Optical-Resolution Photoacoustic Microscopy Using Transparent Ultrasound Transducer
Abstract
1. Introduction
- It exhibits a good electromechanical coupling coefficient (53%);
- It demonstrates a high Curie temperature (>1100 °C), making it easy to process through high-temperature sputtering without losing poling [21];
- The TUT can be fixed onto the imaging object (such as the skull of a mouse) to facilitate wearable imaging without a thick coupling medium. In the future, this will likely help in imaging the brains of freely behaving or awake mice in combination with ultrasound stimulation;
- Depending on the size of the TUT, it enables the high-speed scanning of large areas with single-channel data acquisition.
- In the future, our TUT approach can also be integrated into conventional optical microscopes to realize a multimodal microscopy platform with ultrasound-stimulation capabilities.
2. Materials and Methods
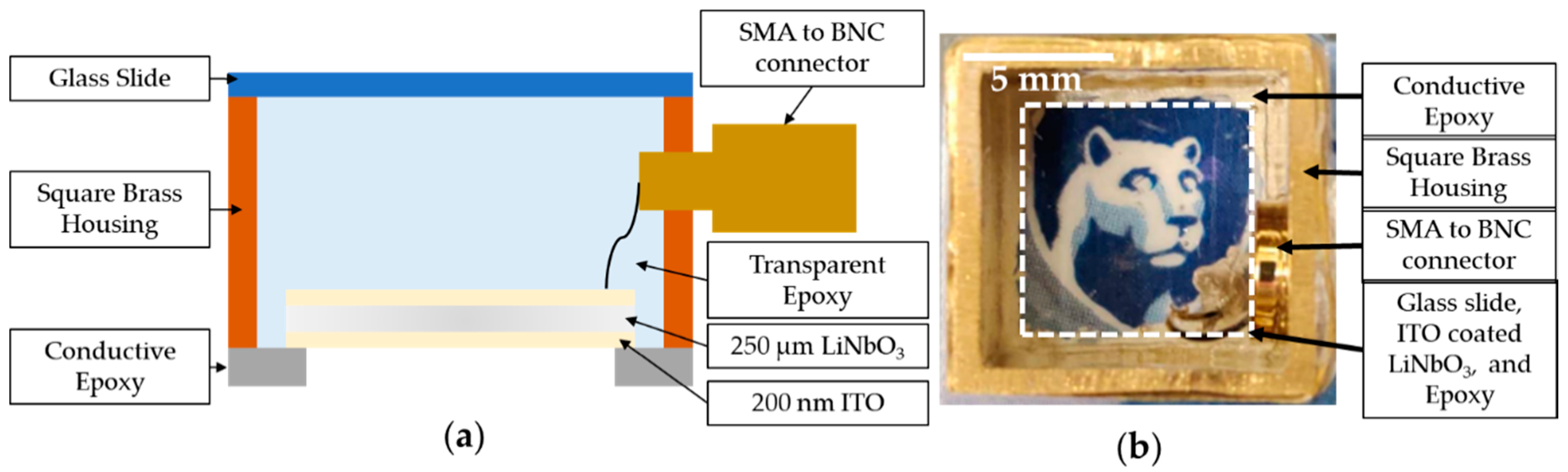
2.1. Transducer-Fabrication Processes
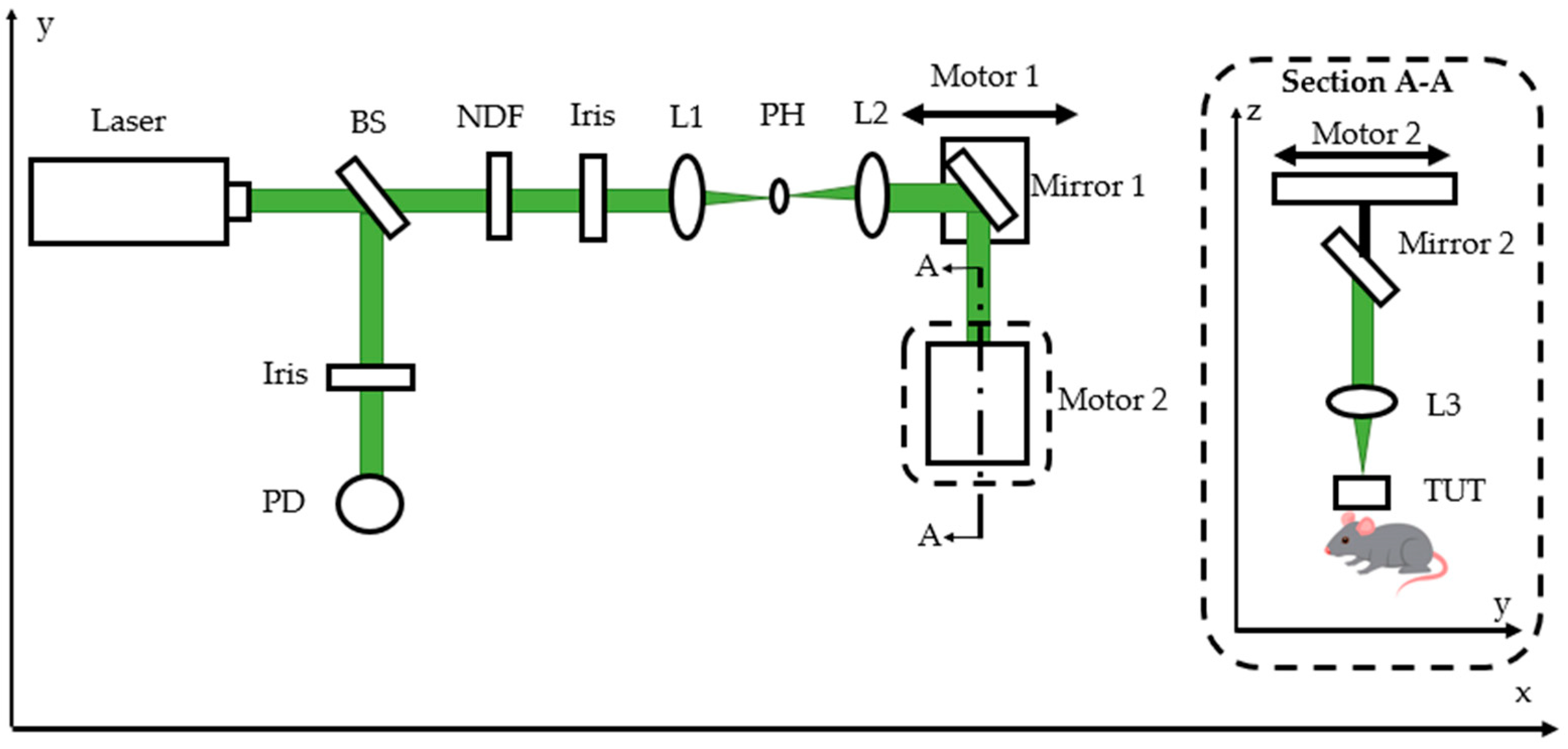
2.2. Optical-Resolution Photoacoustic-Microscopy Experiment Setup
3. Results and Discussion
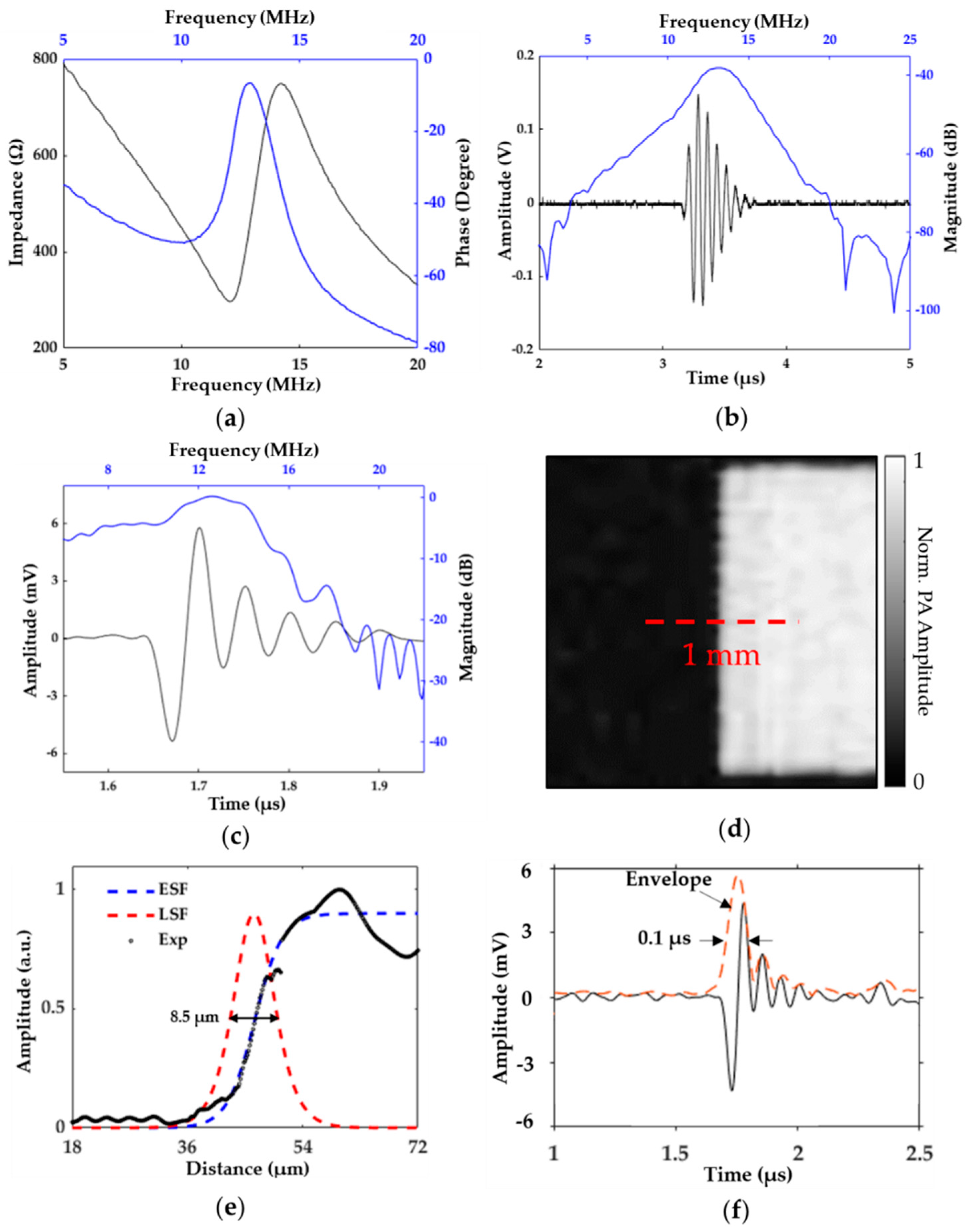
3.1. System and Transducer Characterization
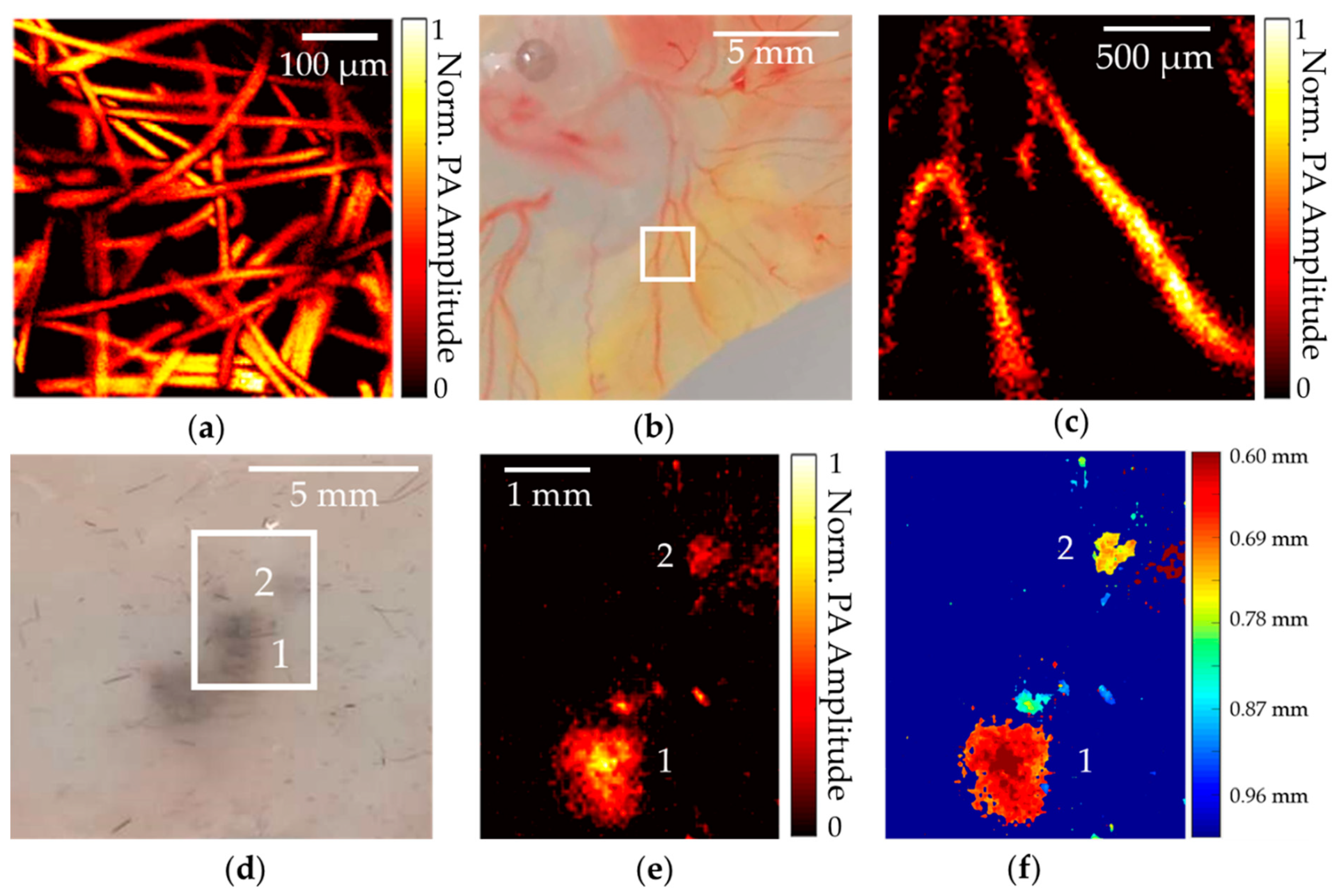
3.2. Phantom- and Biological-Tissue-Imaging Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lan, B.; Liu, W.; Wang, Y.C.; Shi, J.; Li, Y.; Xu, S.; Sheng, H.; Zhou, Q.; Zou, J.; Hoffmann, U.; et al. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed. Opt. Express 2018, 9, 4689–4701. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Yang, J.M.; Maslov, K.I.; Wong, T.T.; Li, L.; Huang, C.H.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Maslov, K.; Wang, L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Nguyen, V.P.; Huang, Z.; Liu, Z.; Wang, X.; Paulus, Y.M. High-resolution, in vivo multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy imaging of rabbit retinal neovascularization. Light Sci. Appl. 2018, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, J.; Ning, B.; Sun, N.; Wang, T.; Zuo, Z.; Hu, S. Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. Neuroimage 2017, 150, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Han, S.; Kim, C. High-speed and high-SNR photoacoustic microscopy based on a galvanometer mirror in non-conducting liquid. Sci. Rep. 2016, 6, 34803. [Google Scholar] [CrossRef]
- Wong, T.T.; Zhang, R.; Zhang, C.; Hsu, H.C.; Maslov, K.I.; Wang, L.; Shi, J.; Chen, R.; Shung, K.K.; Zhou, Q.; et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, H.; Jin, T.; Qi, W.; Xie, H.; Xi, L. Ultracompact high-resolution photoacoustic microscopy. Opt. Lett. 2018, 43, 1615–1618. [Google Scholar] [CrossRef]
- Rebling, J.; Estrada, H.; Gottschalk, S.; Sela, G.; Zwack, M.; Wissmeyer, G.; Ntziachristos, V.; Razansky, D. Dual-wavelength hybrid optoacoustic-ultrasound biomicroscopy for functional imaging of large-scale cerebral vascular networks. J. Biophotonics 2018, 11, e201800057. [Google Scholar] [CrossRef]
- Dangi, A.; Agrawal, S.; Tiwari, S.; Jadhav, S.; Cheng, C.; Datta, G.R.; Trolier-McKinstry, S.; Pratap, R.; Kothapalli, S.R. Ring PMUT array based miniaturized photoacoustic endoscopy device. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2019, International Society for Optics and Photonics, San Francisco, CA, USA, 27 February 2019; Volume 10878, p. 1087811. [Google Scholar]
- Dangi, A.; Agrawal, S.; Datta, G.R.; Srinivasan, V.; Kothapalli, S.R. Towards a Low-Cost and Portable Photoacoustic Microscope for Point-of-Care and Wearable Applications. IEEE Sens. J. 2019. [Google Scholar] [CrossRef]
- Ansari, R.; Zhang, E.Z.; Desjardins, A.E.; Beard, P.C. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy. Light Sci. Appl. 2018, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Zhang, X.; Shu, X.; Chen, X.; Hai, R.; Czaplewski, D.A.; Zhang, H.F.; Sun, C. Disposable ultrasound-sensing chronic cranial window by soft nanoimprinting lithography. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Sun, C.; Zhang, H.F. Optical detection of ultrasound in photoacoustic imaging. IEEE Trans. Biomed. Eng. 2016, 64, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Gougheri, H.S.; Dangi, A.; Kothapalli, S.R.; Kiani, M. A Comprehensive Study of Ultrasound Transducer Characteristics in Microscopic Ultrasound Neuromodulation. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 835–847. [Google Scholar] [CrossRef]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.H.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Adelegan, O.J.; Yamaner, F.Y.; Oralkan, Ö. Backward-mode photoacoustic imaging using illumination through a CMUT with improved transparency. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 65, 85–94. [Google Scholar] [CrossRef]
- Li, Z.; Ilkhechi, A.K.; Zemp, R. Transparent capacitive micromachined ultrasonic transducers (CMUTs) for photoacoustic applications. Opt. Express 2019, 27, 13204–13218. [Google Scholar] [CrossRef]
- Dangi, A.; Agrawal, S.; Kothapalli, S.R. Lithium niobate-based transparent ultrasound transducers for photoacoustic imaging. Opt. Lett. 2019, 44, 5326–5329. [Google Scholar] [CrossRef]
- Baba, A.; Searfass, C.T.; Tittmann, B.R. High temperature ultrasonic transducer up to 1000 C using lithium niobate single crystal. Appl. Phys. Lett. 2010, 97, 232901. [Google Scholar]
- Fei, C.; Chiu, C.T.; Chen, X.; Chen, Z.; Ma, J.; Zhu, B.; Shung, K.K.; Zhou, Q. Ultrahigh frequency (100 MHz–300 MHz) ultrasonic transducers for optical resolution medical imagining. Sci. Rep. 2016, 6, 28360. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shcherbakova, D.M.; Kurupassery, N.; Li, Y.; Zhou, Q.; Verkhusha, V.V.; Yao, J. Quad-mode functional and molecular photoacoustic microscopy. Sci. Rep. 2018, 8, 11123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cha, J.H.; Huang, Y.; Zhang, R.; Cao, W.; Shung, K.K. Alumina/epoxy nanocomposite matching layers for high-frequency ultrasound transducer application. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 213–219. [Google Scholar] [PubMed]
- Shung, K.K.; Zippuro, M. Ultrasonic transducers and arrays. IEEE Eng. Med. Biol. Mag. 1996, 15, 20–30. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.M. The effective electromechanical coupling coefficient of piezoelectric thin-film resonators. Appl. Phys. Lett. 2005, 86, 022904. [Google Scholar] [CrossRef]
- Zhang, C.; Maslov, K.I.; Yao, J.; Wang, L.V. In vivo photoacoustic microscopy with 7.6-µm axial resolution using a commercial 125-MHz ultrasonic transducer. J. Biomed. Opt. 2012, 17, 116016. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Marcacci, M.; Bravo, S.; Kurz, T.; Tremblay, J.; Rusing, J.C. Ex ovo model for directly visualizing chick embryo development. Am. Boil. Teach. 2012, 74, 628–634. [Google Scholar]
- Laufer, J.G.; Zhang, E.Z.; Treeby, B.E.; Cox, B.T.; Beard, P.C.; Johnson, P.; Pedley, B. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. J. Biomed. Opt. 2012, 44817, 056016. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.J.; Gershenwald, J.E.; Thompson, J.F.; Reintgen, D.S.; Cascinelli, N.; Urist, M.; McMasters, K.M.; Ross, M.I.; Kirkwood, J.M.; et al. Prognostic factors analysis of 17,600 melanoma of 14patients: Validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 2001, 19, 3622–3634. [Google Scholar] [CrossRef]
- Ansari, M.A.; Mohajerani, E. Mechanisms of laser-tissue interaction: Optical properties of tissue. J. Lasers Med. Sci. 2011, 2, 119. [Google Scholar]
- American National Standards Institute. American National Standard for Safe Use of Lasers; Laser Institute of America: New York, NY, USA, 2007. [Google Scholar]
- Szabo, T.L.; Lewin, P.A. Piezoelectric materials for imaging. J. Ultrasound Med. 2007, 26, 283–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shung, K.K.; Cannata, J.; Zhou, M.Q.; Lee, J. High Frequency Ultrasound: A New Frontier for Ultrasound. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hilton Minneapolis, Minnesota, 2–6 September 2009; pp. 1953–1955. [Google Scholar]
- Kothapalli, S.R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Trans. Med. 2019, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qin, Y.; Chen, Y.; Pan, J.; Xu, C.; Wu, D.; Chao, W.Y.; Wei, J.T.; Tomlins, S.A.; Wang, X.; et al. Interstitial assessment of aggressive prostate cancer by physio-chemical photoacoustics: An ex vivo study with intact human prostates. Med. Phys. 2018, 45, 9. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.R.; Hasan, A.; Ertsey, R.; et al. Intraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Agrawal, S.; Dangi, A.; Wible, C.; Osman, M.; Abune, L.; Jia, H.; Rossi, R.; Wang, Y.; Kothapalli, S.-R. Optical-Resolution Photoacoustic Microscopy Using Transparent Ultrasound Transducer. Sensors 2019, 19, 5470. https://doi.org/10.3390/s19245470
Chen H, Agrawal S, Dangi A, Wible C, Osman M, Abune L, Jia H, Rossi R, Wang Y, Kothapalli S-R. Optical-Resolution Photoacoustic Microscopy Using Transparent Ultrasound Transducer. Sensors. 2019; 19(24):5470. https://doi.org/10.3390/s19245470
Chicago/Turabian StyleChen, Haoyang, Sumit Agrawal, Ajay Dangi, Christopher Wible, Mohamed Osman, Lidya Abune, Huizhen Jia, Randall Rossi, Yong Wang, and Sri-Rajasekhar Kothapalli. 2019. "Optical-Resolution Photoacoustic Microscopy Using Transparent Ultrasound Transducer" Sensors 19, no. 24: 5470. https://doi.org/10.3390/s19245470
APA StyleChen, H., Agrawal, S., Dangi, A., Wible, C., Osman, M., Abune, L., Jia, H., Rossi, R., Wang, Y., & Kothapalli, S.-R. (2019). Optical-Resolution Photoacoustic Microscopy Using Transparent Ultrasound Transducer. Sensors, 19(24), 5470. https://doi.org/10.3390/s19245470




