Zinc/Aluminium–Quinclorac Layered Nanocomposite Modified Multi-Walled Carbon Nanotube Paste Electrode for Electrochemical Determination of Bisphenol A
Abstract
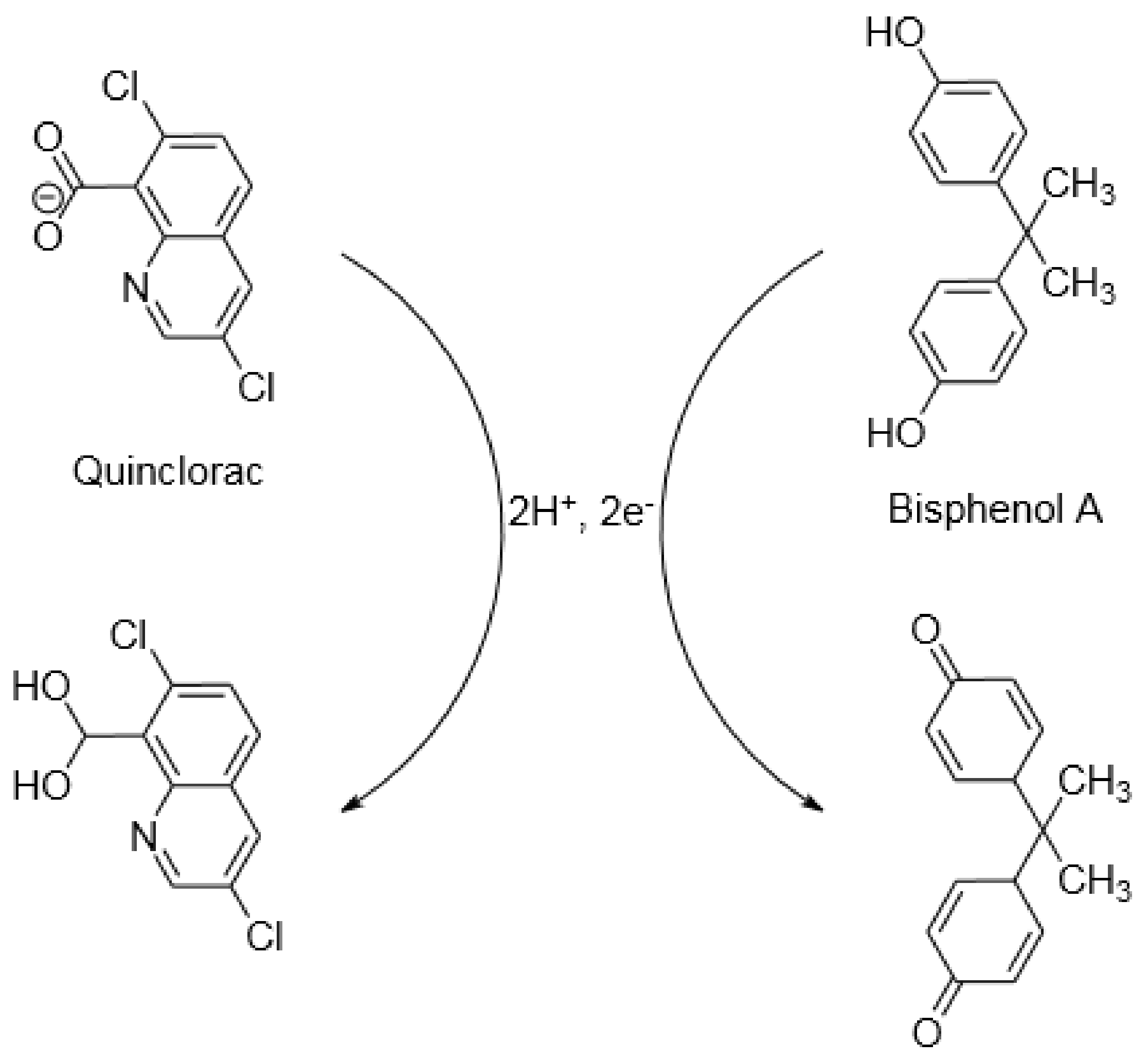
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
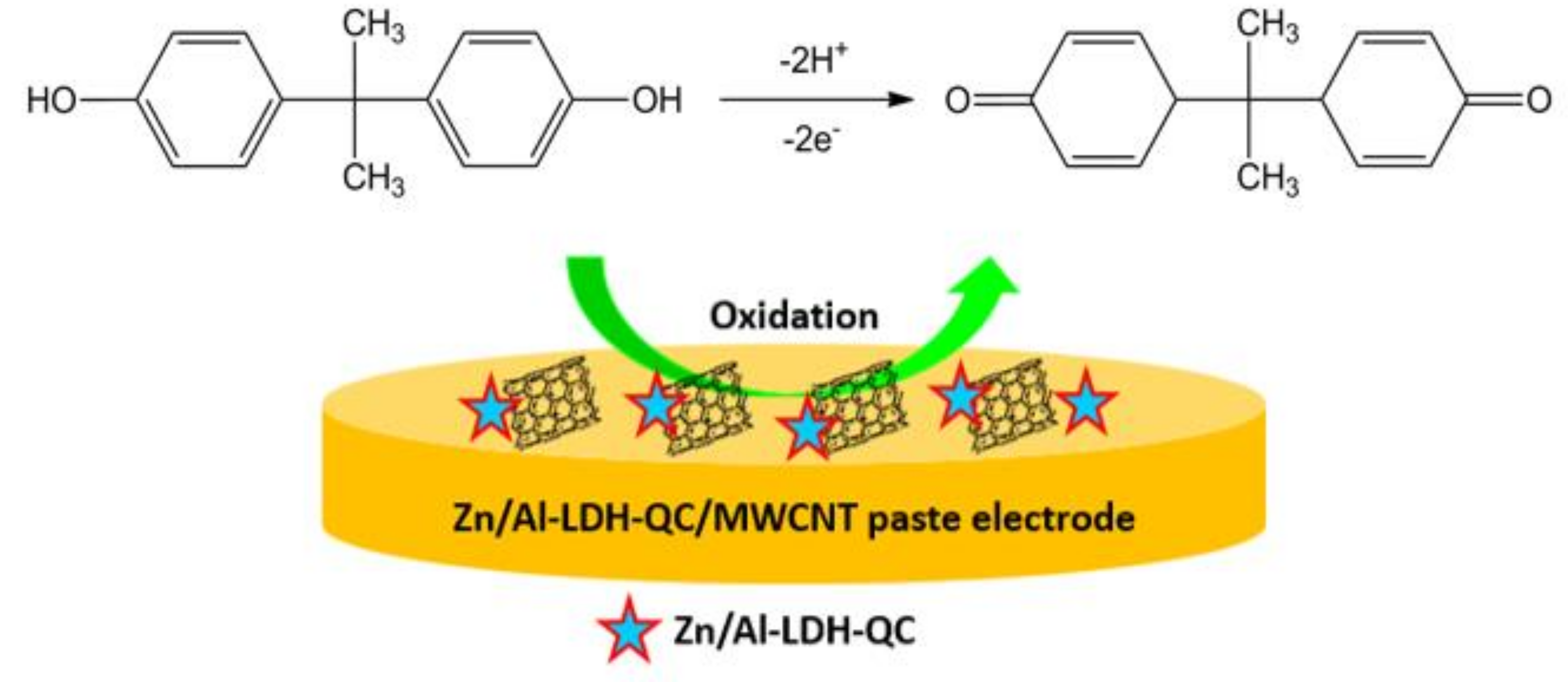
2.3. Synthesis of Zn/Al-LDH-QC Nanocomposite
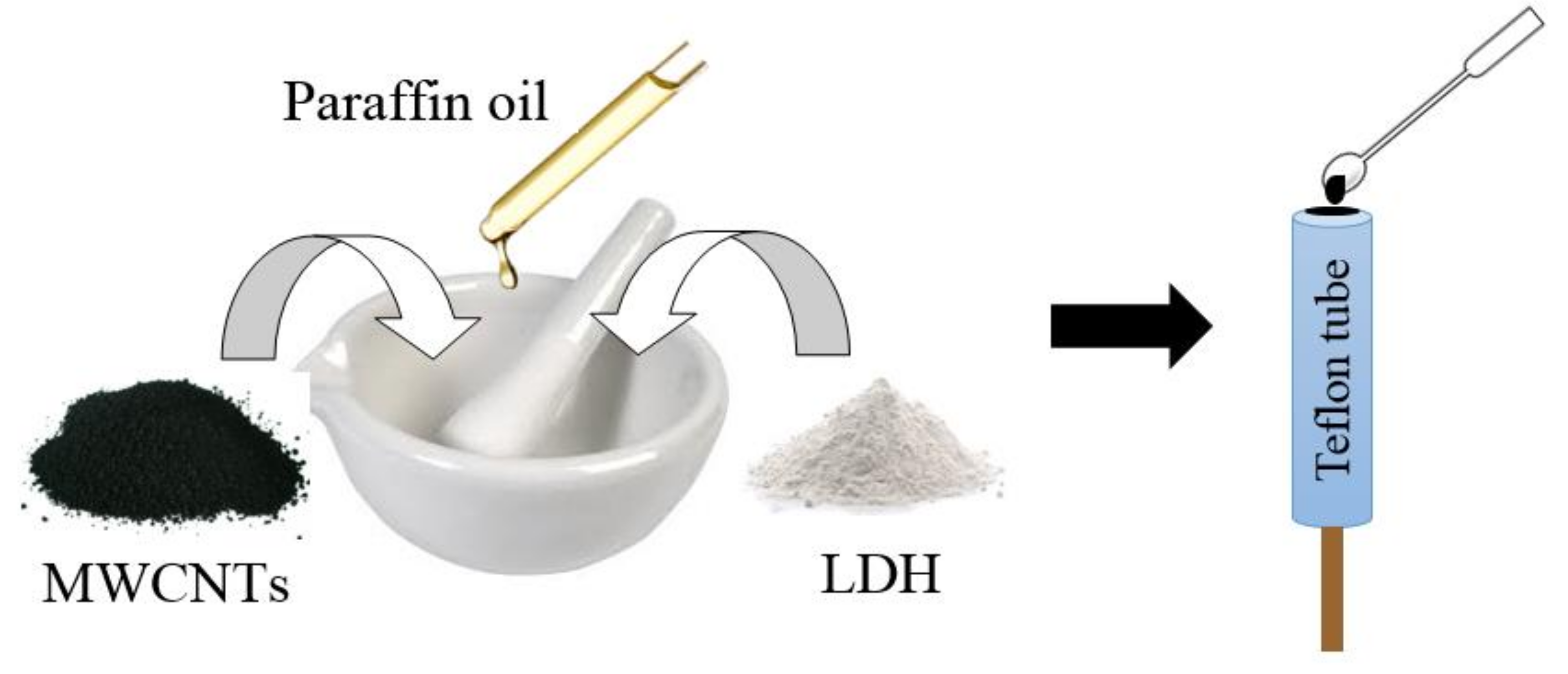
2.4. Preparation of Electrodes
2.5. Measurement Procedure
2.6. Real Samples
3. Results
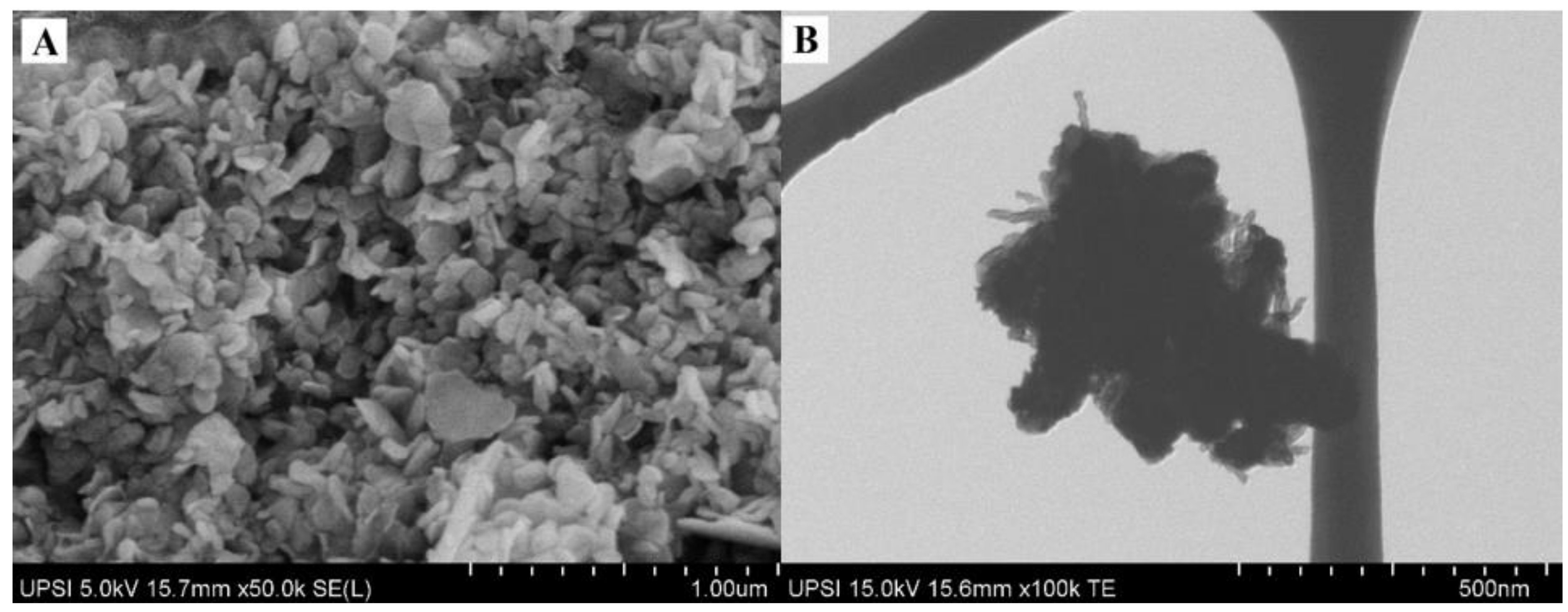
3.1. Surface Morphology Studies of Zn/Al-LDH-QC-MWCNT
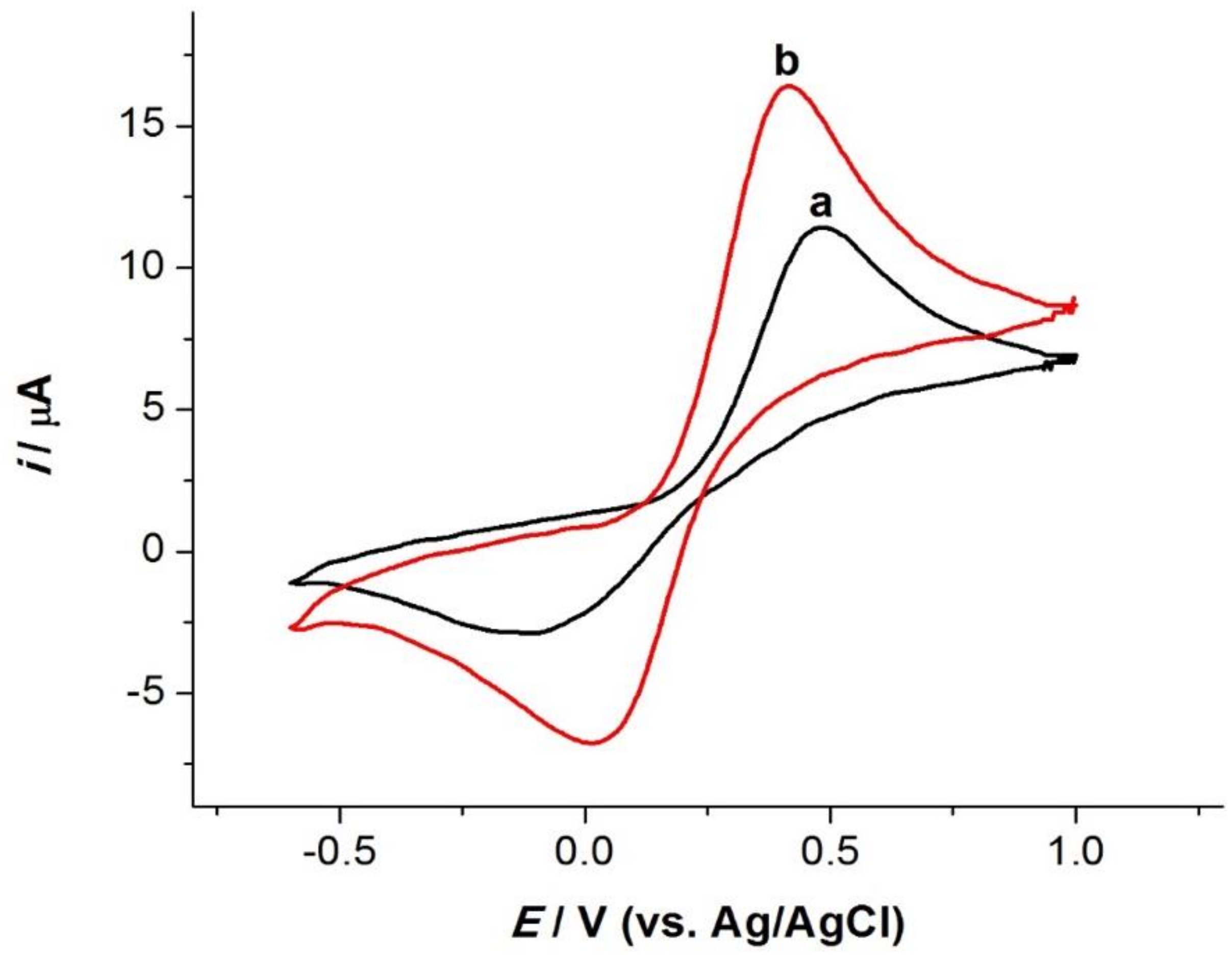
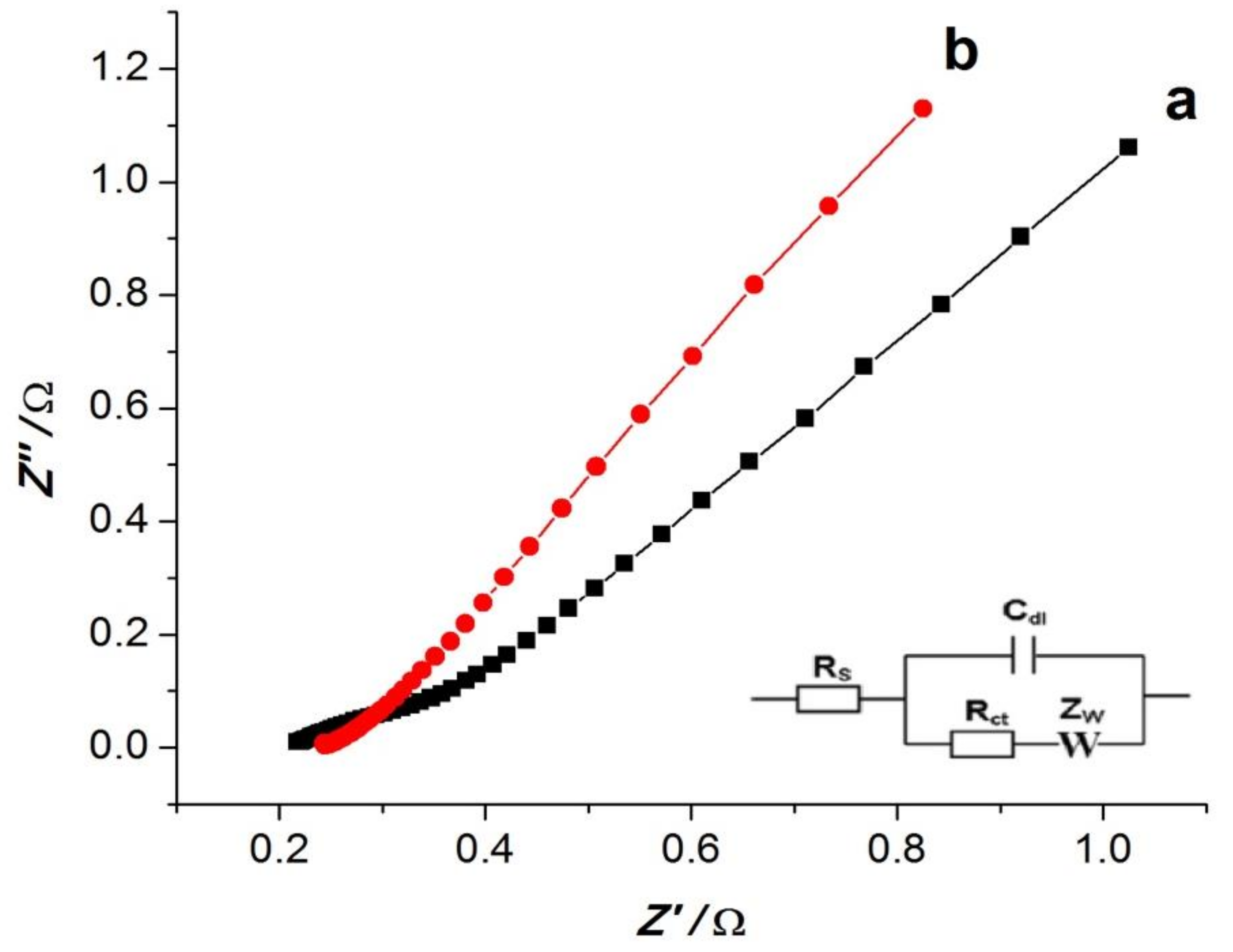
3.2. Electrode Characterization
3.3. Optimization of the Experimental Conditions
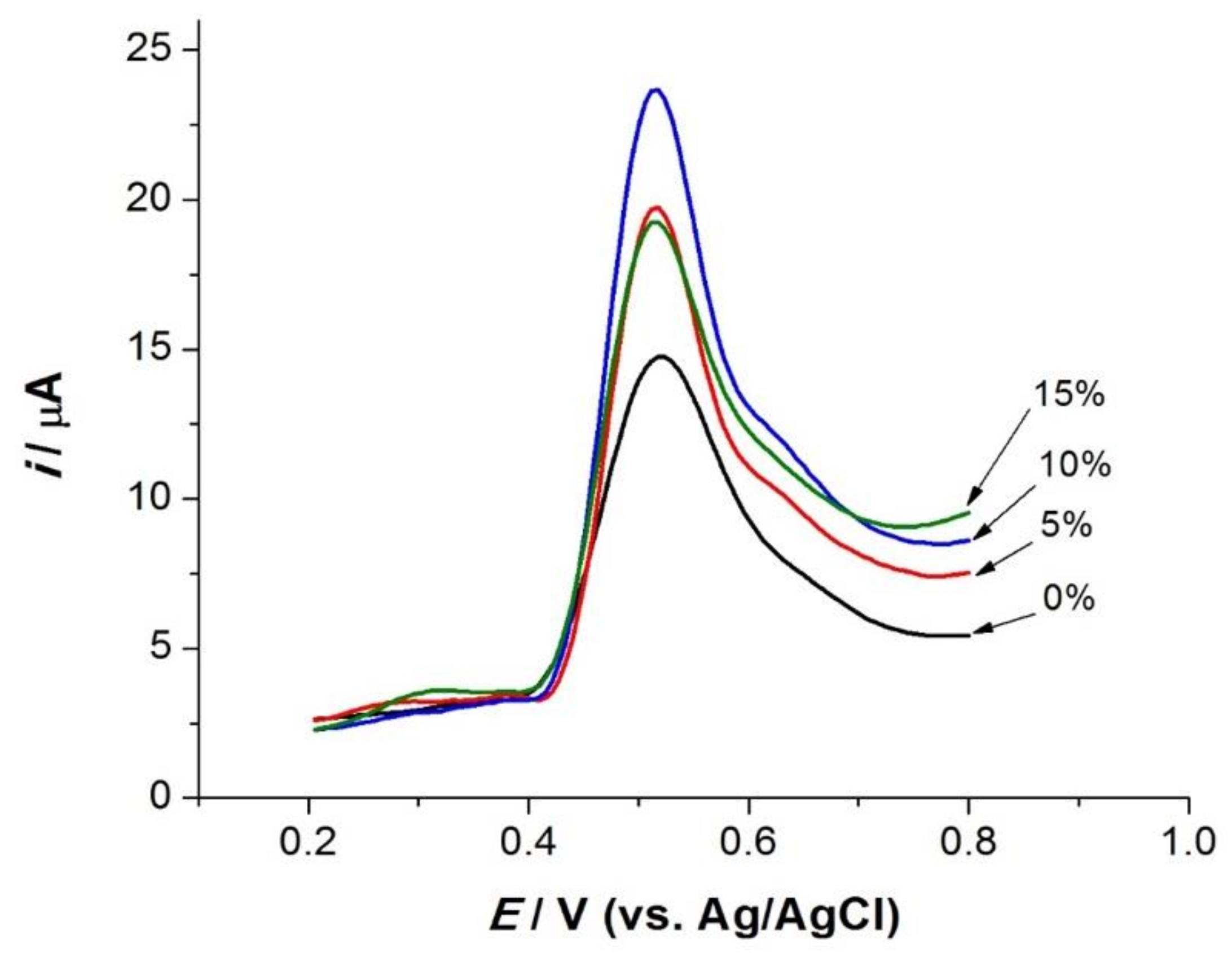
3.3.1. Effect of Modifier Content
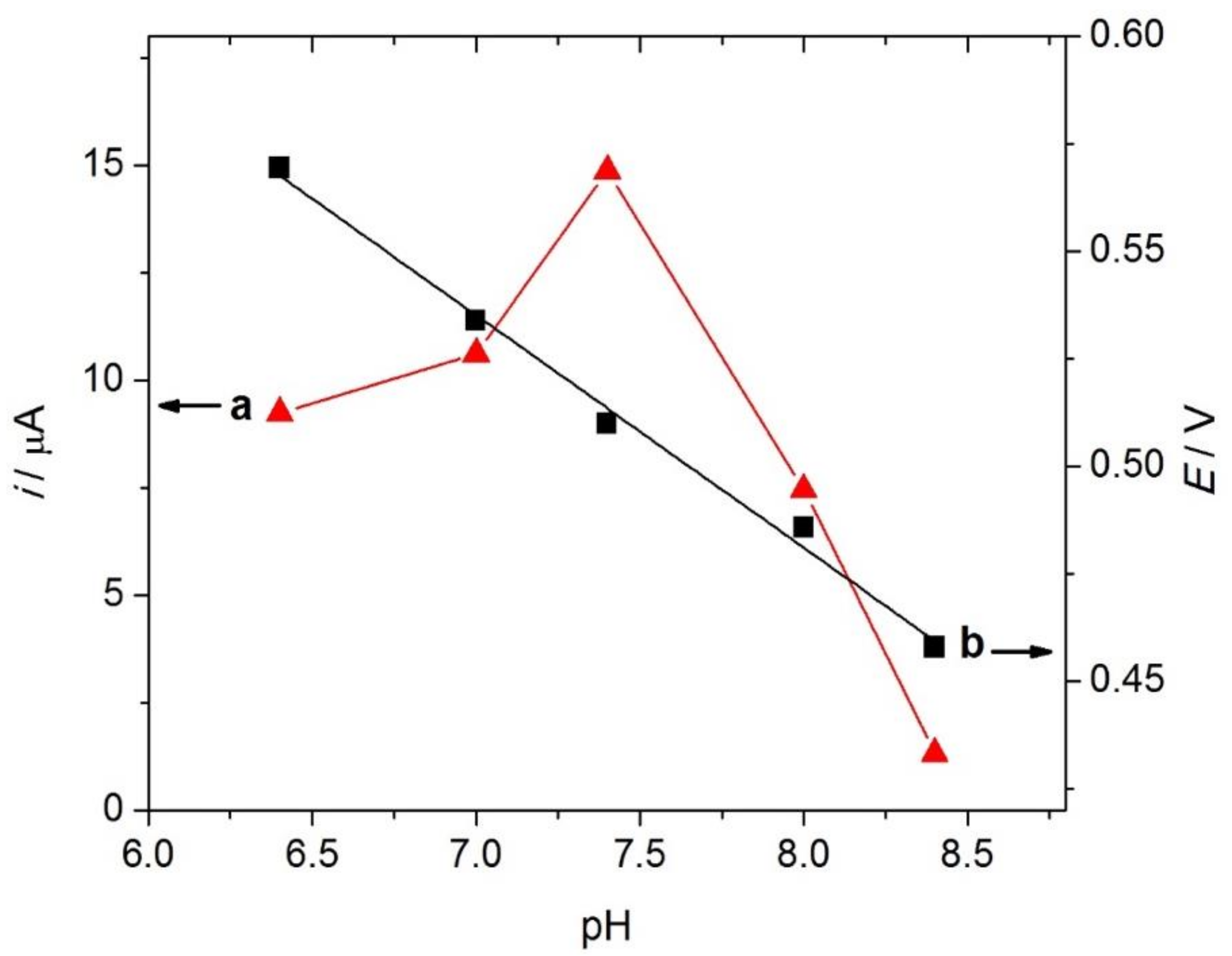
3.3.2. Effect of pH
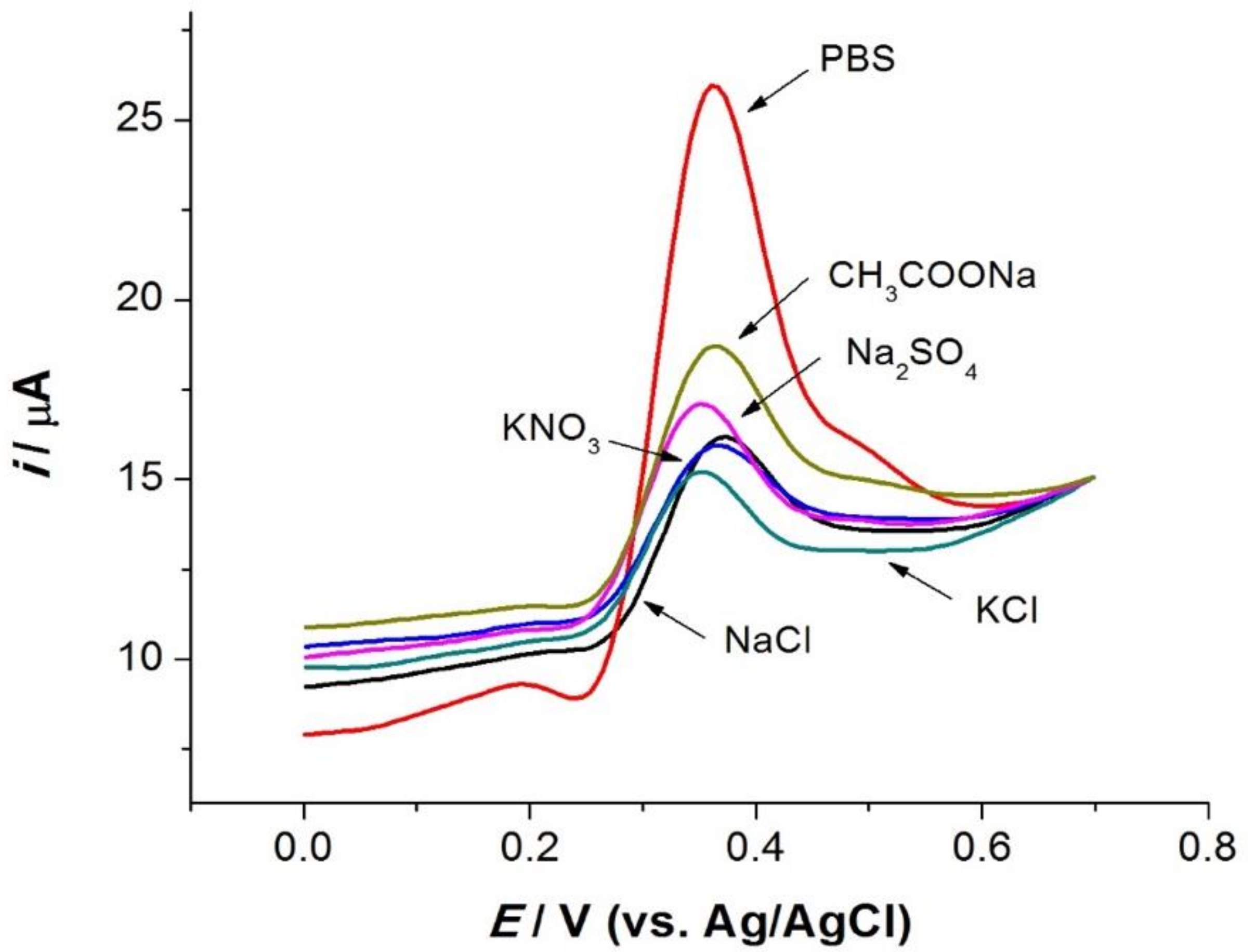
3.3.3. Effect of Electrolytes
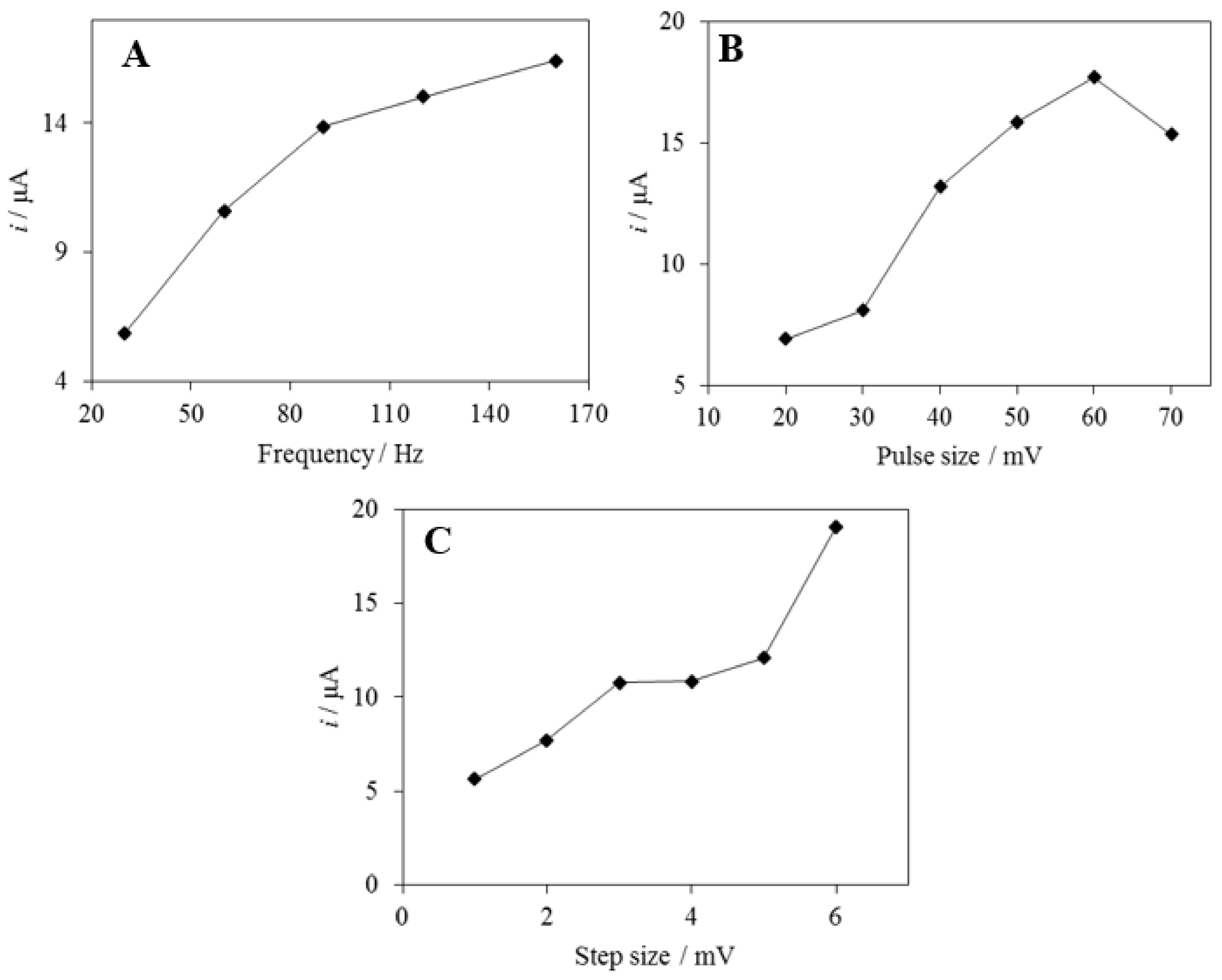
3.3.4. Effect of SWV Parameters
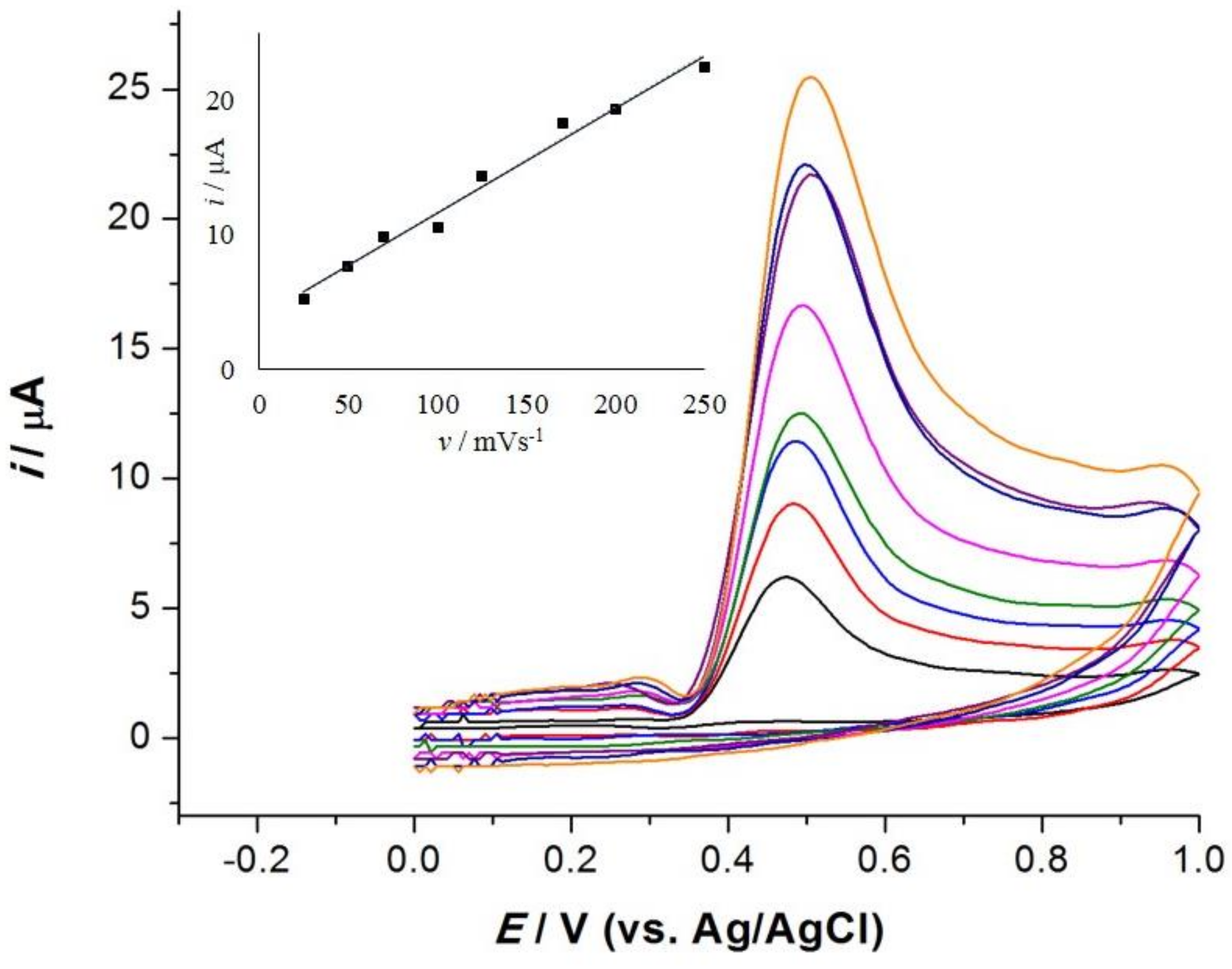
3.4. Effects of Scan Rate
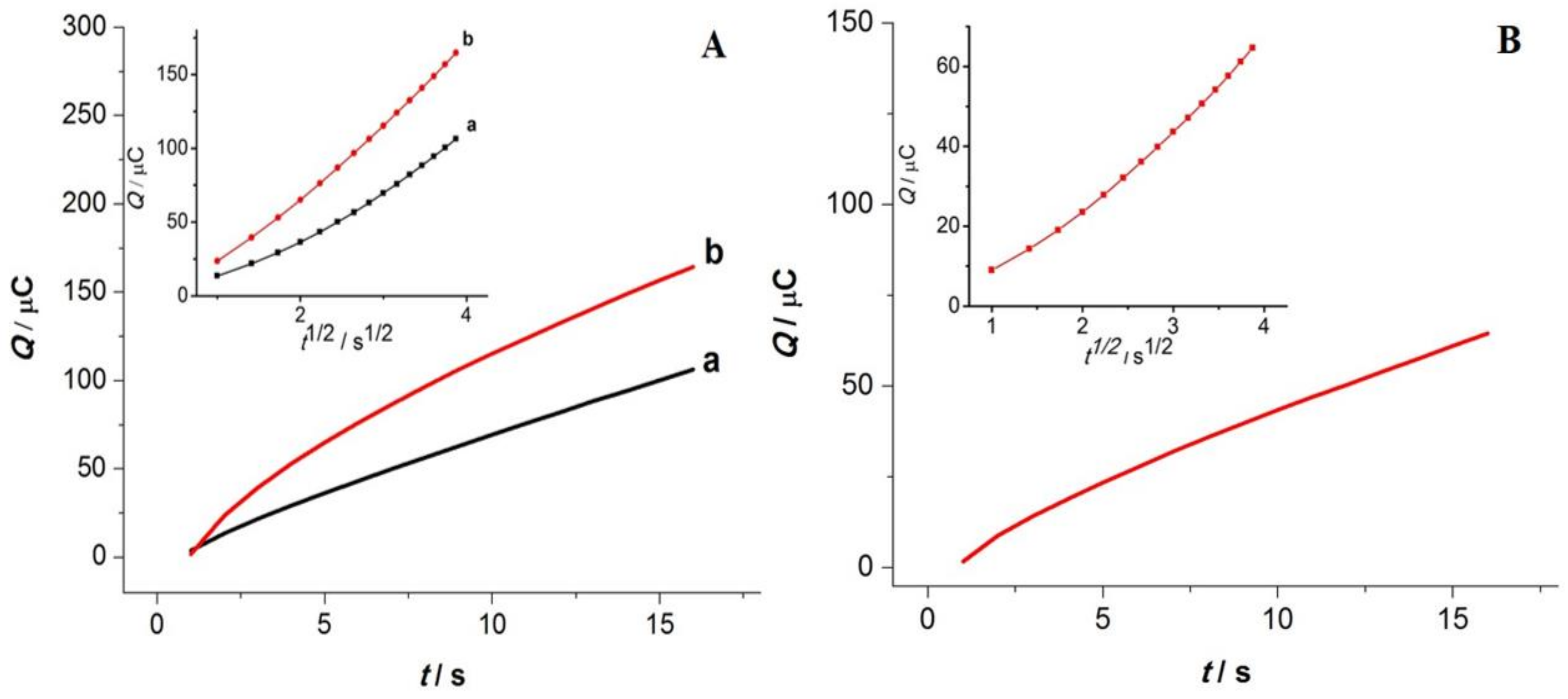
3.5. Chronocoulometry Studies
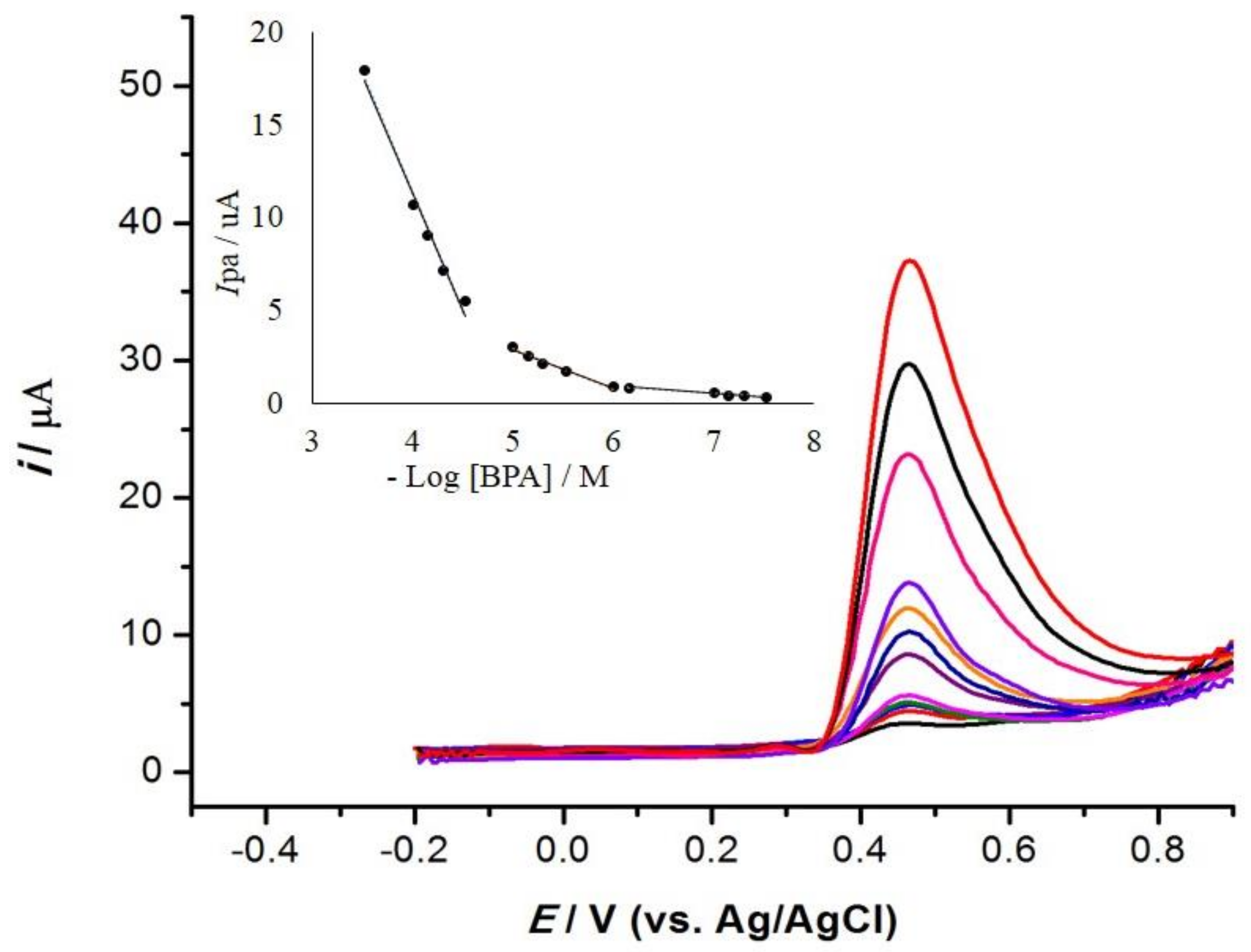
3.6. Calibration Curve
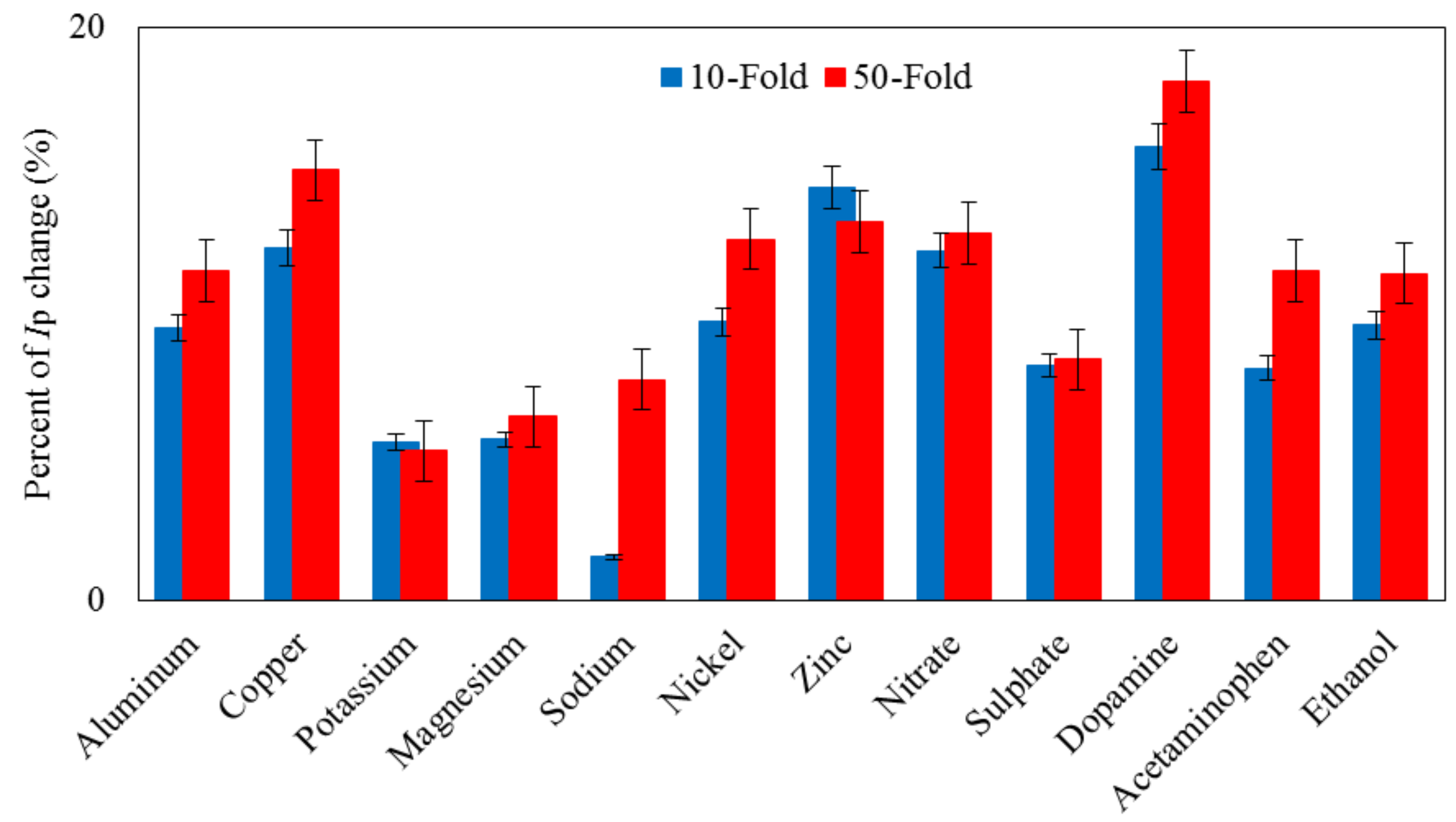
3.7. Reproducibility, Stability and Interferences
3.8. Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosu, D.; Mustata, F.; Tudorachi, N.; Musteata, V.E.; Rosu, L.; Varganici, C.D. Novel bio-based flexible epoxy resin from diglycidyl ether of bisphenol A cured with castor oil maleate. RSC Adv. 2015, 5, 45679–45687. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Deng, D.; Jin, J.; Lu, X.; Chen, J. Nanographene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens. Bioelectron. 2012, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Navas, J.M.; Schäfers, C.; Wenzel, A. Potencies of estrogenic compounds in in vitro screening assays and in life cycle tets with zebrafish in vivo. Ecotoxicol. Environ. Saf. 2003, 54, 315–322. [Google Scholar] [CrossRef]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Gray, L.E.; Hayward, S.W.; Lees, P.S.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defect Res. B 2008, 83, 157–395. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Irie, M.; Kishikawa, N.; Wada, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Santoni, E.; Vittori, S.; Font, G.; Mañes, J.; Sagratini, G. Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry in powdered milk and infant formulas. Food Chem. 2011, 126, 360–367. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomedic. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.F.; Zhou, M.; Gu, J.; Li, X.M. Spectrophotometric and high performance liquid chromatographic methods for sensitive determination of bisphenol A. Spectrochim. Acta A 2014, 122, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zhu, D.; Huang, C.P.; Sun, Y.H.; Lee, Y.I. Sensitive detection of bisphenol A in complex samples by in-column molecularly imprinted solid-phase extraction coupled with capillary electrophoresis. Microchem. J. 2015, 121, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.Q.; Zhang, K.; Zho, J.Q. Cd-doped ZnO quantum dots-based immunoassay for the quantitative determination of bisphenol A. Chemosphere 2014, 95, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T. A novel chemiluminescence method for determination of bisphenol A based on the carbon dot-enhanced HCO3—H2O2. J. Lumin. 2015, 158, 160–164. [Google Scholar] [CrossRef]
- Kaddar, N.; Bendridi, N.; Harthé, C.; de Ravel, M.R.; Bienvenu, A.-L.; Cuilleron, C.-Y.; Mappus, E.; Pugeat, M; Déchaud, H. Development of radioimmunoassay for the measurement of bisphenol A in biological samples. Anal. Chim. Acta 2009, 645, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H.; Zhang, Q. Disposable paper-based electrochemical sensor based on stacked gold nanoparticles supported carbon nanotubes for the determination of bisphenol A. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zheng, S. A novel and label-free immunosensor for bisphenol A using rutin as the redox probe. Talanta 2016, 160, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Liu, Y.J.; Liu, Y.M.; Wang, L.L. Molybdenum disulfide nanoflower-chitosan-Au nanoparticles composites based electrochemical sensing platform for bisphenol A determination. J. Hazard. Mater. 2014, 276, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Goulart, L.A.; Mascaro, L.H. GC electrode modified with carbon nanotubes and NiO for the simultaneous determination of bisphenol A, hydroquinone and catechol. Electrochim. Acta 2016, 196, 48–55. [Google Scholar] [CrossRef]
- Nilkahd, B.; Khalilzadeh, M.A. Liquid phase determination of bisphenol A in food samples using novel nanostructure ionic liquid modified sensor. J. Mol. Liq. 2016, 215, 253–257. [Google Scholar] [CrossRef]
- Santana, E.R.; de Lima, C.A.; Piovesa, J.V.; Spinelli, A. An original ferroferric oxide and gold nanoparticles-modified glassy carbon electrode for the determination of bisphenol A. Sens. Actuators B 2017, 240, 487–496. [Google Scholar] [CrossRef]
- Hou, C.; Tang, W.; Zhang, C.; Wang, Y.; Zhu, N. A novel and sensitive electrochemical sensor for bisphenol A determination based on carbon black supporting ferroferric oxide nanoparticles. Electrochim. Acta 2014, 144, 324–331. [Google Scholar] [CrossRef]
- Peng, L.; Dong, S.; Xie, H.; Gu, G.; He, Z.; Lu, J.; Huang, T. Sensitive simultaneous determination of diethylstilbestrol and bisphenol A based on Bi2WO6 nanoplates modified carbon paste electrode. J. Electroanal. Chem. 2014, 726, 15–20. [Google Scholar] [CrossRef]
- Wardani, N.I.; Isa, I.M.; Hashim, N.; Ghani, S.A. Zinc layered hydroxide-2(3-chlorophenoxy)propionate modified multiwalled carbon nanotubes paste electrode for the determination of nanomolar levels copper(II). Sens. Actuators B 2014, 198, 243–248. [Google Scholar] [CrossRef]
- Isa, I.M.; Sharif, S.N.M.; Hashim, N.; Ghani, S.A. Amperometric determination of nanomolar mercury (II) by layered double nanocomposite of zinc/aluminium hydroxide-3(4-methoxyphenyl) propionate modified single-walled carbon nanotube paste electrode. Ionics 2015, 21, 2949–2958. [Google Scholar] [CrossRef]
- Isa, I.M.; Fasyir, M.R.; Hashim, N.; Ghani, S.A.; Bakar, S.A.; Mohamed, A.; Kamari, A. A highly sensitive mercury (II) sensor using Zn/Al layered double hydroxide-3(4-hydroxyphenyl) propionate modified multi-walled carbon nanotube paste electrode. Int. J. Electrochem. Sci. 2015, 10, 6227–6240. [Google Scholar]
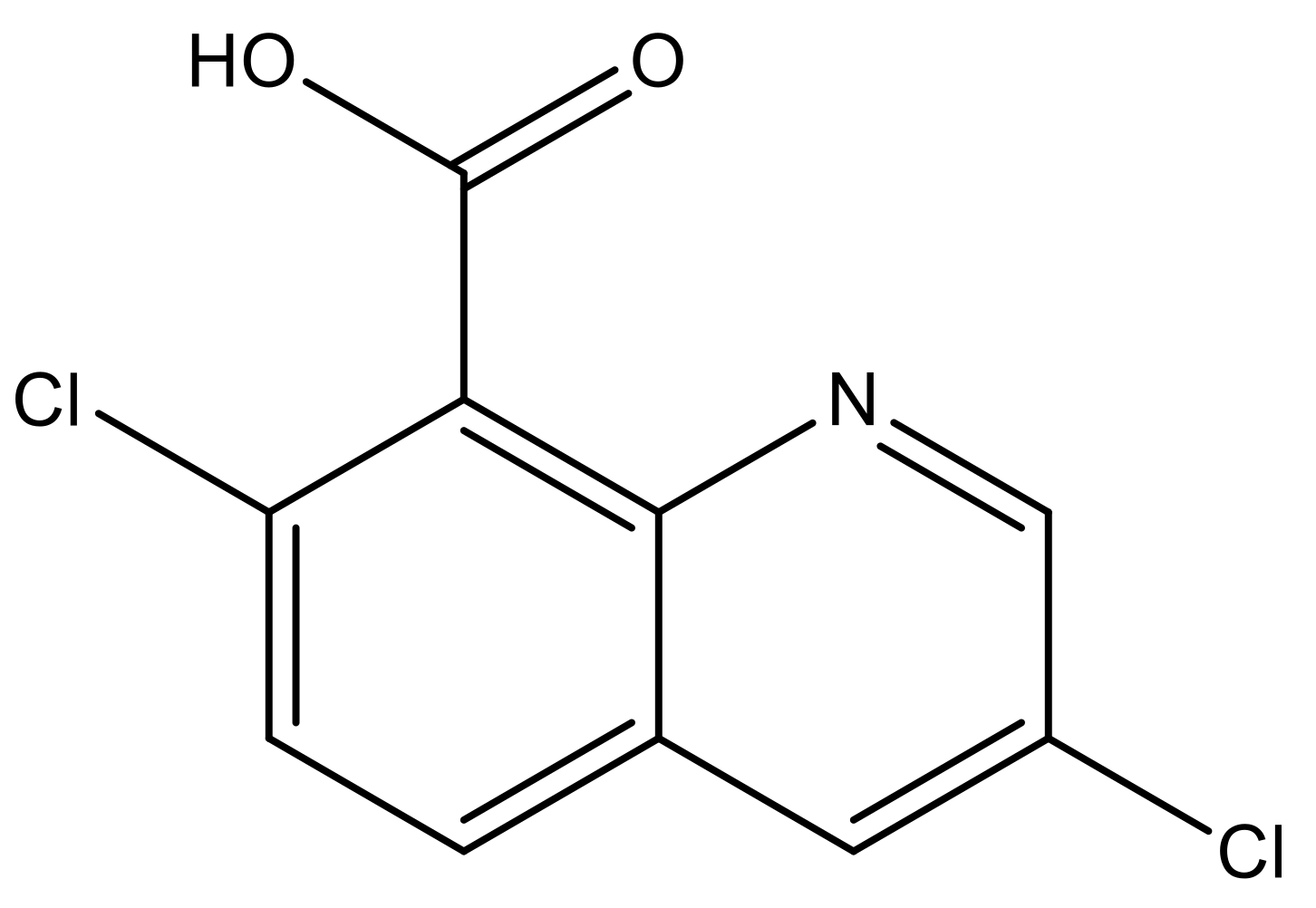
- Sharif, S.N.M.; Hashim, N.; Isa, I.M.; Ali, N.M.; Bakar, S.A.; Hussein, M.Z.; Mamat, M.; Bakar, N.A.; Mahamod, W.R.W. Preparation and characterization of novel paddy cultivation herbicide nanocomposite from zinc/aluminium layered double hydroxide and quinclorac anion. Mater. Res. Innov. 2018, 1–6. [Google Scholar] [CrossRef]
- Concenco, G.; Silva, A.F.; Ferreira, E.A.; Galon, L.; Noldin, J.A.; Aspiazu, I.; Ferreira, F.A.; Silva, A.A. Effect of dose and application site on quinclorac absorption by barnyardgrass biotypes. Planta Daninha 2009, 27, 541–548. [Google Scholar] [CrossRef]
- Sljukic, B.; Banks, C.E.; Crossley, A.; Compton, R.G. Iron (III) oxide graphite composite electrodes: application to the electroanalytical detection of hydrazine and hydrogen peroxide. Electroanalysis 2006, 18, 1757–1762. [Google Scholar] [CrossRef]
- Keyvanfard, M.; Shakeri, R.; -Maleh, H.K.; Alizad, K. Highly selective and sensitive voltammetric sensor based on modified multiwall carbon nanotube paste electrode for simultaneous determination of ascorbic acid, acetaminophen and tryptophan. Mater. Sci. Eng. C 2013, 33, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Thomas, T.; Basavaraja, B.M.; Saxena, A.K.; Mukhopadhyay, K.; Roy, D. Platinum decorated multi-walled carbon nanotubes/Triton X-100 modified carbon paste electrode for the sensitive amperometric determination of paracetamol. J. Elec. Chem. 2015, 739, 49–57. [Google Scholar] [CrossRef]
- Apodaca, D.C.; Pernites, R.B.; Ponnapati, R.; Mundo, F.R.D.; Advincula, R.C. Electropolymerized molecularly imprinted polymer film: EIS sensing of bisphenol A. Macromoleculec 2011, 44, 6669–6682. [Google Scholar] [CrossRef]
- Su, B.; Shao, H.; Li, N.; Chen, X.; Chai, Z.; Chen, X. A sensitive bisphenol A voltammetric sensor relying on AuPd nanoparticles/graphene composites modified glassy carbon electrode. Talanta 2017, 166, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, J.G.; Osteryoung, R.A. Square-wave voltammetry. Anal. Chem. 1985, 57, 101A–102A. [Google Scholar] [CrossRef]
- Dong, X.; Qi, X.; Liu, N.; Yang, Y.; Piao, Y. Direct electrochemical detection of bisphenol A using a highly conductive graphite nanoparticles film electrode. Sensors 2017, 17, 836. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.S.; Pellicano, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Ghanam, A.; Lahcen, A.A.; Amine, A. Electroanalytical determination of bisphenol A: Investigation of electrode surface fouling using various carbon materials. J. Electroanal. Chem. 2017, 789, 58–66. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Li, X.; Hou, W. Electrochemical sensor for bisphenol A based on ionic liquid functionalized Zn-Al layered double hydroxide modified electrode. Mater. Sci. Eng. C 2016, 64, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Song, Y.; Tan, Z.; Hou, W. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Li, S.; Dang, Y. A novel electrochemical sensor for highly sensitive detection of bisphenol A based on the hydrothermal synthesized Na-doped WO3 nanorods. Sens. Actuators B 2017, 245, 238–246. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Ghica, M.E.; Dridi, C.; Ali, M.B.; Brett, C.M.A. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Shim, K.; Kim, J.; Shahabuddin, M.; Yamauchi, Y.; Hossain, M.S.A.; Kim, J.H. Efficient wide range electrochemical bisphenol-A sensor by self-supported dendritic nanoparticles on screen-printed carbon electrode. Sens. Actuators B 2017, 255, 2800–2808. [Google Scholar] [CrossRef]
- Hu, X.; Feng, Y.; Wang, H.; Zhao, F.; Zeng, B. A novel bisphenol A electrochemical sensor based on a molecularly imprinted polymer/carbon nanotubes-Au nanoparticles/boron-doped ordered mesoporous carbon composite. Anal. Methods 2018, 10, 4543–4548. [Google Scholar] [CrossRef]
- Koyun, O.; Gorduk, S.; Gencten, M.; Sahin, Y. A novel copper (II) phthalocyanine-modified multiwalled carbon nanotube-based electrode for sensitive electrochemical detection of bisphenol A. New J. Chem. 2019, 43, 85–92. [Google Scholar] [CrossRef]
- Shi, R.; Yuan, X.; Liu, A.; Xu, M.; Zhao, Z. Determination of bisphenol A in beverages by an electrochemical sensor based on Rh2O3/reduced grapheme oxide composites. Appl. Sci. 2018, 8, 2535. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Lahcen, A.A.; Dridi, C.; Amine, A. Ultrasound assisted magnetic imprinted polymer combined sensor based on carbon black and gold nanoparticles for selective and sensitive electrochemical detection of bisphenol A. Sens. Actuators B 2018, 276, 304–312. [Google Scholar] [CrossRef]
- Butmee, P.; Tumcharern, G.; Saejueng, P.; Stankovic, D.; Ortner, A.; Jitcharoen, J.; Kalcher, K.; Samphao, A. A direct and sensitive electrochemical sensing platfor baed on ionic liquid functionalized grapheme nanoplatelets for the detection of bisphenol A. J. Electroanal. Chem. 2019, 833, 370–379. [Google Scholar] [CrossRef]
| Modifier | Electrode | Linear Range (µM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| ILs/Zn-Al-LDH | GCE | 0.02–3.00 | 4.6 | [39] |
| Exfoliated Ni2Al-LDH nanosheets | GCE 1 | 0.02–1.51 | 6.8 | [40] |
| Fe3O4/AuNPs | GCE | 0.02–1.40 | 7.0 | [21] |
| Na-doped WO3 nanorods | CPE 2 | 0.08–22.5 | 28.0 | [41] |
| MWCNT/AuNPs | GCE | 0.01–0.70 | 4.0 | [42] |
| DPNs/PEI-PC | SPCE 3 | 0.01–1.00, and 1.00–300 | 6.63 | [43] |
| MIP/CNTs-Au/BOMC | GCE | 0.01–10.0 | 5.0 | [44] |
| CuPC/MWCNT-COOH | PGE 4 | 0.10–27.5 | 18.9 | [45] |
| Rh2O3/rGO | GCE | 0.60–40.0 | 120.0 | [46] |
| US-MagMIP/CBNPs | SPCE | 0.07–10.0 | 8.8 | [47] |
| IL/GNPs | GCPE 5 | 0.02–5.00 | 6.4 | [48] |
| Zn/Al-LDH-quinclorac/MWCNT | CPE | 0.03–0.70, 1.00–10.0, and 30.0–300 | 4.4 | This work |
| Real Aample | BPA Detected (μM) | BPA Added (μM) | BPA Found (μM) | Recovery (%) |
|---|---|---|---|---|
| Baby bottle | N.D. 1 | 8 | 7.985 ± 0.19 2 | 99.81 |
| Mineral water 1 | N.D. | 15 | 14.73 ± 0.56 | 98.22 |
| Mineral water 2 | N.D. | 15 | 15.15 ± 0.60 | 101.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainul, R.; Abd Azis, N.; Md Isa, I.; Hashim, N.; Ahmad, M.S.; Saidin, M.I.; Mukdasai, S. Zinc/Aluminium–Quinclorac Layered Nanocomposite Modified Multi-Walled Carbon Nanotube Paste Electrode for Electrochemical Determination of Bisphenol A. Sensors 2019, 19, 941. https://doi.org/10.3390/s19040941
Zainul R, Abd Azis N, Md Isa I, Hashim N, Ahmad MS, Saidin MI, Mukdasai S. Zinc/Aluminium–Quinclorac Layered Nanocomposite Modified Multi-Walled Carbon Nanotube Paste Electrode for Electrochemical Determination of Bisphenol A. Sensors. 2019; 19(4):941. https://doi.org/10.3390/s19040941
Chicago/Turabian StyleZainul, Rahadian, Nurashikin Abd Azis, Illyas Md Isa, Norhayati Hashim, Mohamad Syahrizal Ahmad, Mohamad Idris Saidin, and Siriboon Mukdasai. 2019. "Zinc/Aluminium–Quinclorac Layered Nanocomposite Modified Multi-Walled Carbon Nanotube Paste Electrode for Electrochemical Determination of Bisphenol A" Sensors 19, no. 4: 941. https://doi.org/10.3390/s19040941
APA StyleZainul, R., Abd Azis, N., Md Isa, I., Hashim, N., Ahmad, M. S., Saidin, M. I., & Mukdasai, S. (2019). Zinc/Aluminium–Quinclorac Layered Nanocomposite Modified Multi-Walled Carbon Nanotube Paste Electrode for Electrochemical Determination of Bisphenol A. Sensors, 19(4), 941. https://doi.org/10.3390/s19040941




