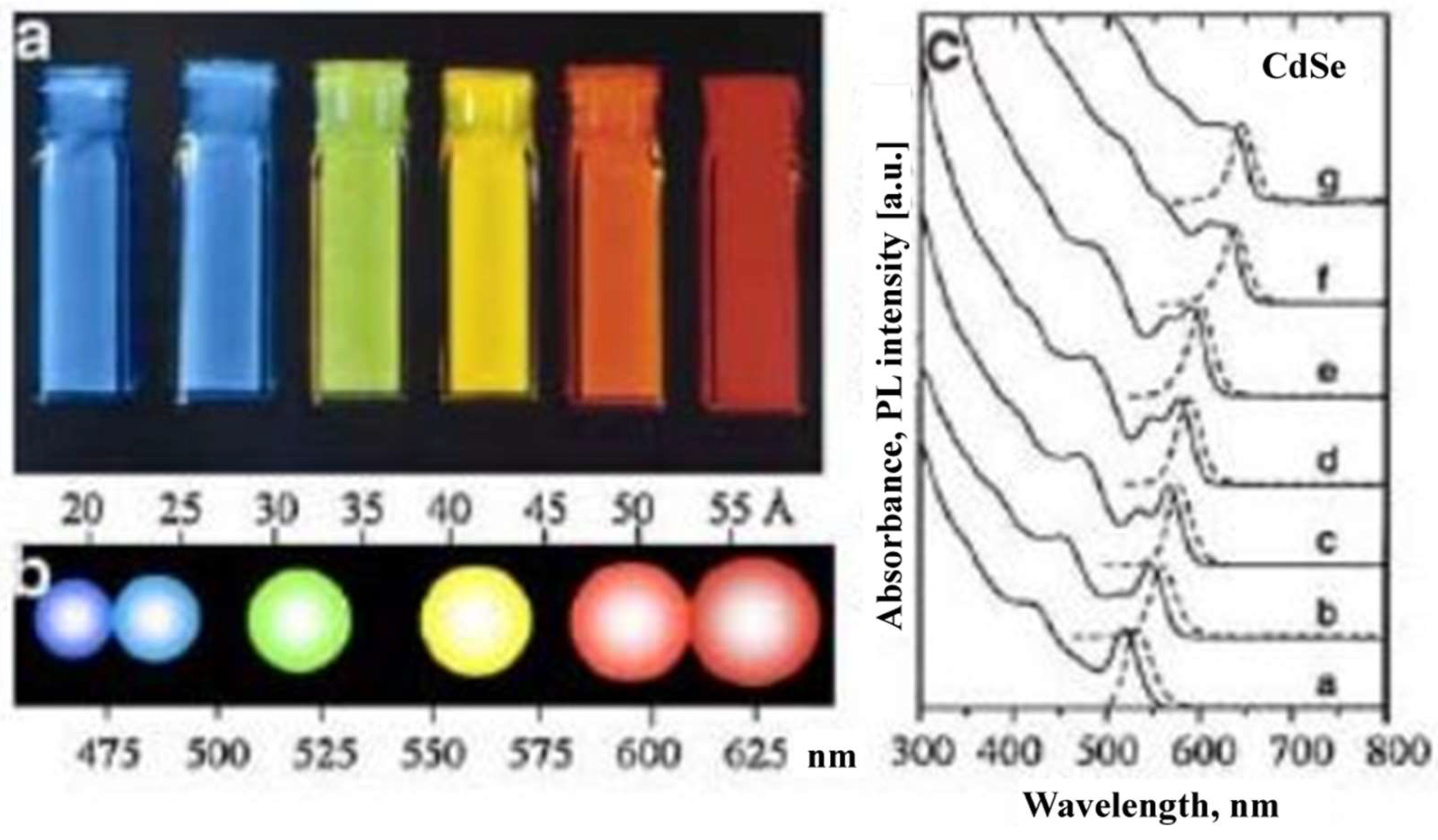
5. Nanotechnology in Transdermal Biosensing
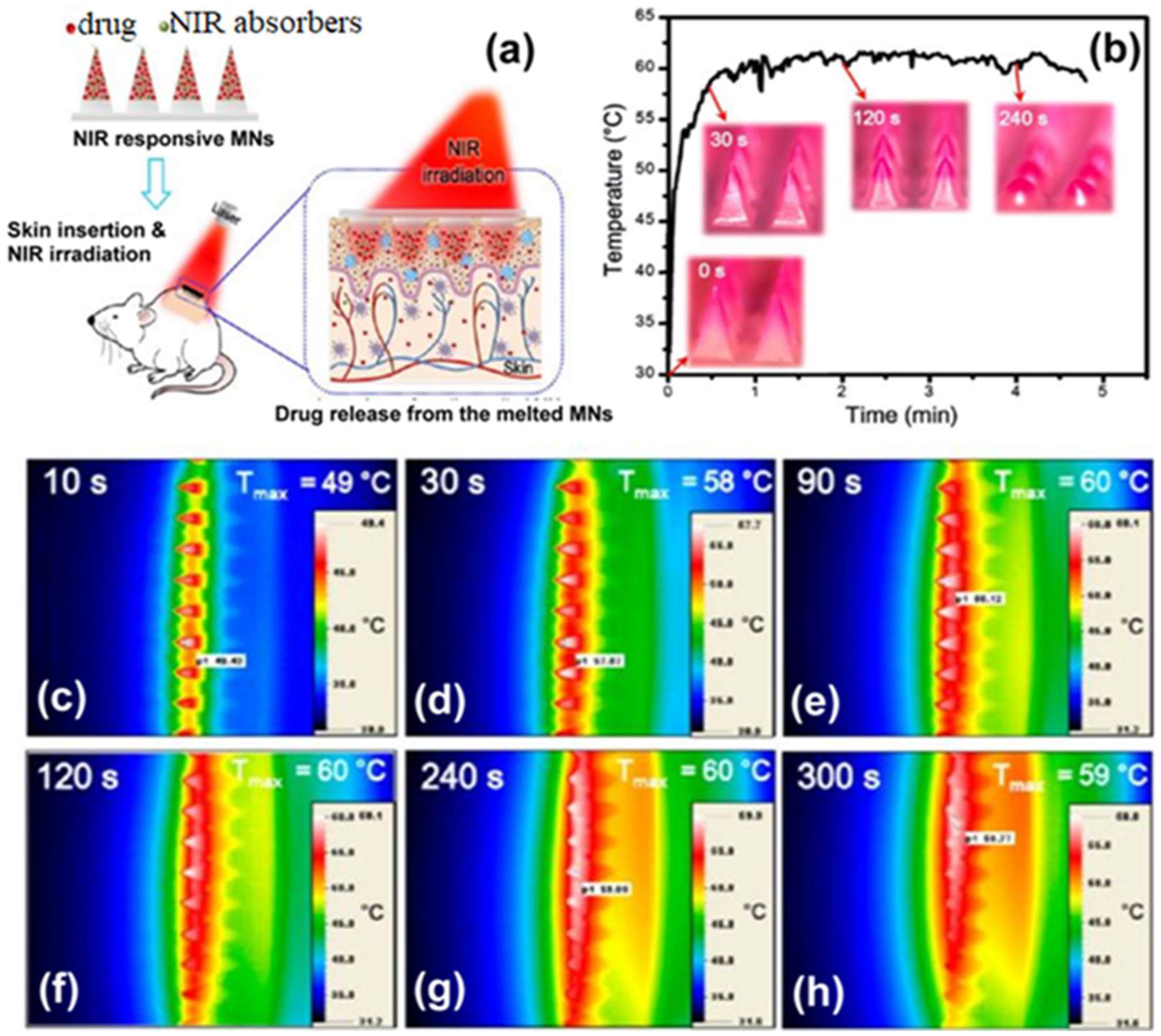
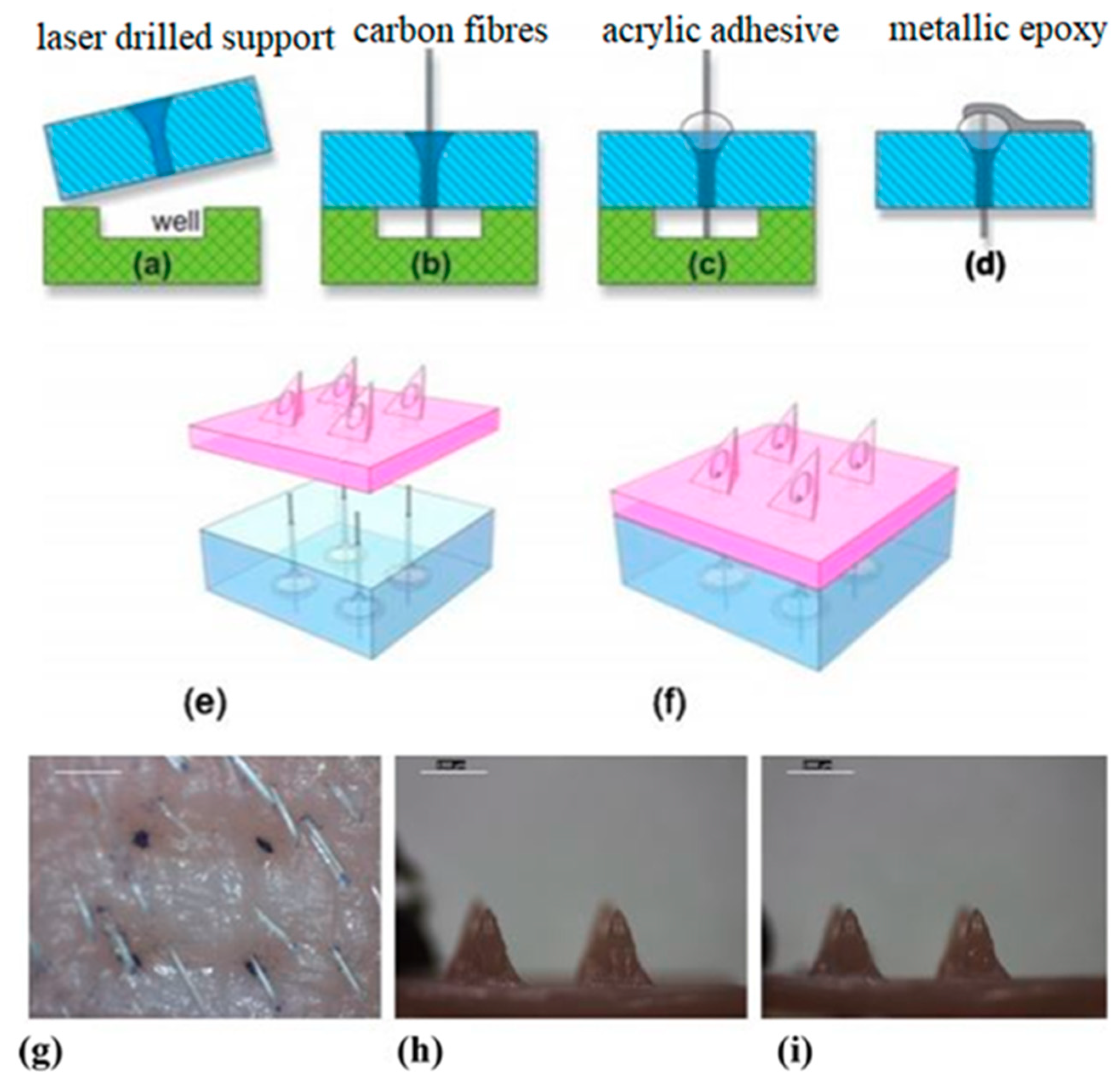
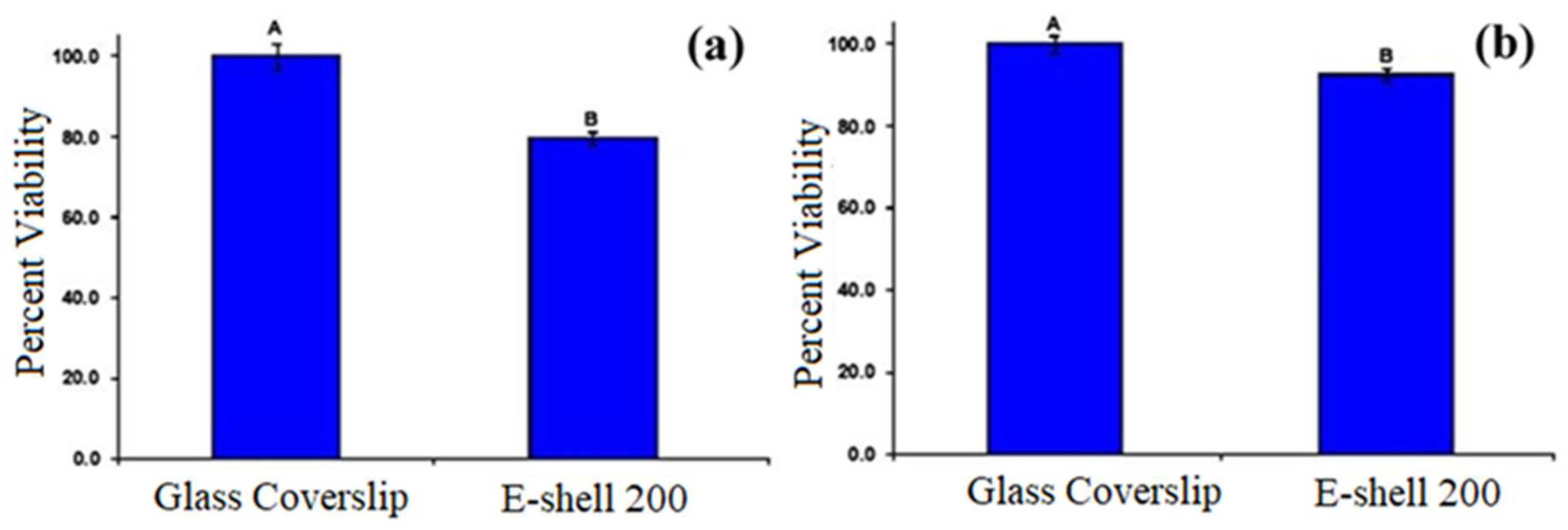
6. Microneedles Technique in Transdermal Sensing and Drug Delivery
6.1. Polymer-Based Microneedles for Drug Delivery
6.2. Microneedles for Chemical and Electrochemical Biosensing
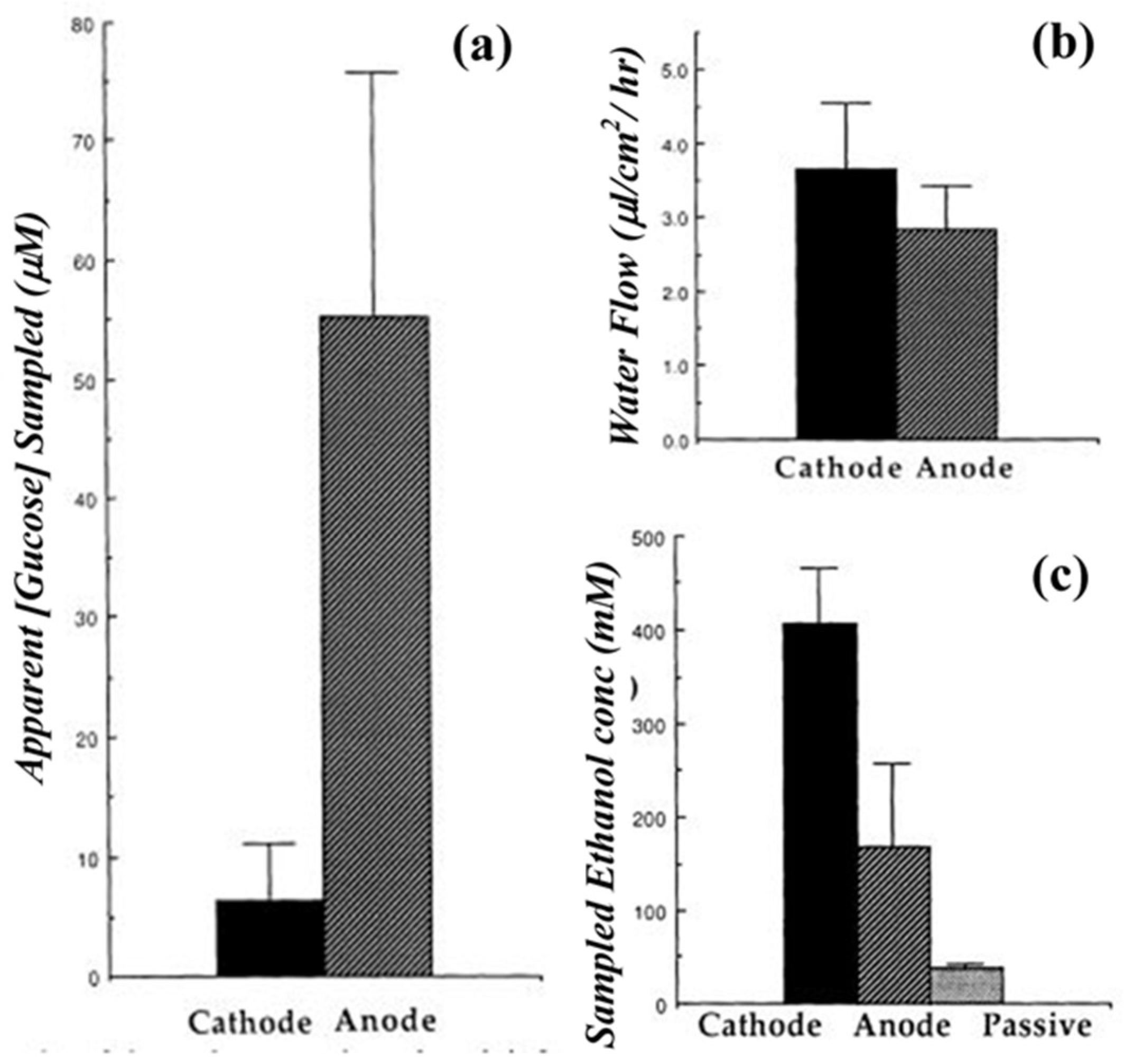
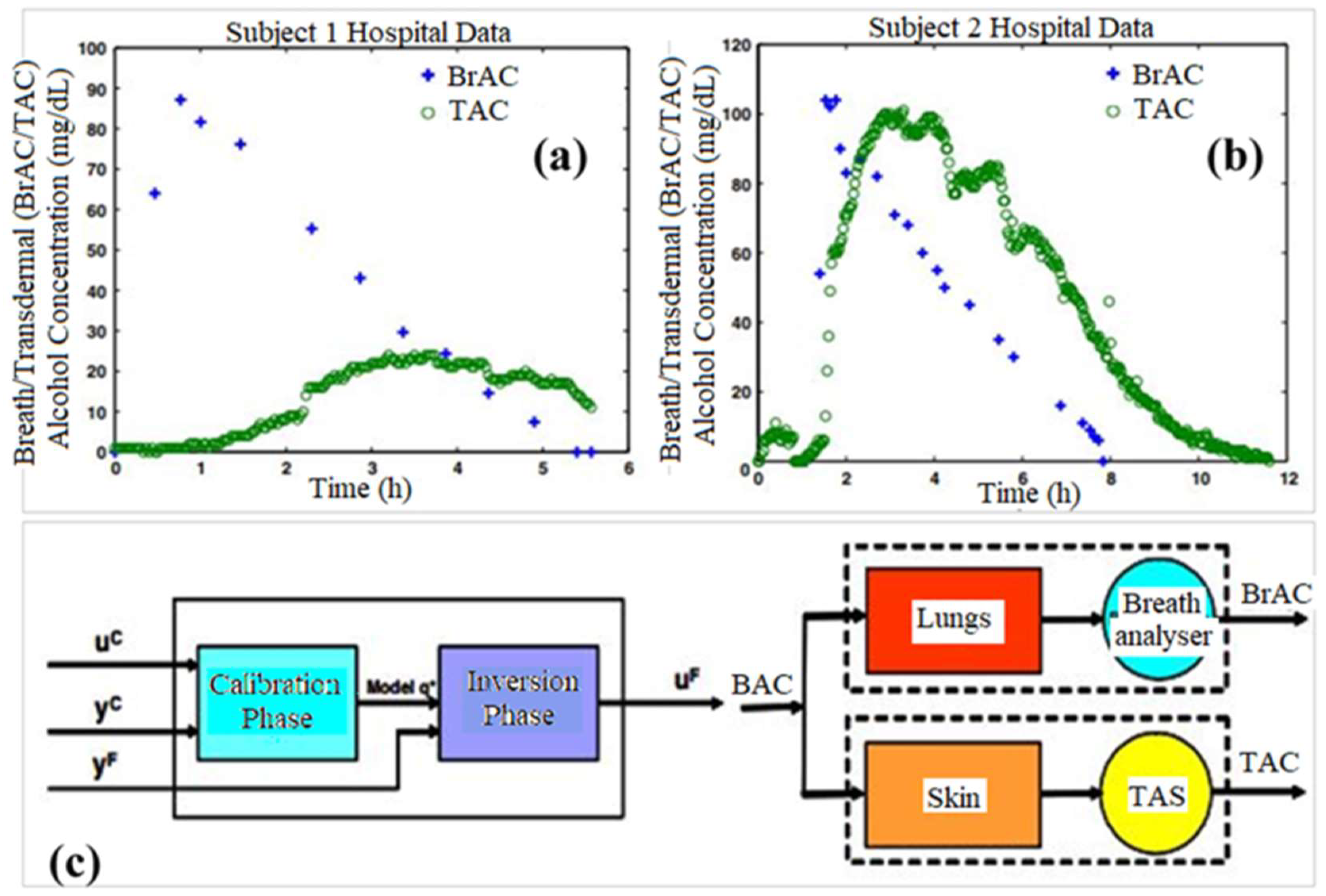
7. Transdermal Alcohol Sensors
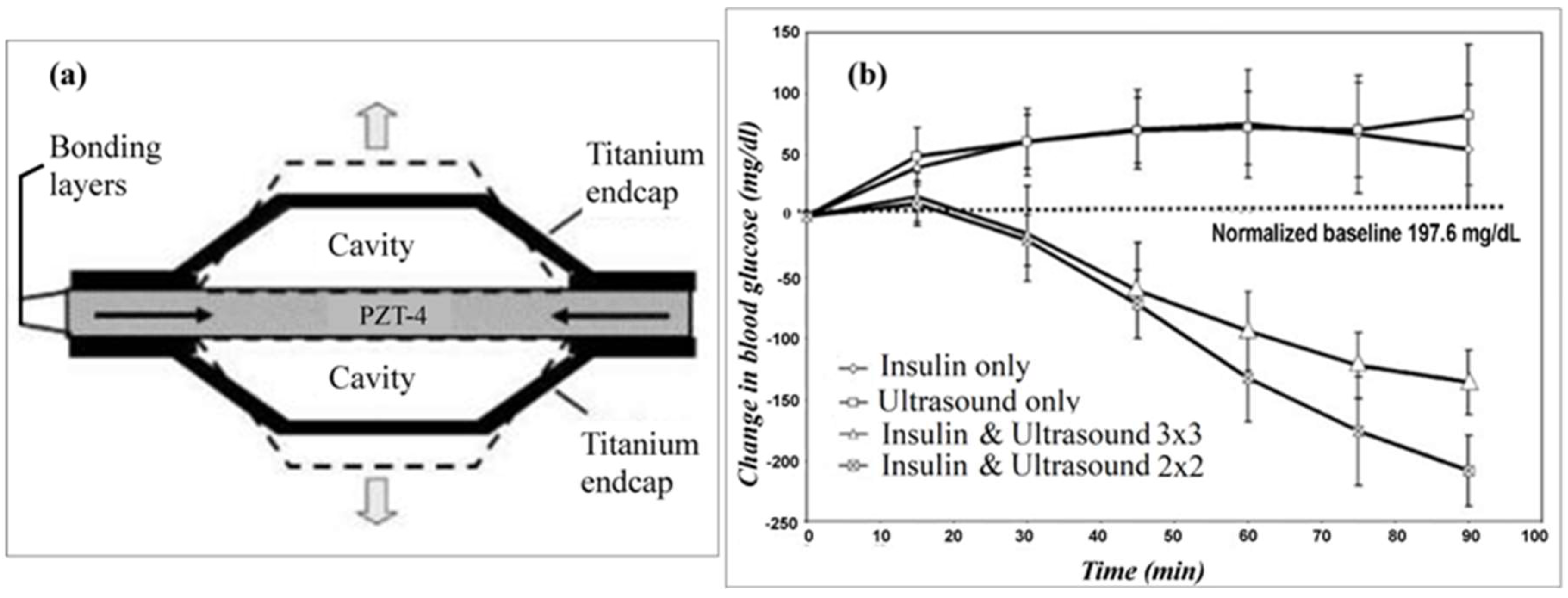
8. Ultrasound Technique for Transdermal Biosensing
9. Microfluidics Approach in Transdermal Sensing
10. Optical Transdermal Sensors: A Condensed Overview
10.1. Fluorophore Based Glucose Sensor
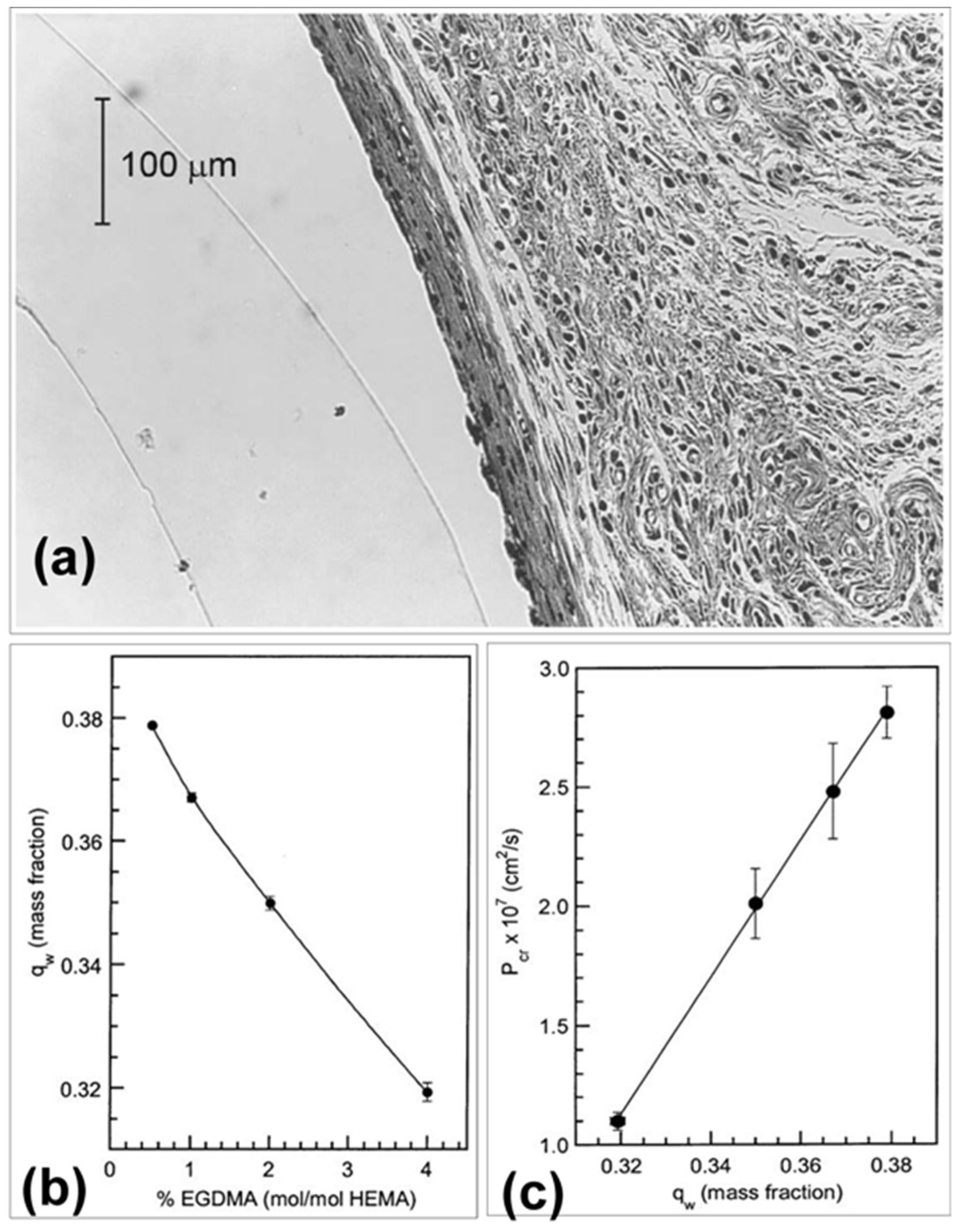
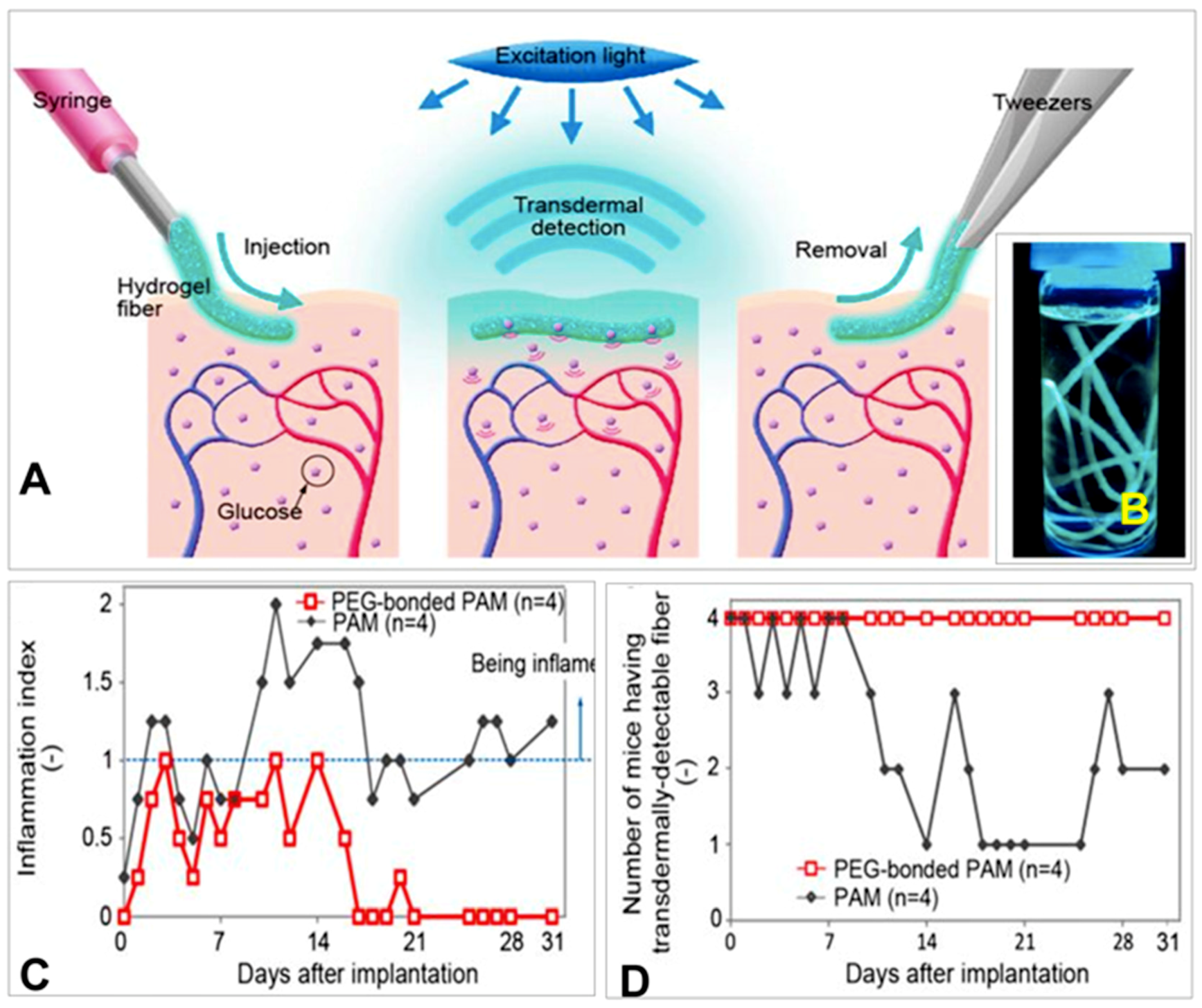
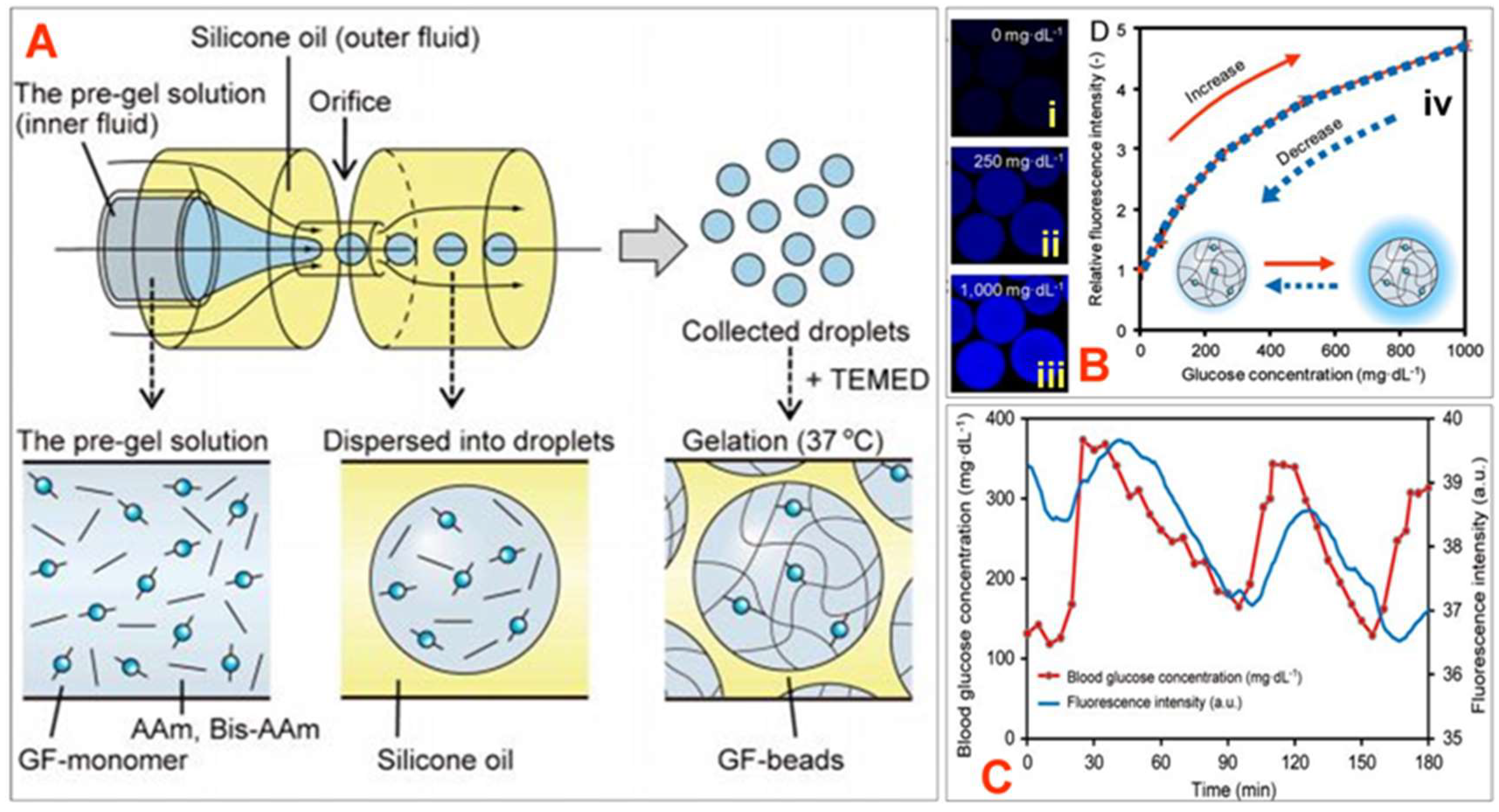
10.2. Hydrogel-Based Fluorescent Glucose Sensors
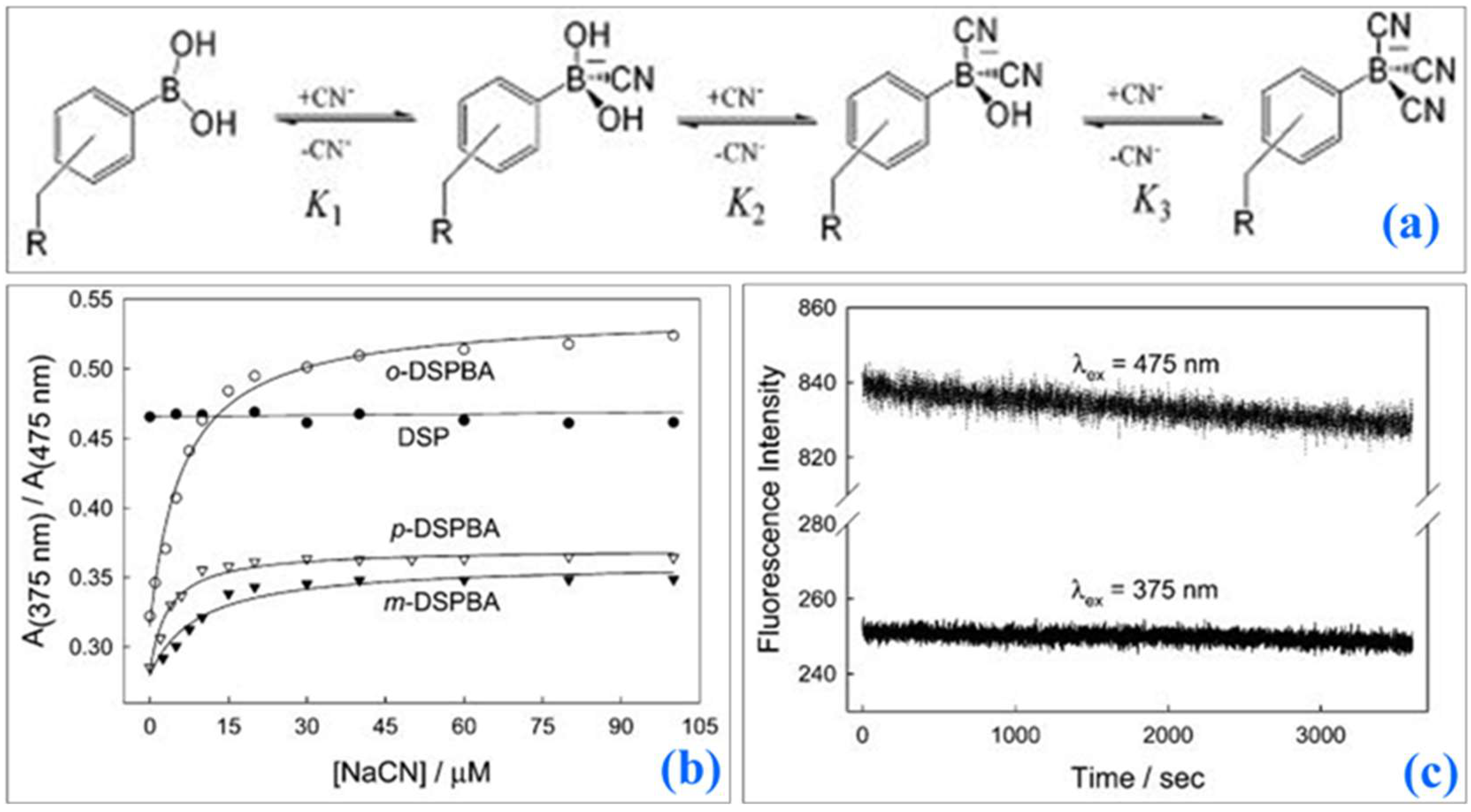
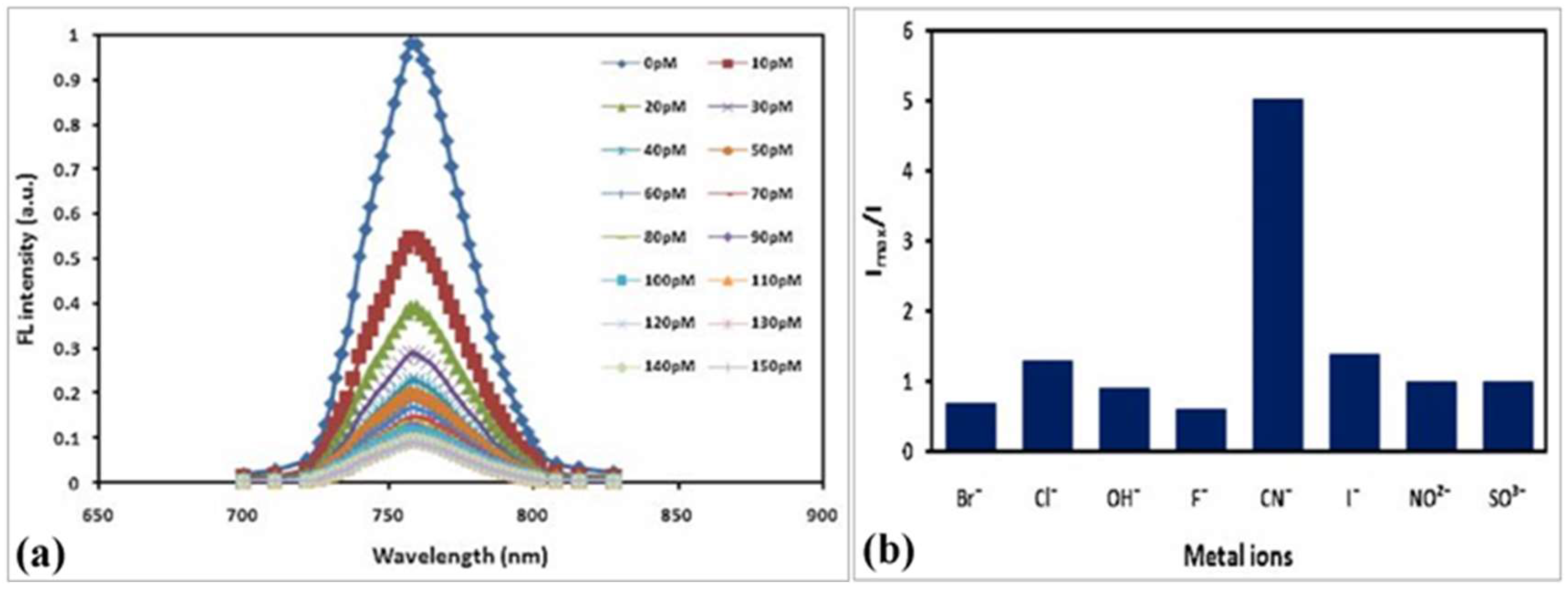
10.3. Fluorescence-Based Ion Sensors
10.4. Luminescent Sensors
10.5. Nanomaterial-Based Optical Sensors
10.6. Raman Spectroscopy Based Transdermal Biosensors
11. Miscellaneous Transdermal Biosensors
12. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ita, K. Transdermal delivery of drugs with microneedles: Strategies and outcomes. J. Drug. Deliv. Sci. Technol. 2015, 29, 16–23. [Google Scholar] [CrossRef]
- Ita, K.B.; Du Preez, J.; Lane, M.E. Dermal delivery of selected hydrophilic drugs from elastic liposomes: Effect of phospholipid formulation and surfactants. J. Pharm. Pharmacol. 2007, 59, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2018, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Lipani, L.; Dupont, B.G.; Doungmene, F.; Marken, F.; Tyrrell, R.M.; Guy, R.H.; Ilie, A. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 2018, 13, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Djabri, A.; Guy, R.H.; Delgado-Charro, M.B. Transdermal iontophoresis of ranitidine: An opportunity in paediatric drug therapy. Int. J. Pharm. 2012, 435, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlen, L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control. Release 2018, 283, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19. [Google Scholar] [CrossRef]
- Shim, G.; Kim, D.; Park, G.T.; Jin, H.; Suh, S.K.; Oh, Y.K. Therapeutic gene editing: Delivery and regulatory perspectives. Acta Pharmacol. Sin. 2017, 38, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal delivery of drugs with microneedles—Potential and challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K. Transdermal delivery of insulin to alloxan-diabetc rabbits by ultrasound exposure. Pharm. Res. 1992, 9, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Azagury, A.; Khoury, L.; Enden, G.; Kost, J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Tamada, Y.; Shimizu, J.; Ohshima, Y.; Matsumoto, Y.; Sugenoya, J.I. Effectiveness of iontophoresis with alternating current (AC) in the treatment of patients with palmoplantar hyperhidrosis. J. Dermatol. 2003, 30, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Banga, A.K. Transdermal delivery of proteins. Aaps Pharmscitech. 2011, 12, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Cázares-Delgadillo, J.; Naik, A.; Ganem-Rondero, A.; Quintanar-Guerrero, D.; Kalia, Y.N. Transdermal Delivery of Cytochrome C—A 12.4 kDa protein—Across Intact Skin by constant–current Iontophoresis. Pharm. Res. 2007, 24, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Manda, P.; Angamuthu, M.; Hiremath, S.R.; Raman, V.; Murthy, S.N. Iontophoretic drug delivery for the treatment of scars. J. Pharm. Sci. 2014, 103, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Charro, M.B.; Guy, R.H. Transdermal reverse iontophoresis of valproate: A noninvasive method for therapeutic drug monitoring. Pharm. Res. 2003, 20, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Subramony, J.A.; Sharma, A.; Phipps, J.B. Microprocessor controlled transdermal drug delivery. Int. J. Pharm. 2006, 317, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; Heinemann, L. Sensors for glucose monitoring: Technical and clinical aspects. Diabetes Metab. Res Rev. 2001, 17, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Glikfeld, P.; Guy, R.H. Reverse iontophoresis: Development of a noninvasive approach for glucose monitoring. Pharm. Res. 1993, 10, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Liu, H.; Jiang, L.; Yang, Q.; Cai, X. Study of noninvasive sampling of subcutaneous glucose by reverse iontophoresis. In Proceedings of the 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS’07), Bangkok, Thailand, 16–19 January 2007. [Google Scholar] [CrossRef]
- Ching, C.T.S.; Sun, T.P.; Huang, S.H.; Shieh, H.L.; Chen, C.Y. A mediated glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Ann. Biomed. Eng. 2010, 38, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.T.S.; Chou, T.R.; Sun, T.P.; Huang, S.Y.; Shieh, H.L. Simultaneous, noninvasive, and transdermal extraction of urea and homocysteine by reverse iontophoresis. Int. J. Nanomed. 2011, 6, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. A Process for Making Microneedle-Based Transdermal Biosensor Involving Poyethyleninmine-Modified Prussian Blue-Gold Nanohybrids Enzyme Ink for On-Chip Electrochemical Biosensing in Body Fluid. India Patent 201811044930, 28 November 2018. [Google Scholar]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. A Process for Making Solid-State Ion Sensor for On-Chip Determination of Potassium Ion in Body Fluid. India Patent 201811041978, 6 November 2018. [Google Scholar]
- Wei, C.; Dong, X.; Zhang, Y.; Liang, J.; Yang, A.; Zhu, D.; Liu, T.; Kong, D.; Lv, F. Simultaneous fluorescence imaging monitoring of the programMed. release of dual drugs from a hydrogel-carbon nanotube delivery system. Sens. Actuators B Chem. 2018, 273, 264–275. [Google Scholar] [CrossRef]
- Cinteza, L.O. Quantum dots in biomedical applications: Advances and challenges. J. Nanophotonics 2010, 4, 042503. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.J.J.L.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Hejazi, Z.; Armagan, E.; Zhao, S.; Schmid, M.; Zhang, H.; Guo, H.; Weidenbacher, L.; Rossi, R.M.; Koebel, M.M.; et al. Carbon dots and fluorescein: The ideal FRET pair for the fabrication of a precise and fully reversible ammonia sensor. Sens. Actuators B Chem. 2017, 253, 714–722. [Google Scholar] [CrossRef]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef] [PubMed]
- Hanks, D.F.; Lu, Z.; Sircar, J.; Salamon, T.R.; Antao, D.S.; Bagnall, K.R.; Barabadi, B.; Wang, E.N. Nanoporous membrane device for ultra high heat flux thermal management. Microsyst Nanoeng. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, D.H. Preparation of LaB6 nanoparticles as a novel and effective near-infrared photothermal conversion material. Chem. Eng. J. 2012, 180, 337–342. [Google Scholar] [CrossRef]
- Lui, B.H.; Chen, D.H. LaB6 nanoparticles with carbon-doped silica coating for fluorescence imaging and near-IR photothermal therapy of cancer cells. Acta Biomater. 2013, 9, 7556–7563. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Ling, M.H.; Wang, K.W.; Lin, Z.W.; Lai, B.H.; Chen, D.H. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules 2015, 16, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Leong, Y.K.; Saunders, M.; Martyniuk, M.; Faraone, L.; Keating, A.; Dell, J.M. Uniform dispersion of lanthanum hexaboride nanoparticles in a silica thin film: Synthesis and optical properties. ACS Appl. Mater. Interfaces 2012, 4, 5833–5838. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ranamukhaarachchi, S.A.; Padeste, C.; Dubner, M.; Hafeli, U.O.; Stoeber, B.; Cadarso, V.J. Integrated hollow microneedle-optofluidic biosensor for therapeutic drug monitoring in sub-nanoliter volumes. Sci. Rep. 2016, 6, 29075. [Google Scholar] [CrossRef] [PubMed]
- Sanaee, Z.; Mohajerzadeh, S. Ultrafine hollow needle formation on silicon. J. Appl. Phys. 2010, 107, 083711. [Google Scholar] [CrossRef]
- Miller, P.R.; Gittard, S.D.; Edwards, T.L.; Lopez, D.M.; Xiao, X.; Wheeler, D.R.; Monteiro-Riviere, N.A.; Brozik, S.M.; Polsky, R.; Narayan, R.J. Integrated carbon fiber electrodes within hollow polymer microneedles for transdermal electrochemical sensing. Biomicrofluidics 2011, 5, 013415. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Zhou, N.; Chuang, M.C.; Valdés-Ramírez, G.; Santhosh, P.; Miller, P.R.; Narayan, R.; Wang, J. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 2011, 136, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Dumett, M.A.; Rosen, I.G.; Sabat, J.; Shaman, A.; Tempelman, L.; Wang, C.; Swift, R.M. Deconvolving an estimate of breath measured blood alcohol concentration from biosensor collected transdermal ethanol data. Appl. Math. Comput. 2008, 196, 724–743. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.M.; Charles, N.E.; Acheson, A.; John, S.; Furr, R.M.; Hill-Kapturczak, N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp. Clin. Psychopharmacol. 2012, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gurvich, E.M.; Kenna, G.A.; Leggio, L. Use of novel technology-based techniques to improve alcohol-related outcomes in clinical trials. Alcohol. Alcohol. 2013, 48, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell, T.R.; Cooney, N.J.; Murphy, J.G.; Luczak, S.; Rosen, G.; Dougherty, D.M.; Barnett, N.P. Continuous objective monitoring of alcohol use: Twenty-first century measurement using transdermal sensors. Alcohol. Clin. Exp. Res. 2013, 37, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Luczak, S.E.; Rosen, I.G.; Weiss, J. Determining blood and/or breath alcohol concentration from transdermal alcohol data. In Proceedings of the American Control Conference (ACC), Washington, DC, USA, 17–19 June 2013; pp. 473–478. [Google Scholar] [CrossRef]
- Barnett, N.P.; Meade, E.B.; Glynn, T.R. Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Exp. Clin. Psychopharmacol. 2014, 22, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nayak, V.; Dodds, J.; Pishko, M.; Smith, N.B. Glucose measurements with sensors and ultrasound. Ultrasound Med. Biol. 2005, 31, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Smith, N.B.; Markley, D.; Snyder, B.; Uzgur, A.E.; Pishko, M.; Newnham, R.E. Composite transducer arrays for the treatment of diabetes. Int. J. Appl. Ceram. Technol. 2005, 2, 308–316. [Google Scholar] [CrossRef]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 1–19. [Google Scholar] [CrossRef]
- Gadre, A.P.; Nijdam, A.J.; Garra, J.A.; Monica, A.H.; Cheng, M.C.; Luo, C.; Srivastava, Y.N.; Schneider, T.W.; Long, T.J.; White, R.C.; et al. Fabrication of a fluid encapsulated dermal patch using multilayered SU-8. Sens. Actuators A Phys. 2004, 114, 478–485. [Google Scholar] [CrossRef]
- Shoji, E. Fabrication of a diaphragm micropump system utilizing the ionomer-based polymer actuator. Sens. Actuators B Chem. 2016, 237, 660–665. [Google Scholar] [CrossRef]
- Li, D.; Ji, Y.; Liang, W.; Zhang, X.; Yu, H.; Xu, K. A portable instrument for continuous glucose monitoring by the integration of microfluidic chip and micro-glucose sensor. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XI, San Francisco, CA, USA, 9 March 2013; International Society for Optics and Photonics; Volume 8615, p. 861519. [Google Scholar] [CrossRef]
- Yu, H.X.; Li, D.C.; Ji, Y.J.; Zhang, X.L.; Xu, K.X. An Interstitial Fluid Transdermal Extraction Chip with Vacuum Generator and Volume Sensor for Continuous Glucose Monitoring. Key Eng. Mater. 2013, 562, 571–575. [Google Scholar] [CrossRef]
- Cordeiro, C.A.; Sias, A.; Koster, T.; Westerink, B.H.C.; Cremers, T.I.F.H. In vivo “real-time” monitoring of glucose in the brain with an amperometric enzyme-based biosensor based on gold coated tungsten (W-Au) microelectrodes. Sens. Actuators B Chem. 2018, 263, 605–613. [Google Scholar] [CrossRef]
- Ballerstadt, R.; Schultz, J.S. A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring. Anal. Chem. 2000, 72, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Ballerstadt, R.; Gowda, A.; McNichols, R. Fluorescence resonance energy transfer-based near-infrared fluorescence sensor for glucose monitoring. Diabetes Technol. Ther. 2004, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ballerstadt, R.; Polak, A.; Beuhler, A.; Frye, J. In vitro long-term performance study of a near-infrared fluorescence affinity sensor for glucose monitoring. Biosens. Bioelectron. 2004, 19, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Ballerstadt, R.; Evans, C.; Gowda, A.; McNichols, R. In vivo performance evaluation of a transdermal near-infrared fluorescence resonance energy transfer affinity sensor for continuous glucose monitoring. Diabetes Technol. Ther. 2006, 8, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat. Chem. 2017, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Meller, K.; Szumski, M.; Buszewski, B. Microfluidic reactors with immobilized enzymes characterization, dividing, perspectives. Sens. Actuators B Chem. 2017, 244, 84–106. [Google Scholar] [CrossRef]
- Kermis, H.R.; Rao, G.; Barbari, T.A. Transport properties of pHEMA membranes for optical glucose affinity sensors. J. Membr. Sci. 2003, 212, 75–86. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Fukumura, D.; Jain, R.K.; Bouma, B.E. Cancer imaging by optical coherence tomography: Preclinical progress and clinical potential. Nat. Rev. Cancer 2012, 12, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Saxl, T.E.; Pickup, J.C. Fluorescence intensity-and lifetime-based glucose sensing using an engineered high-Kd mutant of glucose/galactose-binding protein. Anal. Biochem. 2010, 399, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Shibata, H.; Okitsu, T.; Kawanishi, T.; Takeuchi, S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc. Natl. Acad. Sci. USA 2011, 108, 13399–13403. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.M.; Hasan, M.H.; Alamgir, A.; Xu, L.; Powell, D.R.; Wong, B.M.; Tandon, R.; Hossain, M.A. Highly selective and sensitive macrocycle-based dinuclear foldamer for fluorometric and colorimetric sensing of citrate in water. Sci. Rep. 2018, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Enhanced fluorescence cyanide detection at physiologically lethal levels: Reduced ICT-based signal transduction. J. Am. Chem. Soc. 2005, 127, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Al-Agel, F.A.; Mahmoud, W.E. “Turn-off-on” fluorescence probe based functionalized InP quantum wires for detection of cyanide ions. Sens. Actuators B Chem. 2013, 183, 441–445. [Google Scholar] [CrossRef]
- Long, R.; McShane, M.J. Three-dimensional, multiwavelength Monte Carlo simulations of dermally implantable luminescent sensors. J. Biomed. Opt. 2010, 15, 027011. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Nanogold plasmon resonance-based glucose sensing. 2. Wavelength-ratiometric resonance light scattering. Anal. Chem. 2005, 77, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Uskoković-Marković, S.; Jelikić-Stankov, M.; Holclajtner-Antunović, I.; Đurđević, P. Raman spectroscopy as a new biochemical diagnostic tool. J. Med. Biochem. 2013, 32, 96–103. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, C.; Shen, Y.; Chen, Z.; Silveira, E.S.; Zhang, L.; Wei, M.; Liu, C.; de Sena-Tomas, C.; Targoff, K.; et al. Optical Imaging of Metabolic Dynamics in Animals. Nat. Commun. 2018, 9, 2995. [Google Scholar] [CrossRef] [PubMed]
- Enejder, A.M.; Scecina, T.G.; Oh, J.; Hunter, M.; Shih, W.; Sasic, S.; Horowitz, G.L.; Feld, M.S. Raman spectroscopy for noninvasive glucose measurements. J. Biomed. Opt. 2005, 10, 031114. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In vivo blood glucose quantification using Raman spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef] [PubMed]
- Lyandres, O.; Yuen, J.M.; Shah, N.C.; VanDuyne, R.P.; Walsh, J.T., Jr.; Glucksberg, M.R. Progress toward an in vivo surface-enhanced Raman spectroscopy glucose sensor. Diabetes Technol. Ther. 2008, 10, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.C.; Van Duyne, R.P.; Chalmers, J.M.; Griffiths, P.R. Electromagnetic Mechanism of Surface-Enhanced Spectroscopy. In Handbook of Vibrational Spectroscopy; Wiley & Sons: New York, NY, USA, 2002; pp. 759–774. [Google Scholar] [CrossRef]
- Ju, J.; Liu, W.; Perlaki, C.M.; Chen, K.; Feng, C.; Liu, Q. Sustained and Cost Effective Silver Substrate for Surface Enhanced Raman Spectroscopy Based Biosensing. Sci. Rep. 2017, 7, 6917. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.M.; Shah, N.C.; Walsh, J.T., Jr.; Glucksberg, M.R.; Van Duyne, R.P. Transcutaneous glucose sensing by surface-enhanced spatially offset Raman spectroscopy in a rat model. Anal. Chem. 2010, 82, 8382–8385. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Clark, I.P.; Draper, E.R.C.; Morris, M.D.; Goodship, A.E.; Everall, N.; Towrie, M.; Finney, W.F.; Parker, A.W. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl. Spectrosc. 2005, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yuen, J.M.; Shah, N.C.; Walsh, J.T., Jr.; Glucksberg, M.R.; Van Duyne, R.P. In vivo, transcutaneous glucose sensing surface-enhanced spatially offset Raman spectroscopy: Multiple rats, improved hypoglycemic accuracy, low incident power, and continuous monitoring for greater than 17 days. Anal. Chem. 2011, 83, 9146–9152. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.F.; Sann, E.E.; Lou, X.; Liu, R.; Dai, J.; Zuo, X.; Xia, F.; Jiang, L. Naked-eye point-of-care testing platform based on a pH-responsive superwetting surface: Toward the non-invasive detection of glucose. NPG Asia Mater. 2018, 11, 117. [Google Scholar] [CrossRef]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabetic Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Brauker, J. Continuous glucose sensing: Future technology developments. Diabetes Technol. Ther. 2009, 11, S25–S36. [Google Scholar] [CrossRef] [PubMed]
- Aye, T.; Block, J.; Buckingham, B. Toward closing the loop: An update on insulin pumps and continuous glucose monitoring systems. Endocrinol. Metab. Clin. N. Am. 2010, 39, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Weigl, W.; Milej, D.; Gerega, A.; Toczylowska, B.; Sawosz, P.; Kacprzak, M.; Janusek, D.; Wojtkiewicz, S.; Maniewski, R.; Liebert, A. Confirmation of brain death using optical methods based on tracking of an optical contrast agent: Assessment of diagnostic feasibility. Sci. Rep. 2018, 8, 7332. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.A.; Majumder, S.K.; Ellis, D.L.; Billheimer, D.D.; Mahadevan-Jansen, A. In vivo nonmelanoma skin cancer diagnosis using Raman microspectroscopy. Laser Surg. Med. 2008, 40, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Zhao, J.; McLean, D.I.; Zeng, H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012, 72, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.G.; Li, X.; Lee, H.W.; Singh, T.D.; Lee, S.B.; Ji, H.D.; Yoon, G.; Cho, S.J.; Lee, I.K.; Jeong, S.Y.; et al. Non-invasive visualization of mast cell recruitment and its effects in lung cancer by optical reporter gene imaging and glucose metabolism monitoring. Biomaterials 2017, 112, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Tsalikian, E.; Fox, L.; Weinzimer, S.; Buckingham, B.; White, N.H.; Beck, R.; Kollman, C.; Xing, D.; Ruedy, K. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr. Diabetes 2012, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ichimori, S.; Nishida, K.; Shimoda, S.; Sekigami, T.; Matsuo, Y.; Ichinose, K.; Shichiri, M.; Sakakida, M.; Araki, E. Development of a highly responsive needle-type glucose sensor using polyimide for a wearable artificial endocrine pancreas. J. Artif. Organs 2006, 9, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Li, Y.; Zhu, H.; Banerjee, P.; Zhou, S. Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 2012, 33, 7115–7125. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.C.; Shukla, S.; Skoog, S.A.; Boehm, R.D.; Narayan, R.J. Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors 2019, 19, 1028. https://doi.org/10.3390/s19051028
Pandey PC, Shukla S, Skoog SA, Boehm RD, Narayan RJ. Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors. 2019; 19(5):1028. https://doi.org/10.3390/s19051028
Chicago/Turabian StylePandey, Prem C., Shubhangi Shukla, Shelby A. Skoog, Ryan D. Boehm, and Roger J. Narayan. 2019. "Current Advancements in Transdermal Biosensing and Targeted Drug Delivery" Sensors 19, no. 5: 1028. https://doi.org/10.3390/s19051028
APA StylePandey, P. C., Shukla, S., Skoog, S. A., Boehm, R. D., & Narayan, R. J. (2019). Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors, 19(5), 1028. https://doi.org/10.3390/s19051028






