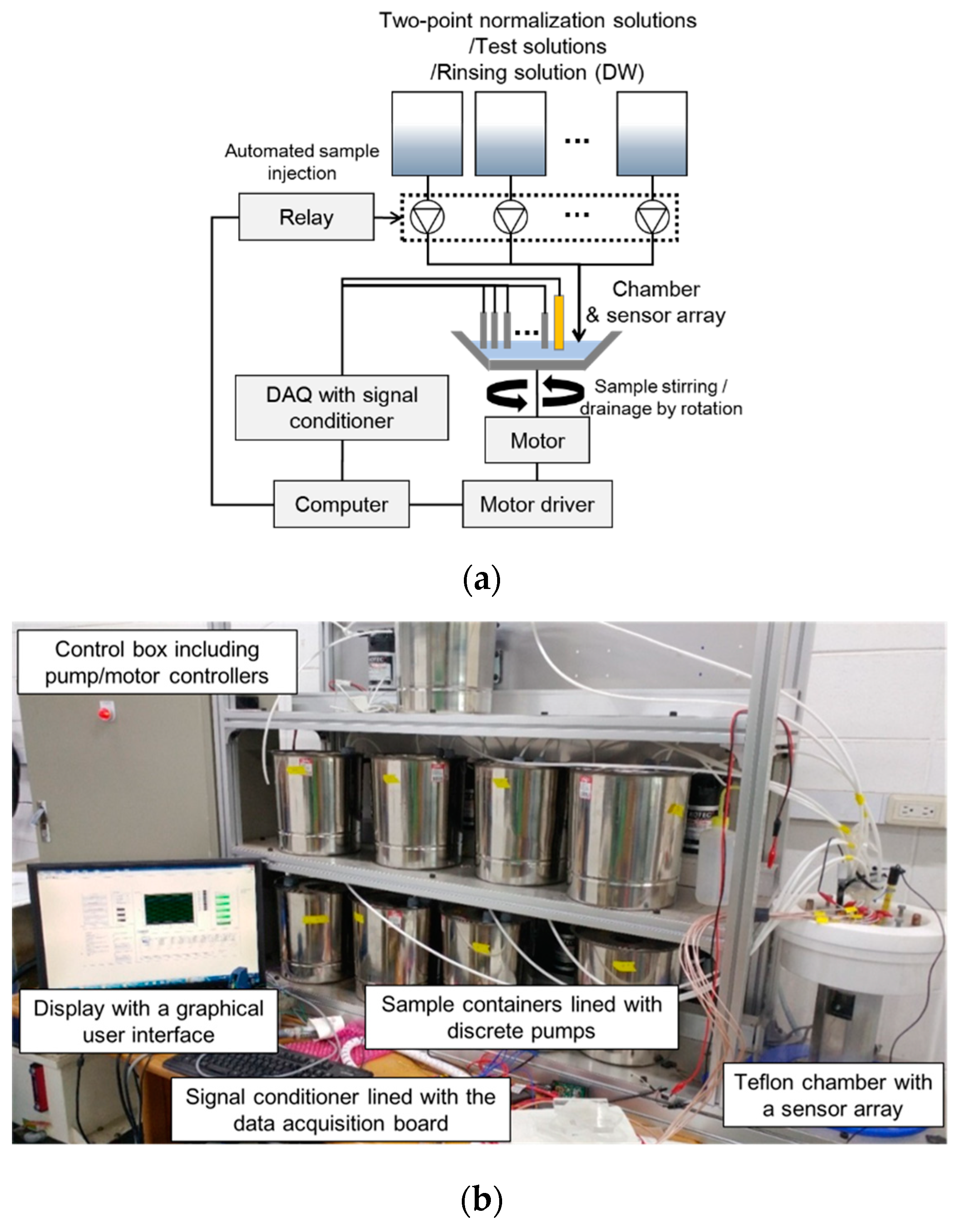
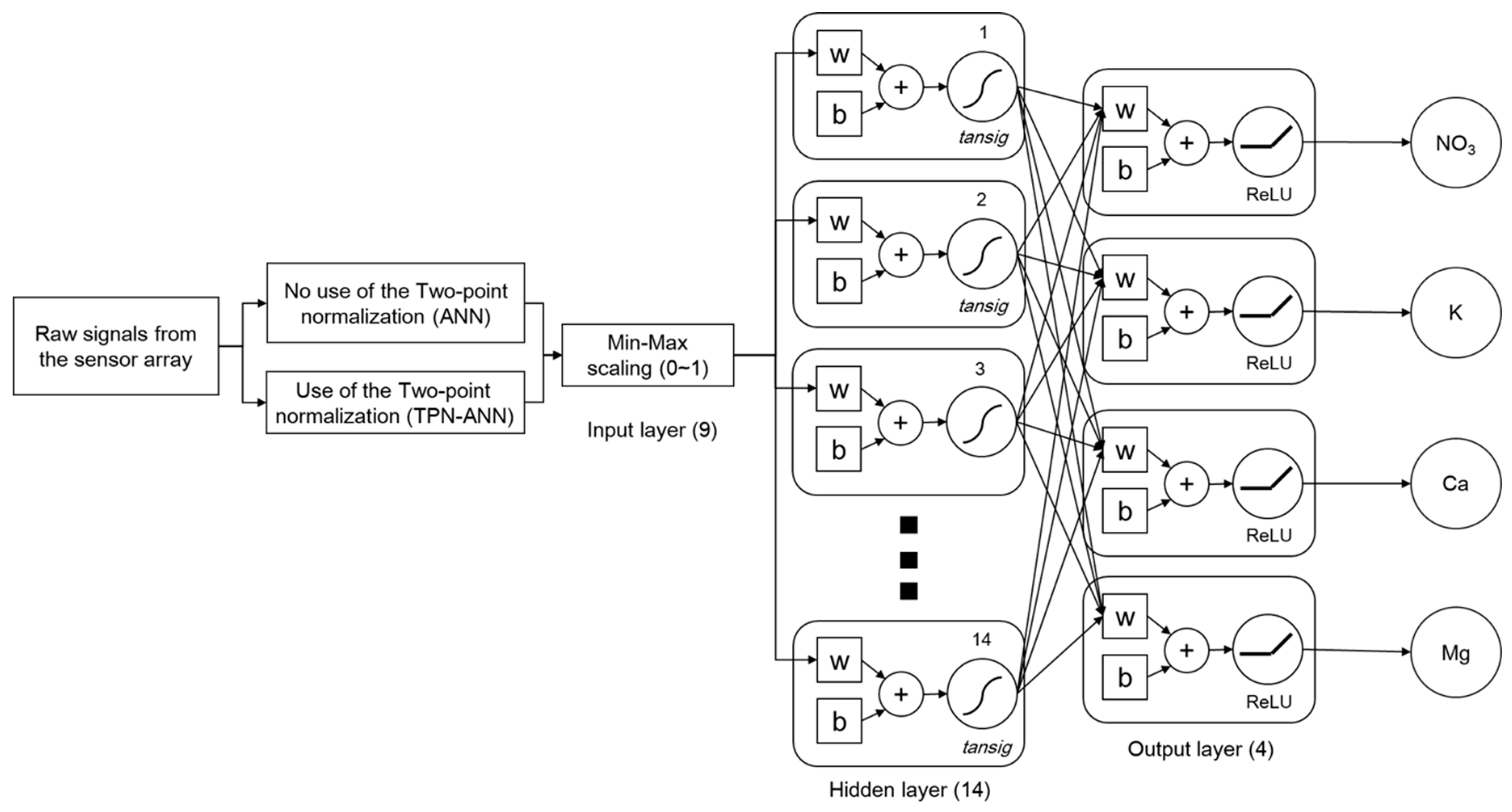
1. Introduction
Hydroponics is a cultivation method that grows plants using nutrient solutions composed of water and nutrient salts without soil. Recently, hydroponics has been widely and rapidly utilized in agricultural industries because it is the most intensive and effective production method that can be designed to support year-round production with high yields and good quality [
1,
2]. Hydroponics is usually classified into open and closed types. In open hydroponics, the nutrient solution flows through the growing bed and is discarded, which can result in the pollution of ground- and surface water [
3,
4]. In closed hydroponics, which collects drainage solutions and reuses these by replenishing water and nutrients, the use and discharge of water and nutrients are less than for open hydroponics [
5,
6,
7]. Therefore, a transition from open hydroponics to closed hydroponics is seen increasingly often due to the more environmentally-friendly aspect of closed hydroponics [
7]. However, current practices for closed hydroponics still have several limitations, as described below.
In closed hydroponics, the management of the reused solutions is mostly conducted by the conductivity and pH probes. However, the probes can only provide a total ion activity and pH, so the imbalance of nutrient ratios may occur in reused nutrient solutions due to the lack of information about the individual ion concentrations [
8,
9,
10,
11,
12]. This makes the crop quality and productivity decrease. Therefore, growers usually flush the nutrient solutions and replace all solutions periodically, despite the environmental pollution and loss of fertilizers [
13]. Although growers can analyze the individual ion concentrations of the nutrient solutions by periodic laboratory analysis, a time delay between the sampling and the analysis limits the instantaneous feedback control of the nutrient solution composition [
14,
15]. In this regard, fast and continuous measurement of individual nutrient concentrations is necessary for the precise correction of the reused nutrient solutions, thereby allowing both improved efficiency of fertilizer use and reduced environmental pollution [
9,
16,
17].
Ion-selective electrodes (ISEs) could be one of the most attractive tools to measure the individual ion concentrations of hydroponic solutions due to their advantages such as simplicity of use, fast response time, direct measurement of analyte, sensitivity over a wide concentration range, and portability [
18,
19,
20]. Specifically, the concept of a sensor array makes it possible to simultaneously determine individual ion concentrations in complex samples [
9,
21,
22]. However, several disadvantages of ISEs such as signal drift and distortions due to interfering ions make the application for hydroponics difficult [
9,
13,
17,
23]. Therefore, it is essential to develop an effective data-processing method to compensate for the signal drift and interference [
24,
25].
One such method is a two-point normalization (TPN) method in conjunction with the use of the Nernst equation that consists of a sensitivity adjustment followed by an offset adjustment applied to all of the signal data measured with the ISEs [
18,
23,
26]. In previous studies, the TPN method was employed and shown to be effective in compensating for the signal drifts of a sensor array consisting of NO
3, K, and Ca ISEs which were used for measuring hydroponic solutions [
10,
17,
18,
23,
26,
27]. However, the TPN method is relatively weak for the interference because the standard curve for the TPN is constructed based on the simplified Nernst equation. The simplified Nernst equation assumes the ion-selective membrane would be specific to the ion of interest, but the actual membrane responds to other interfering ions. As a result, electromotive forces (EMFs) generated from the ISEs are affected by the complex ion matrix in hydroponic solutions, thereby inducing errors in the ion concentrations predicted by the TPN method. In addition, use of the TPN method is still limited in measuring other ions, such as P and Mg, present in hydroponic solutions, because ionophores for selective recognition of the P and Mg with an acceptable level are not yet commercially available.
Considering the complexity of ions present in hydroponic solutions, an artificial neural network (ANN) would be a proper method for compensating for the interferences on ISEs because ANN conducts the processing of non-linear multivariate interactions based on knowledge storage and learning and its property of controlling the number of hidden neurons and hidden layers makes it more flexible than other machine-learning techniques [
15,
25,
28,
29,
30]. In addition, ANN could be utilized as a predictive tool through the reflection of inherent chemical relationships [
28]. However, ANN is vulnerable to signal drifts. For example, drifts can make the signals different from the signals obtained during the training, then the predicted ion concentrations by the ANN model would deviate from the actual values. This indicates that the ANN model would be difficult to use in ISE measurements without the drift compensation [
17].
Based on the complementary properties of the TPN and ANN methods, in this study, we proposed a hybrid signal processing approach to effectively compensate for the signal drifts and interferences from other ions, thereby improving the accuracy of ISEs in hydroponic applications. The specific objectives of this study were (1) to evaluate the hybrid processing method when compared to the TPN or ANN methods using a sensor array consisting of ion-selective electrodes for macronutrients (NO3, K, and Ca) and an electrical conductivity electrode, and (2) to investigate the possibility of an ANN-based prediction model for Mg concentration in hydroponic solutions, of which there are few robust ISEs.
4. Discussion
In this study, we suggested a hybrid signal-processing method to improve the accuracy and feasibility of ISEs in hydroponic application by effectively compensating for the signal drifts and interferences from other ions.
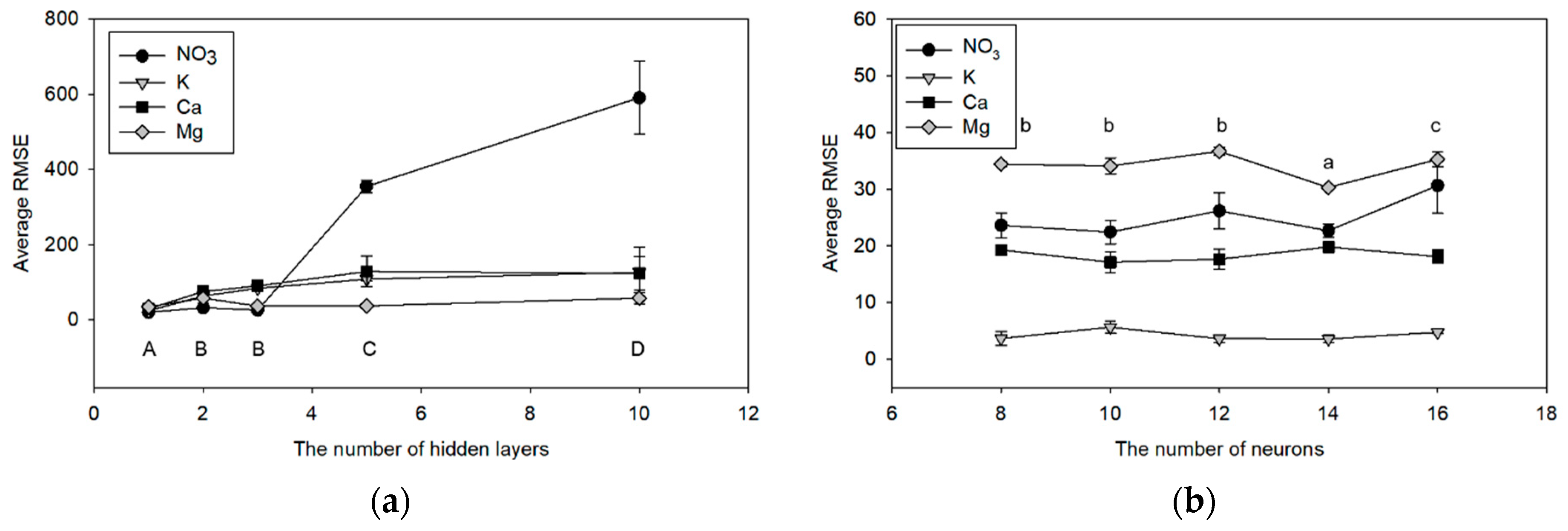
The optimization result of the number of hidden layers showed a single hidden layer ANN had the lowest RMSE for NO
3, K, Ca, and Mg prediction (
Figure 3a). In actual fact, the ANN models with more hidden layers do not guarantee better performance than those with fewer layers if the number of hidden layers is sufficient for the given non-linear problem [
40]. Similarly, the performance of the ANN model was not increased according to the number of hidden neurons (
Figure 3b), as reported in the previous study [
29].
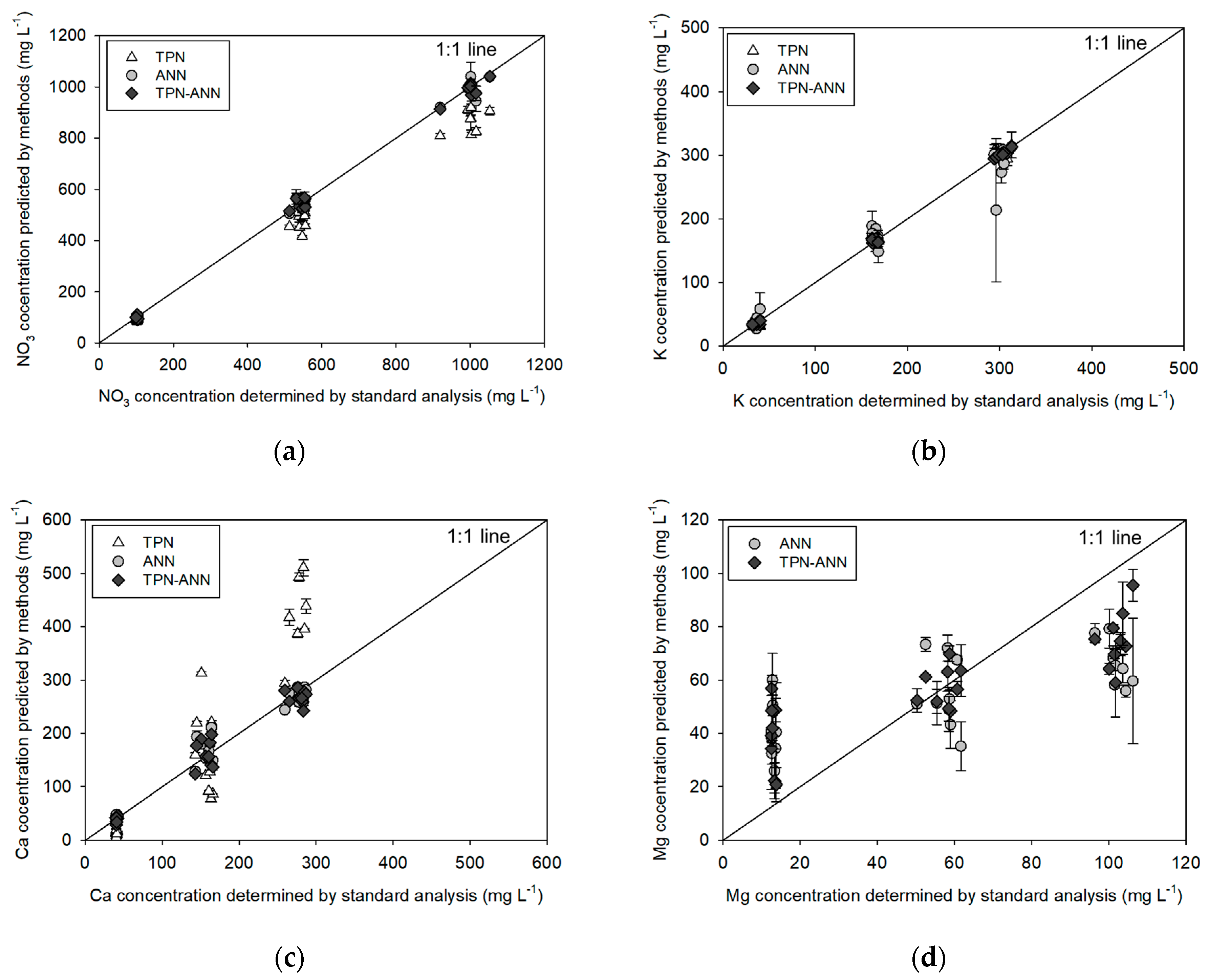
In the training sample measurements (
Figure 5,
Table 4), the application of the TPN showed a strongly linear relationship with R
2 of 0.99 despite a slight underestimation of NO
3 concentrations between the actual and predicted concentrations similar to that reported in previous studies [
23,
26,
27]. This might be caused by signal interferences from other ions, such as Cl and SO
4 in training samples. Moreover, TPN-based Ca prediction has a deviated slope of 1.57, which might be due to the presence of Mg ions, which have a similar chemical behavior to Ca ions [
41]. The cross-interference would affect the Nernstian slope, thereby inducing inaccuracy in prediction [
42]. To solve the interference issue, the ANN, which would possibly compensate for the interfering responses by training the various backgrounds, was employed and improved the performance of the actual test (
Figure 5a,c). It supports the theory that ANN would be effective for the non-linear interference by adjusting the relationship as reported in the previous studies [
9,
15,
28,
31,
42]. In addition, we applied the ANN to predict the Mg concentration because we expected the ANN would extract the signals from the Mg ions through the training with defined background samples. Although the results were not satisfactory (
Figure 5d,
Table 4), the ANN-based models could be used to discriminate between high and low concentrations of Mg according to the criterion of the previous study [
39].
In real sample application, the TPN-ANN was the best processing method, followed by TPN and ANN (
Figure 6,
Table 5). As mentioned above, the Ca prediction by the TPN was vulnerable to interferences. Although the TPN made Ca predictions more precise than the ANN in several samples, e.g., Basil 1,
Atractylodes japonica, Glehnia littoralis 1, and
Glehnia littoralis 2, relatively high variations in Ca predictions depending on the samples showed that the TPN could be affected by the changes of background ions. In contrast, the ANN-based methods were effective in managing the interferences in actual tests, showing they were less affected by the samples in most cases (
Figure 6c). However, the ANN method showed high RMSEs in the predictions of NO
3, K, and Mg. The main reason for the errors in the ANN-based prediction would be due to the signal drifts. This limitation of the ANN was similar to the results of several studies [
9,
15,
29]. The TPN method proved its effectiveness in drift compensation with improved accuracy. However, there were deviations in Ca prediction similar to those in the training sample measurement, which could be due to the interference by the various background ions. The hybrid method showed the best predictability in real hydroponic sample application by successfully combining the strengths of the TPN and the ANN, as expected. It meant the hybrid method could compensate for the signal drifts and then calculate the concentrations considering the non-linear influences from the interference through the neural network. As a result, the hybrid method improved the accuracy and the precision of the prediction of the ion concentrations with the lowest RMSEs of 47.2, 13.2, and 18.9 mg∙L
−1 and CVs below 10% for NO
3, K, and Ca, respectively.
In Mg prediction, the RMSE of 29.4 mg∙L−1 in the ANN-based prediction is high considering the range of 10–60 mg∙L−1 in real samples. However, by applying the hybrid method, the RMSE of the prediction was reduced to 14.6 mg∙L−1. Considering the lack of the ISEs for the direct measurement of Mg, it would be possible to improve the predictability by adding more ISEs which are more closely related to the Mg ion.
5. Conclusions
In this study, a hybrid signal-processing approach combining the TPN and the ANN was proposed to improve the applicability of the ISEs in hydroponics by effectively managing the signal drift and the interference. The parameters of the method were optimized by the 27 training samples, which imitated the hydroponic background. The feasibility and the performance of the method was validated through eight of the real hydroponic sample applications.
From the results, the conventional processing methods such as the TPN and the ANN were sometimes unsatisfactory for prediction of the ion concentrations in hydroponic samples due to their vulnerability to the interference or the drift. The hybrid method improved the RMSEs to 47.2, 13.2, 18.9, and 14.6 mg∙L−1, which were approximately half the values of the conventional methods, with CVs below 10% for NO3, K, Ca, and Mg, respectively. Furthermore, the hybrid method showed potential as an approximate diagnostic tool for Mg prediction despite the lack of direct Mg ISEs in the sensor array. The structure of the hybrid method can be utilized fundamentally for other ISEs. Therefore, the TPN-ANN method enables the ISEs to measure the individual ions in hydroponic solutions by minimizing the effects of signal drifts and the interference, thereby contributing to both improved efficiency of fertilizer use and reduced environmental pollution when growing plants in closed hydroponics.