Fluorescent Single-Walled Carbon Nanotubes for Protein Detection
Abstract
1. Introduction
2. Single-Walled Carbon Nanotubes
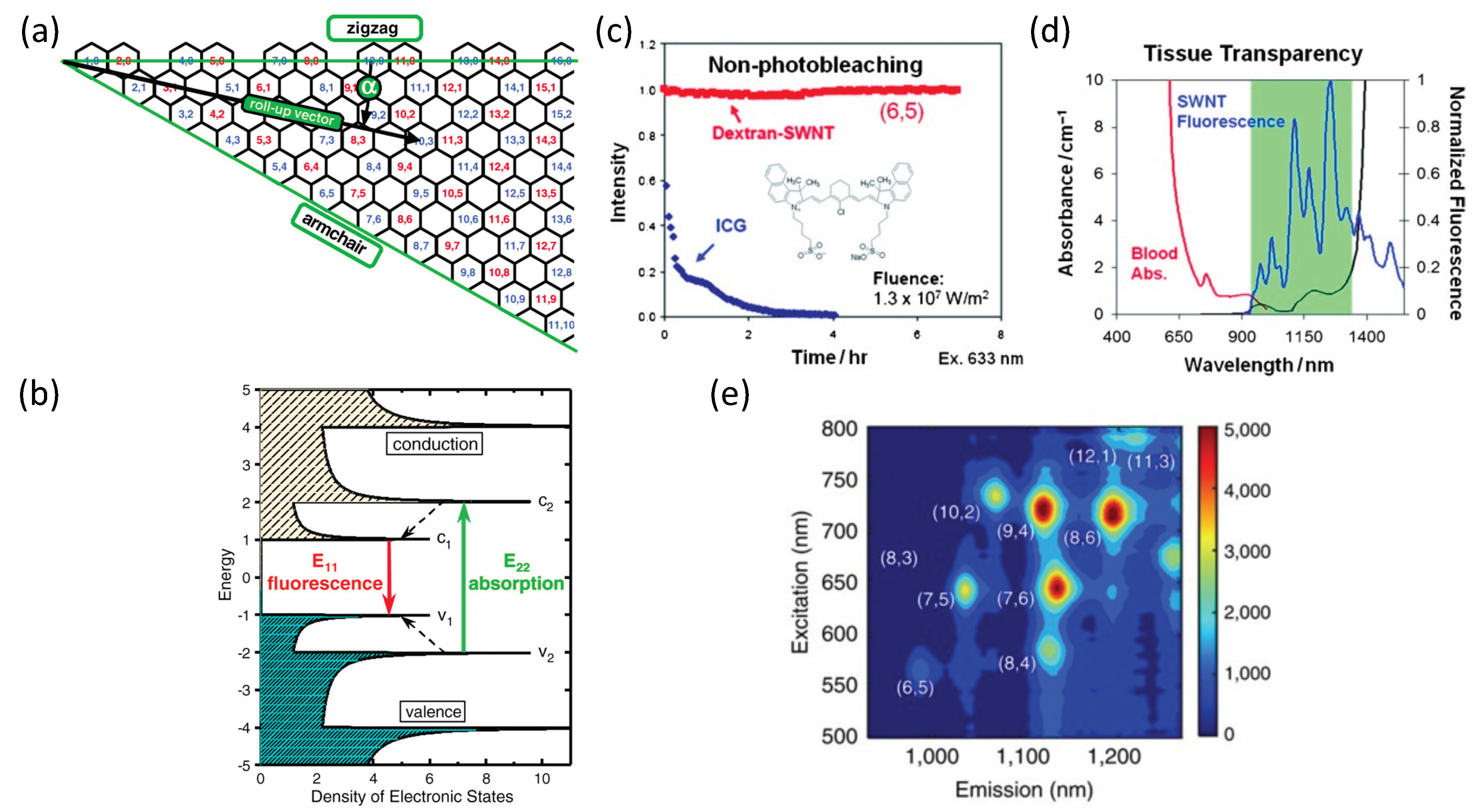
2.1. SWCNTs Properties
2.2. SWCNTs as Optical Sensors
3. Protein Recognition with SWCNTs
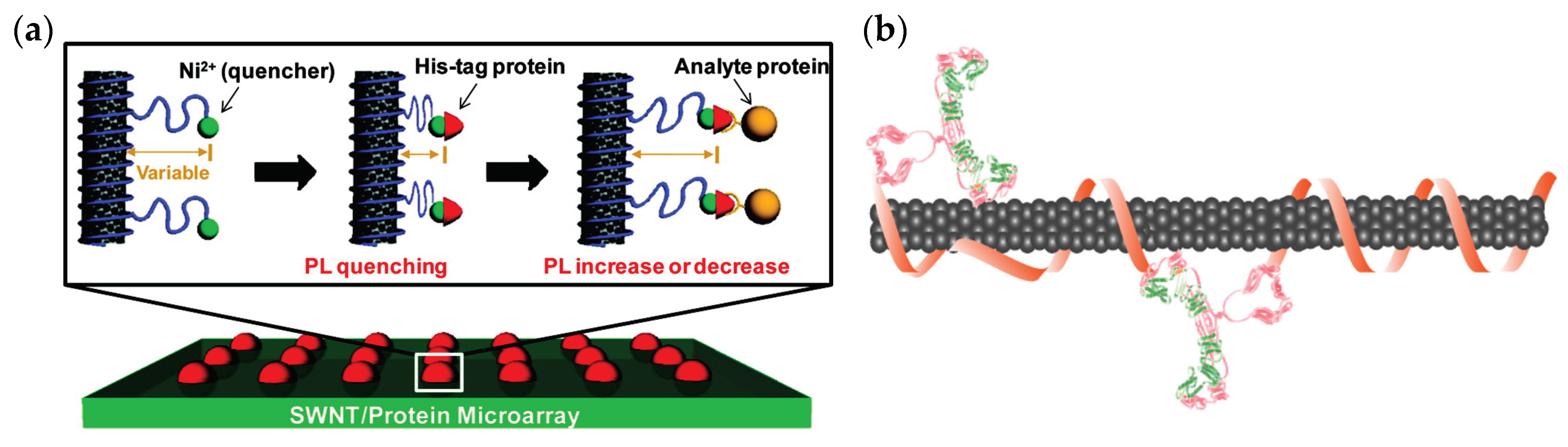
3.1. Natural Protein Recognition
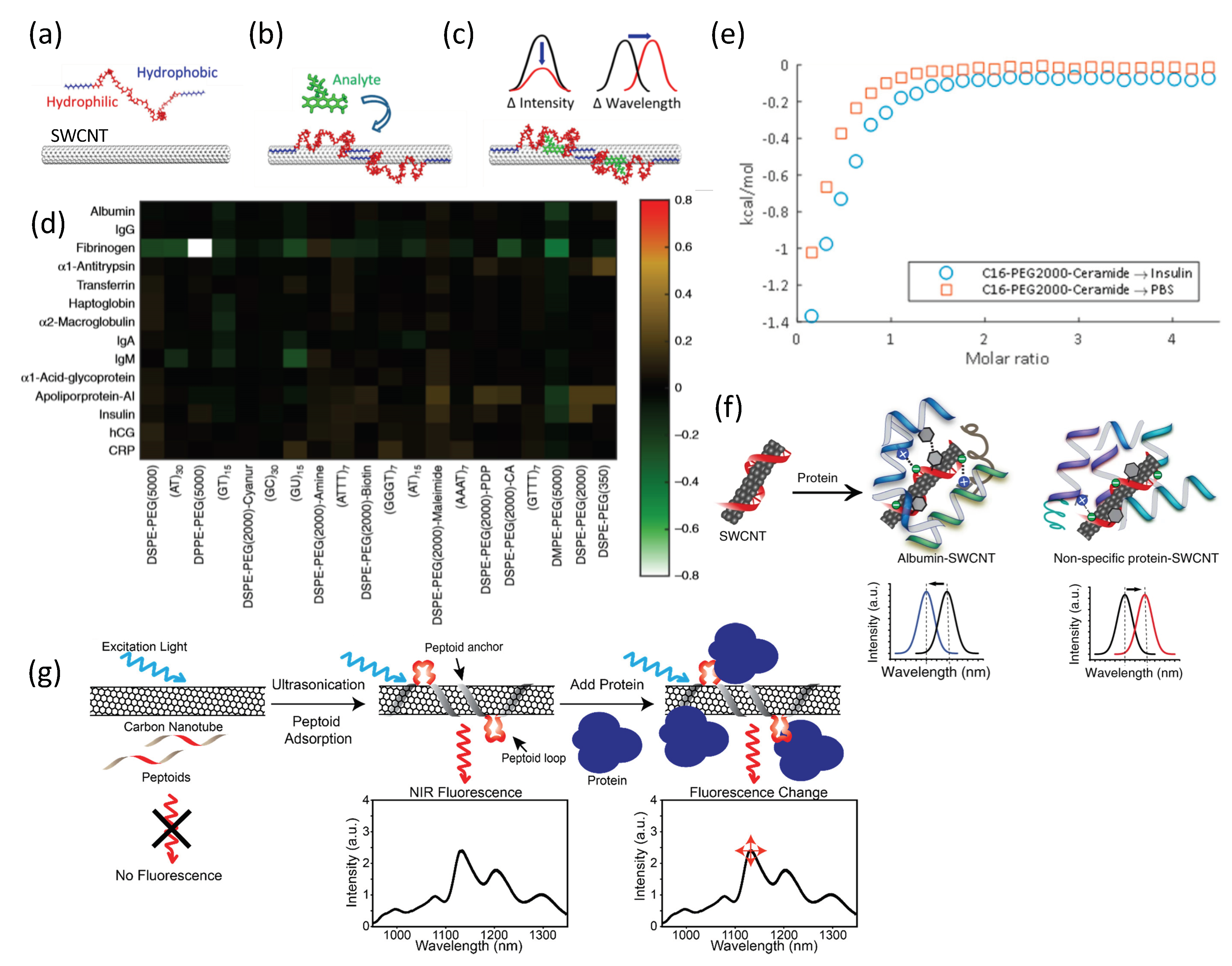
3.2. Synthetic Protein Recognition
4. SWCNTs Advantages
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dervan, P. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 2001, 9, 2215–2235. [Google Scholar] [CrossRef]
- Mann, S. Molecular recognition in biomineralization. Nature 1988, 332, 119–124. [Google Scholar] [CrossRef]
- Rebek, J. Molecular Recognition with Model Systems. Angew. Chem. Int. Ed. Engl. 1990, 29, 245–255. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. In Nanoscience and Technology; Co-Published with Macmillan Publishers Ltd.: London, UK, 2009; pp. 308–319. ISBN 9789814287005. [Google Scholar]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo Biosensing: Progress and Perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, U.; Erdogan, B.; Rotello, V.M. Nanoparticles: Scaffolds for molecular recognition. Chem. A Eur. J. 2004, 10, 5570–5579. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Y.; Yan, F. Semiconductor quantum dots for Biomedicial applications. Sensors 2011, 11, 11736–11751. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Yang, Y.; Zacharias, M. Nanowire-Based Sensors. Small 2010, 6, 1705–1722. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Yang, R.; Zhu, Z.; Chen, Y.; Tan, W. Single-walled carbon nanotubes as optical materials for biosensing. Nanoscale 2011, 3, 1949. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, Z.; Liu, J.-H.; Huang, X.-J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.R.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene. Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, A.A.; Zhang, J.; Barone, P.W.; Reuel, N.F.; Kim, J.H.; Heller, D.A.; Ahn, J.H.; Hilmer, A.J.; Rwei, A.; Arkalgud, J.R.; et al. Near-infrared fluorescent sensors based on single-walled carbon nanotubes for life sciences applications. ChemSusChem 2011. [Google Scholar] [CrossRef]
- Kim, S.-J.J.; Choi, S.-J.J.; Jang, J.-S.S.; Cho, H.-J.J.; Kim, I.-D.D. Innovative Nanosensor for Disease Diagnosis. Acc. Chem. Res. 2017, 50, 1587–1596. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Aizenberg, J.; Analoui, M.; Andrews, A.M.; Bisker, G.; Boyden, E.S.; Kamm, R.D.; Karp, J.M.; Mooney, D.J.; Oklu, R.; et al. Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation. ACS Nano 2017, 11, 5195–5214. [Google Scholar] [CrossRef]
- Cooper, R.M.; Leslie, D.C.; Domansky, K.; Jain, A.; Yung, C.; Cho, M.; Workman, S.; Super, M.; Ingber, D.E. A microdevice for rapid optical detection of magnetically captured rare blood pathogens. Lab Chip 2014, 14, 182–188. [Google Scholar] [CrossRef]
- KÖHLER, G.; MILSTEIN, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Proske, D.; Blank, M.; Buhmann, R.; Resch, A. Aptamers—Basic research, drug development, and clinical applications. Appl. Microbiol. Biotechnol. 2005, 69, 367–374. [Google Scholar] [CrossRef]
- Morales, J.M.; Skipwith, C.G.; Clark, H.A. Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine. Sensors (Basel) 2015, 15, 19912–19924. [Google Scholar] [CrossRef]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Ren, L.; Zhao, H.; Xu, C.; Zhang, L.; Yu, Y.; Wang, H.; Lan, Y.; Roberts, M.F.; Chuang, J.H.; et al. A molecular-imprint nanosensor for ultrasensitive detection of proteins. Nat. Nanotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Warn, R.M.; Flegg, L.; Warn, A. An investigation of microtubule organization and functions in living Drosophila embryos by injection of a fluorescently labeled antibody against tyrosinated alpha-tubulin. J. Cell Biol. 1987, 105, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Prince, A.; Patel, N.; Moore, L.S.; Rosenthal, E.L.; Hughley, B.B.; Warram, J.M. Antiangiogenic antibody improves melanoma detection by fluorescently labeled therapeutic antibodies. Laryngoscope 2016, 126, E387–E395. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Coons, A.H.; Creech, H.J.; Jones, R.N. Immunological Properties of an Antibody Containing a Fluorescent Group. Exp. Biol. Med. 1941, 47, 200–202. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Hunt, H.K.; Armani, A.M. Label-free biological and chemical sensors. Nanoscale 2010, 2, 1544. [Google Scholar] [CrossRef]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and Specificity: Pros and Cons in Sensing. ACS Sens. 2016. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014. [Google Scholar] [CrossRef]
- Tsyboulski, D.A.; Hou, Y.; Fakhri, N.; Ghosh, S.; Zhang, R.; Bachilo, S.M.; Pasquali, M.; Chen, L.; Liu, J.; Weisman, R.B. Do inner shells of double-walled carbon nanotubes fluoresce? Nano Lett. 2009. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, G.; Dresselhaus, M.S. Carbon nanotubes: Advanced topics in the synthesis, structure, properties and applications. Mater. Today 2008, 11, 57. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon nanotubes—The route toward applications. Science (80-.) 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; de Barros, A.L.B.; Soares, D.C.F.; Moss, S.N.; Alisaraie, L. Functionalized single-walled carbon nanotubes: cellular uptake, biodistribution and applications in drug delivery. Int. J. Pharm. 2017, 524, 41–54. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Krishnakumar, K.; Bhatt, A.; Paul, D.; Cherian, J.; John, A.; Suresh, S. Single-walled and multi-walled carbon nanotubes based drug delivery system: Cancer therapy: A review. Indian J. Cancer 2015, 52, 262. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Charlier, J.C.; Rinzler, A.G. Carbon nanotubes: From macromolecules to nanotechnology. Proc. Natl. Acad. Sci. USA 1999, 96, 14199–14200. [Google Scholar] [CrossRef]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat. Mater. 2005, 4, 86–92. [Google Scholar] [CrossRef]
- Fakhri, N.; Tsyboulski, D.A.; Cognet, L.; Weisman, R.B.; Pasquali, M. Diameter-dependent bending dynamics of single-walled carbon nanotubes in liquids. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar] [CrossRef] [PubMed]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dukovic, G.; Brus, L.E.; Heinz, T.F. The optical resonances in carbon nanotubes arise from excitons. Science 2005, 308, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Nishide, D.; Tanaka, T.; Kataura, H. Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography. Nat. Commun. 2011. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; Mclean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Fagan, J.A. Aqueous two-polymer phase extraction of single-wall carbon nanotubes using surfactants. Nanoscale Adv. 2019, 1, 3307–3324. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized Carbon Nanotubes for Probing and Modulating Molecular Functions. Chem. Biol. 2010, 17, 107–115. [Google Scholar] [CrossRef]
- Gillen, A.J.; Boghossian, A.A. Non-covalent Methods of Engineering Optical Sensors Based on Single-Walled Carbon Nanotubes. Front. Chem. 2019, 7, 612. [Google Scholar] [CrossRef]
- Heller, B.D.A.; Baik, S.; Eurell, T.E.; Strano, M.S. Single-Walled Carbon Nanotube Spectroscopy in Live Cells: Towards Long-Term Labels and Optical Sensors. Adv. Mater. 2005, 17, 2793–2799. [Google Scholar] [CrossRef]
- Kallmyer, N.E.; Huynh, T.; Graves, J.C.; Musielewicz, J.; Tamiev, D.; Reuel, N.F. Influence of sonication conditions and wrapping type on yield and fluorescent quality of noncovalently functionalized single-walled carbon nanotubes. Carbon N. Y. 2018. [Google Scholar] [CrossRef]
- Rungnim, C.; Rungrotmongkol, T.; Kungwan, N.; Hannongbua, S. Protein–protein interactions between SWCNT/chitosan/EGF and EGF receptor: A model of drug delivery system. J. Biomol. Struct. Dyn. 2016, 34, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Sanginario, A.; Miccoli, B.; Demarchi, D. Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment. Biosensors 2017, 7, 9. [Google Scholar] [CrossRef]
- Bisker, G.; Iverson, N.M.; Ahn, J.; Strano, M.S. A pharmacokinetic model of a tissue implantable insulin sensor. Adv. Healthc. Mater. 2014, 4, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Giraldo, J.P.; Kwak, S.-Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2016, 16, 264–272. [Google Scholar] [CrossRef]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon N. Y. 2015, 95, 767–779. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.M.; et al. Nanosensor Technology Applied to Living Plant Systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.V.; Roxbury, D.; Galassi, T.V.; Akkari, L.; Horoszko, C.P.; Iaea, D.B.; Budhathoki-Uprety, J.; Pipalia, N.; Haka, A.S.; Harvey, J.D.; et al. A Carbon Nanotube Optical Reporter Maps Endolysosomal Lipid Flux. ACS Nano 2017, 11, 10689–10703. [Google Scholar] [CrossRef] [PubMed]
- Galassi, T.V.; Jena, P.V.; Shah, J.; Ao, G.; Molitor, E.; Bram, Y.; Frankel, A.; Park, J.; Jessurun, J.; Ory, D.S.; et al. An optical nanoreporter of endolysosomal lipid accumulation reveals enduring effects of diet on hepatic macrophages in vivo. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Iverson, N.M.; Barone, P.W.; Shandell, M.; Trudel, L.J.; Sen, S.; Sen, F.; Ivanov, V.; Atolia, E.; Farias, E.; McNicholas, T.P.; et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2013, 8, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Bakh, N.; Bisker, G.; Brown, E.N.; Strano, M.S. A Pharmacokinetic Model of a Tissue Implantable Cortisol Sensor. Adv. Healthc. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.E.; Vig, K.; Dennis, V.A.; Singh, S.R. functionalized-carbon-nanotubes--biomedical-applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar] [PubMed]
- Godin, A.G.; Varela, J.A.; Gao, Z.; Danné, N.; Dupuis, J.P.; Lounis, B.; Groc, L.; Cognet, L. Single-nanotube tracking reveals the nanoscale organization of the extracellular space in the live brain. Nat. Nanotechnol. 2017, 12, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Yeow, J.T.-W. Carbon Nanotubes for Biomedical Applications. IEEE Trans. Nanobiosci. 2005, 4, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C.R. A cell nanoinjector based on carbon nanotubes. Proc. Natl. Acad. Sci. USA 2007, 104, 8218–8222. [Google Scholar] [CrossRef]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef]
- Aliev, A.E.; Jiyoung, O.; Mikhail, K.; Fang, A.A.K.S.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N.; Zhang, M.; et al. Giant-Stroke, Superelastic Carbon Nanotube Aerogel Muscles. Science (80-.) 2009, 323, 1575–1578. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Hernández-Rivera, M.; Zaibaq, N.G.; Wilson, L.J. Toward carbon nanotube-based imaging agents for the clinic. Biomaterials 2016, 101, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Lee, B.Y.; Lee, J.; Hong, S.; Sim, S.J. Enhancement of sensitivity and specificity by surface modification of carbon nanotubes in diagnosis of prostate cancer based on carbon nanotube field effect transistors. Biosens. Bioelectron. 2009, 24, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Lynn Kirkpatrick, D.; Weiss, M.; Naumov, A.; Bartholomeusz, G.; Bruce Weisman, R.; Gliko, O. Carbon nanotubes: Solution for the therapeutic delivery of siRNA? Materials (Basel) 2012, 5, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.; Zhang, H.; Goh, N.; Chang, R.; Landry, M. Nanotubes Effectively Deliver siRNA to Intact Plant Cells and Protect siRNA Against Nuclease Degradation. SSRN Electron. J. 2019, 4. Available online: https://www.ssrn.com/abstract=3352632 (accessed on 7 April 2019). [CrossRef]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science (80-.) 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Heller, D.A.; Jin, H.; Martinez, B.M.; Patel, D.; Miller, B.M.; Ha, T.; Silverman, S.K.; Yeung, T.K.; Jena, P.V.; Ho, C.; et al. Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes. Nat. Nanotechnol. 2009, 4, 114–120. [Google Scholar] [CrossRef]
- Gravely, M.; Safaee, M.M.; Roxbury, D. Biomolecular Functionalization of a Nanomaterial To Control Stability and Retention within Live Cells. Nano Lett. 2019, 19, 6203–6212. [Google Scholar] [CrossRef]
- Gao, Z.; Danné, N.; Godin, A.; Lounis, B.; Cognet, L. Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments. Nanomaterials 2017, 7, 393. [Google Scholar] [CrossRef]
- Danné, N.; Kim, M.; Godin, A.G.; Kwon, H.; Gao, Z.; Wu, X.; Hartmann, N.F.; Doorn, S.K.; Lounis, B.; Wang, Y.; et al. Ultrashort Carbon Nanotubes That Fluoresce Brightly in the Near-Infrared. ACS Nano 2018, 12, 6059–6065. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.M.; Bisker, G.; Farias, E.; Ivanov, V.; Ahn, J.; Wogan, G.N.; Strano, M.S. Quantitative Tissue Spectroscopy of Near Infrared Fluorescent Nanosensor Implants. J. Biomed. Nanotechnol. 2016, 12, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Kodym, A.; Afza, R. Physical and Chemical Mutagenesis. In Plant Functional Genomics; Humana Press: Totowa, NJ, USA, 2003; Volume 236, pp. 189–204. [Google Scholar]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: Lundon, UK; World Scientific Publishing CO.: Singapore, 1998; ISBN 978-1-86094-093-4. [Google Scholar]
- Heller, D.A.; Jeng, E.S.; Yeung, T.K.; Martinez, B.M.; Moll, A.E.; Gastala, J.B.; Strano, M.S. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science (80-.) 2006, 311, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.; Cope, M.; Delpy, D.T.; Wyatt, J.S.; Reynolds, E.O.R. Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation. BBA Bioenerg. 1988. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Chemical analysis and cellular imaging with quantum dots. Analyst 2004, 129, 672. [Google Scholar] [CrossRef] [PubMed]
- Bisker, G.; Dong, J.; Park, H.D.; Iverson, N.M.; Ahn, J.; Nelson, J.T.; Landry, M.P.; Kruss, S.; Strano, M.S. Protein-targeted corona phase molecular recognition. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Salem, D.P.; Gong, X.; Liu, A.T.; Koman, V.B.; Dong, J.; Strano, M.S. Ionic Strength-Mediated Phase Transitions of Surface-Adsorbed DNA on Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2017. [Google Scholar] [CrossRef]
- Zhang, J.; Boghossian, A.A.; Barone, P.W.; Rwei, A.; Kim, J.H.; Lin, D.; Heller, D.A.; Hilmer, A.J.; Nair, N.; Reuel, N.F.; et al. Single molecule detection of nitric oxide enabled by d(AT)15 DNA adsorbed to near infrared fluorescent single-walled carbon nanotubes. J. Am. Chem. Soc. 2011, 133, 567–581. [Google Scholar] [CrossRef]
- Choi, J.H.; Strano, M.S. Solvatochromism in single-walled carbon nanotubes. Appl. Phys. Lett. 2007, 90, 223114. [Google Scholar] [CrossRef]
- Heller, D.A.; Pratt, G.W.; Zhang, J.; Nair, N.; Hansborough, A.J.; Boghossian, A.A.; Reuel, N.F.; Barone, P.W.; Strano, M.S. Peptide secondary structure modulates single-walled carbon nanotube fluorescence as a chaperone sensor for nitroaromatics. PNAS May 2011, 108, 8544–8549. [Google Scholar] [CrossRef]
- Roxbury, D.; Jena, P.V.; Williams, R.M.; Enyedi, B.; Niethammer, P.; Marcet, S.; Verhaegen, M.; Blais-Ouellette, S.; Heller, D.A. Hyperspectral Microscopy of Near-Infrared Fluorescence Enables 17-Chirality Carbon Nanotube Imaging. Sci. Rep. 2015, 5, 14167. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.P.; Landry, M.P.; Bisker, G.; Ahn, J.; Kruss, S.; Strano, M.S. Chirality dependent corona phase molecular recognition of DNA-wrapped carbon nanotubes. Carbon N. Y. 2016, 97. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Kalbacova, M.; Kim, J.H.; Zhang, J.; Boghossian, A.A.; Maheshri, N.; Strano, M.S. Detection of single-molecule H2 O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2010, 5, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.W.; Strano, M.S. Reversible control of carbon nanotube aggregation for a glucose affinity sensor. Angew. Chem. Int. Ed. 2006, 45, 8138–8141. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; Chang, Y.I.; Wang, Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006, 6, 1632–1636. [Google Scholar] [CrossRef]
- Nißler, R.; Mann, F.A.; Chaturvedi, P.; Horlebein, J.; Meyer, D.; Vuković, L.; Kruss, S. Quantification of the Number of Adsorbed DNA Molecules on Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2019, 123, 4837–4847. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, R.; You, M.; Zhang, X.; Wu, Y.; Tan, W. Single-walled carbon nanotube as an effective quencher. Anal. Bioanal. Chem. 2010, 396, 73–83. [Google Scholar] [CrossRef]
- So, H.-M.; Won, K.; Hwan Kim, Y.; Kim, B.-K.; Hwan Ryu, B.; Sun Na, P.; Kim, H.; Lee, J.-O. Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef]
- Wang, S.; Humphreys, E.S.; Chung, S.Y.; Delduco, D.F.; Lustig, S.R.; Wang, H.; Parker, K.N.; Rizzo, N.W.; Subramoney, S.; Chiang, Y.M.; et al. Peptides with selective affinity for carbon nanotubes. Nat. Mater. 2003, 2, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Safaee, M.M.; Gravely, M.; Lamothe, A.; McSweeney, M.; Roxbury, D. Enhancing the Thermal Stability of Carbon Nanomaterials with DNA. Sci. Rep. 2019, 9, 11926. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Schuergers, N.; Lin, K.-H.; Gillen, A.J.; Corminboeuf, C.; Boghossian, A.A. Restriction Enzyme Analysis of Double-Stranded DNA on Pristine Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Kruss, S. Impact of Redox-Active Molecules on the Fluorescence of Polymer-Wrapped Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 3061–3070. [Google Scholar] [CrossRef]
- Kruss, S.; Landry, M.P.; Vander Ende, E.; Lima, B.M.A.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Mann, F.A.; Herrmann, N.; Meyer, D.; Kruss, S. Tuning selectivity of fluorescent carbon nanotube-based neurotransmitter sensors. Sensors 2017, 17, 1521. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Kruss, S. Nanosensors for neurotransmitters. Anal. Bioanal. Chem. 2016, 408, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Del Bonis-O’Donnell, J.T.D.; Page, R.H.; Beyene, A.G.; Tindall, E.G.; McFarlane, I.R.; Landry, M.P. Dual Near-Infrared Two-Photon Microscopy for Deep-Tissue Dopamine Nanosensor Imaging. Adv. Funct. Mater. 2017, 27, 1702112. [Google Scholar] [CrossRef]
- Beyene, A.G.; Delevich, K.; Del Bonis-O’Donnell, J.T.; Piekarski, D.J.; Lin, W.C.; Thomas, A.W.; Yang, S.J.; Kosillo, P.; Yang, D.; Prounis, G.S.; et al. Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci. Adv. 2019, 5, eaaw3108. [Google Scholar] [CrossRef]
- Beyene, A.G.; Delevich, K.; Yang, S.J.; Landry, M.P. New Optical Probes Bring Dopamine to Light. Biochemistry 2018, 57, 6379–6381. [Google Scholar] [CrossRef]
- Beyene, A.G.; Alizadehmojarad, A.A.; Dorlhiac, G.; Goh, N.; Streets, A.M.; Král, P.; Vuković, L.; Landry, M.P. Ultralarge Modulation of Fluorescence by Neuromodulators in Carbon Nanotubes Functionalized with Self-Assembled Oligonucleotide Rings. Nano Lett. 2018, 18, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, M.; Neubert, E.; Meyer, D.; Selvaggio, G.; Mann, F.A.; Erpenbeck, L.; Kruss, S. Near-Infrared Imaging of Serotonin Release from Cells with Fluorescent Nanosensors. Nano Lett. 2019, 19, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.D.; Baker, H.A.; Ortiz, M.V.; Kentsis, A.; Heller, D.A. HIV Detection via a Carbon Nanotube RNA Sensor. ACS Sens. 2019, 4, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.D.; Williams, R.M.; Tully, K.M.; Baker, H.A.; Shamay, Y.; Heller, D.A. An in Vivo Nanosensor Measures Compartmental Doxorubicin Exposure. Nano Lett. 2019, 19, 4343–4354. [Google Scholar] [CrossRef]
- Harvey, J.D.; Jena, P.V.; Baker, H.A.; Zerze, G.H.; Williams, R.M.; Galassi, T.V.; Roxbury, D.; Mittal, J.; Heller, D.A. A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 2017, 1, 0041. [Google Scholar] [CrossRef]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.-H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Janin, J. Dissecting protein-protein recognition sites. Proteins Struct. Funct. Genet. 2002, 47, 334–343. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.-H.; Reuel, N.F.; Barone, P.W.; Boghossian, A.A.; Zhang, J.; Yoon, H.; Chang, A.C.; Hilmer, A.J.; Strano, M.S. Label-Free, Single Protein Detection on a Near-Infrared Fluorescent Single-Walled Carbon Nanotube/Protein Microarray Fabricated by Cell-Free Synthesis. Nano Lett. 2011, 11, 19. [Google Scholar] [CrossRef]
- Nelson, J.T.; Kim, S.; Reuel, N.F.; Salem, D.P.; Bisker, G.; Landry, M.P.; Kruss, S.; Barone, P.W.; Kwak, S.; Strano, M.S. Mechanism of Immobilized Protein A Binding to Immunoglobulin G on Nanosensor Array Surfaces. Anal. Chem. 2015, 87, 8186–8193. [Google Scholar] [CrossRef]
- Reuel, N.F.; Ahn, J.H.; Kim, J.H.; Zhang, J.; Boghossian, A.A.; Mahal, L.K.; Strano, M.S. Transduction of glycan-lectin binding using near-infrared fluorescent single-walled carbon nanotubes for glycan profiling. J. Am. Chem. Soc. 2011, 133, 17923–17933. [Google Scholar] [CrossRef]
- Williams, R.M.; Lee, C.; Heller, D.A. A Fluorescent Carbon Nanotube Sensor Detects the Metastatic Prostate Cancer Biomarker uPA. ACS Sens. 2018, 3, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Satishkumar, B.C.; Brown, L.O.; Gao, Y.; Wang, C.-C.C.; Wang, H.-L.L.; Doorn, S.K. Reversible fluorescence quenching in carbon nanotubes for biomolecular sensing. Nat. Nanotechnol. 2007, 2, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.; Ahn, B. Design of Refolding DNA Aptamer on Single-Walled Carbon Nanotubes for Enhanced Optical Detection of Target Proteins. Anal. Chem. 2019, 91, 12704–12712. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Lee, C.; Galassi, T.V.; Harvey, J.D.; Leicher, R.; Sirenko, M.; Dorso, M.A.; Shah, J.; Olvera, N.; Dao, F.; et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci. Adv. 2018, 4, eaaq1090. [Google Scholar] [CrossRef] [PubMed]
- Bisker, G.; Bakh, N.A.; Lee, M.A.; Ahn, J.; Park, M.; O’Connell, E.B.; Iverson, N.M.; Strano, M.S. Insulin Detection Using a Corona Phase Molecular Recognition Site on Single-Walled Carbon Nanotubes. ACS Sens. 2018, 3, 367–377. [Google Scholar] [CrossRef]
- Beyene, A.G.; Demirer, G.S.; Landry, M.P. Nanoparticle-Templated Molecular Recognition Platforms for Detection of Biological Analytes. In Current Protocols in Chemical Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 197–223. [Google Scholar]
- Bisker, G.; Ahn, J.; Kruss, S.; Ulissi, Z.W.; Salem, D.P.; Strano, M.S. A mathematical formulation and solution of the CoPhMoRe inverse problem for helically wrapping polymer corona phases on cylindrical substrates. J. Phys. Chem. C 2015, 119, 13876–13886. [Google Scholar] [CrossRef]
- Landry, M.P.; Vuković, L.; Kruss, S.; Bisker, G.; Landry, A.M.; Islam, S.; Jain, R.; Schulten, K.; Strano, M.S.; Vuković, L.; et al. Comparative Dynamics and Sequence Dependence of DNA and RNA Binding to Single Walled Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 10048–10058. [Google Scholar] [CrossRef]
- Ulissi, Z.W.; Zhang, J.; Sresht, V.; Blankschtein, D.; Strano, M.S. 2D Equation-of-State Model for Corona Phase Molecular Recognition on Single-Walled Carbon Nanotube and Graphene Surfaces. Langmuir 2015, 31, 628–636. [Google Scholar] [CrossRef]
- Landry, M.P.; Kruss, S.; Nelson, J.T.; Bisker, G.; Iverson, N.M.; Reuel, N.F.; Strano, M.S. Experimental tools to study molecular recognition within the nanoparticle corona. Sensors 2014, 14, 16196–16211. [Google Scholar] [CrossRef]
- Safaee, M.M.; Gravely, M.; Rocchio, C.; Simmeth, M.; Roxbury, D. DNA Sequence Mediates Apparent Length Distribution in Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Yermolenko, I.S.; Lishko, V.K.; Ugarova, T.P.; Magonov, S.N. High-Resolution Visualization of Fibrinogen Molecules and Fibrin Fibers with Atomic Force Microscopy. Biomacromolecules 2011, 12, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, H.A. The thrombin-fibrinogen interaction. Biophys. Chem. 2004, 112, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and Qualitative Evaluation of Adsorption/Desorption of Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces. Langmuir 2009, 25, 11614–11620. [Google Scholar] [CrossRef]
- Sweryda-Krawiec, B.; Devaraj, H.; Jacob, G.; Hickman, J.J. A New Interpretation of Serum Albumin Surface Passivation. Langmuir 2004, 20, 2054–2056. [Google Scholar] [CrossRef]
- Vakilian, M.; Tahamtani, Y.; Ghaedi, K. A review on insulin trafficking and exocytosis. Gene 2019, 706, 52–61. [Google Scholar] [CrossRef]
- Sonksen, P.; Sonksen, J. Insulin: Understanding its action in health and disease. Br. J. Anaesth. 2000, 85, 69–79. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Kwak, S.Y.; Jain, R.M.; Wong, M.H.; Iverson, N.M.; Ben-Naim, M.; Strano, M.S. A Ratiometric Sensor Using Single Chirality Near-Infrared Fluorescent Carbon Nanotubes: Application to in Vivo Monitoring. Small 2015, 11, 3973–3984. [Google Scholar] [CrossRef]
- Hofferber, E.M.; Stapleton, J.A.; Adams, J.; Kuss, M.; Duan, B.; Iverson, N.M. Implantable Nanotube Sensor Platform for Rapid Analyte Detection. Macromol. Biosci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Budhathoki-Uprety, J.; Shah, J.; Korsen, J.A.; Wayne, A.E.; Galassi, T.V.; Cohen, J.R.; Harvey, J.D.; Jena, P.V.; Ramanathan, L.V.; Jaimes, E.A.; et al. Synthetic molecular recognition nanosensor paint for microalbuminuria. Nat. Commun. 2019, 10, 3605. [Google Scholar] [CrossRef] [PubMed]
- Chio, L.; Travis, J.; Bonis-O’donnell, D.; Kline, M.A.; Kim, J.H.; Mcfarlane, I.R.; Zuckermann, R.N.; Landry, M.P. Electrostatic Assemblies of Single-Walled Carbon Nanotubes and Sequence-Tunable Peptoid Polymers Detect a Lectin Protein and Its Target Sugars. Nano Lett. 2019, 19, 7563–7572. [Google Scholar] [CrossRef] [PubMed]
- Olivier, G.K.; Cho, A.; Sanii, B.; Connolly, M.D.; Tran, H.; Zuckermann, R.N. Antibody-Mimetic Peptoid Nanosheets for Molecular Recognition. ACS Nano 2013, 7, 9276–9286. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Kim, J.H.; Dehigaspitiya, D.C.; Proulx, C.; Robertson, E.J.; Murray, D.J.; Rad, B.; Kirshenbaum, K.; Zuckermann, R.N. Glycosylated Peptoid Nanosheets as a Multivalent Scaffold for Protein Recognition. ACS Nano 2018, 12, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J. Recombinant antibody constructs in cancer therapy. Curr. Opin. Immunol. 1999, 11, 548–557. [Google Scholar] [CrossRef]
- Trikha, M.; Yan, L.; Nakada, M.T. Monoclonal antibodies as therapeutics in oncology. Curr. Opin. Biotechnol. 2002, 13, 609–614. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar]
- Miersch, S.; Sidhu, S.S. Synthetic antibodies: Concepts, potential and practical considerations. Methods 2012, 57, 486–498. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Fellouse, F.A. Synthetic therapeutic antibodies. Nat. Chem. Biol. 2006, 2, 682–688. [Google Scholar] [CrossRef]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B. Molecularly imprinted polymers—Potential and challenges in analytical chemistry. Anal. Chim. Acta 2005. [Google Scholar] [CrossRef]
- Wu, Y.; Phillips, J.A.; Liu, H.; Yang, R.; Tan, W. Carbon Nanotubes Protect DNA Strands during Cellular Delivery. ACS Nano 2008, 2, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sadoqi, M.; SHAO, J. Degradation Kinetics of Somatostatin in Aqueous Solution. Drug Dev. Ind. Pharm. 2003, 29, 1027–1033. [Google Scholar]
- Gartner, L.P.; Hiatt, J.L. Color Textbook of Histology; Saunders/Elsevier: Philadelphia, PA, USA, 2007; ISBN 9781437700817. [Google Scholar]
- Conn, H.J. Progress in the Standardization of Stains the Haematoxylin Problem. Stain Technol. 1927, 2, 1–3. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science (80-.) 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum Dots as Cellular Probes. Annu. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science (80-.) 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Howarth, M.; Takao, K.; Hayashi, Y.; Ting, A.Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA 2005, 102, 7583–7588. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003, 21, 47–51. [Google Scholar] [CrossRef]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; De Grand, A.M.; Lee, J.; Nakayama, A.; Parker, J.A.; Mihaljevic, T.; Laurence, R.G.; Dor, D.M.; et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar] [CrossRef]
- Michalet, X. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science (80-.) 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Dubach, J.M.; Harjes, D.I.; Clark, H.A. Ion-Selective Nano-optodes Incorporating Quantum Dots. J. Am. Chem. Soc. 2007, 129, 8418–8419. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Langer, R.; Jaklenec, A. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Adv. Mater. 2018, 30, 1–18. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Efros, A.L.; Nesbitt, D.J. Origin and control of blinking in quantum dots. Nat. Nanotechnol. 2016, 11, 661–671. [Google Scholar] [CrossRef]
- Gao, Z.; Varela, J.A.; Groc, L.; Lounis, B.; Cognet, L. Toward the suppression of cellular toxicity from single-walled carbon nanotubes. Biomater. Sci. 2016, 4, 230–244. [Google Scholar] [CrossRef]
- Pan, J.; Li, F.; Choi, J.H. Single-walled carbon nanotubes as optical probes for bio-sensing and imaging. J. Mater. Chem. B 2017, 5, 6511–6522. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendler-Neumark, A.; Bisker, G. Fluorescent Single-Walled Carbon Nanotubes for Protein Detection. Sensors 2019, 19, 5403. https://doi.org/10.3390/s19245403
Hendler-Neumark A, Bisker G. Fluorescent Single-Walled Carbon Nanotubes for Protein Detection. Sensors. 2019; 19(24):5403. https://doi.org/10.3390/s19245403
Chicago/Turabian StyleHendler-Neumark, Adi, and Gili Bisker. 2019. "Fluorescent Single-Walled Carbon Nanotubes for Protein Detection" Sensors 19, no. 24: 5403. https://doi.org/10.3390/s19245403
APA StyleHendler-Neumark, A., & Bisker, G. (2019). Fluorescent Single-Walled Carbon Nanotubes for Protein Detection. Sensors, 19(24), 5403. https://doi.org/10.3390/s19245403




