Competitive USB-Powered Hand-Held Potentiostat for POC Applications: An HRP Detection Case
Abstract
:1. Introduction
2. Materials and Methods
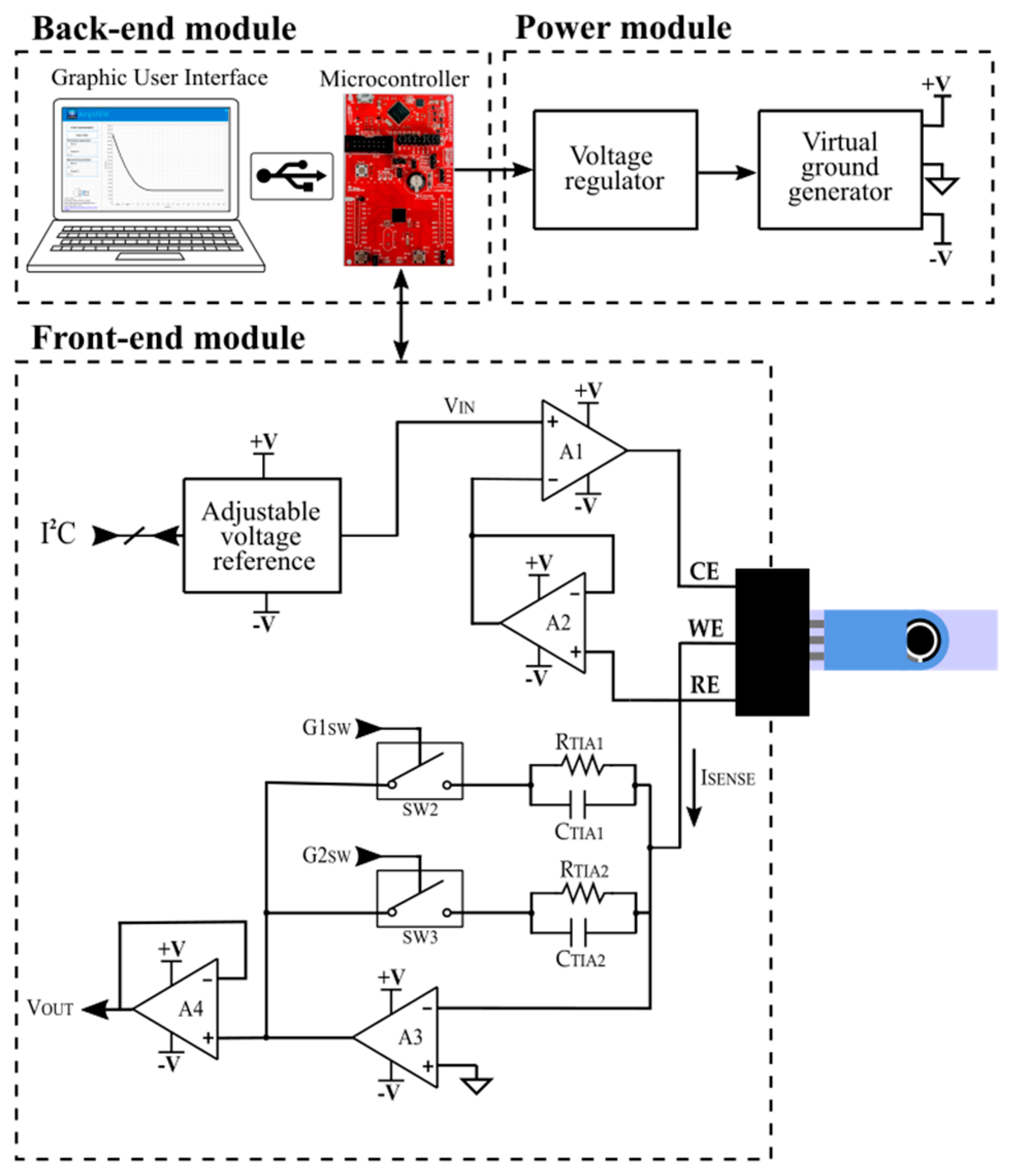
2.1. Portable Potentiostat
2.1.1. Front-End Module (FEM): Signal Acquisition Module Description
2.1.2. Back-End Module (BEM): Processing and Display Module Description
2.1.3. Power Module (PM): Power Supply Module Description
2.2. Electrochemical Measurements
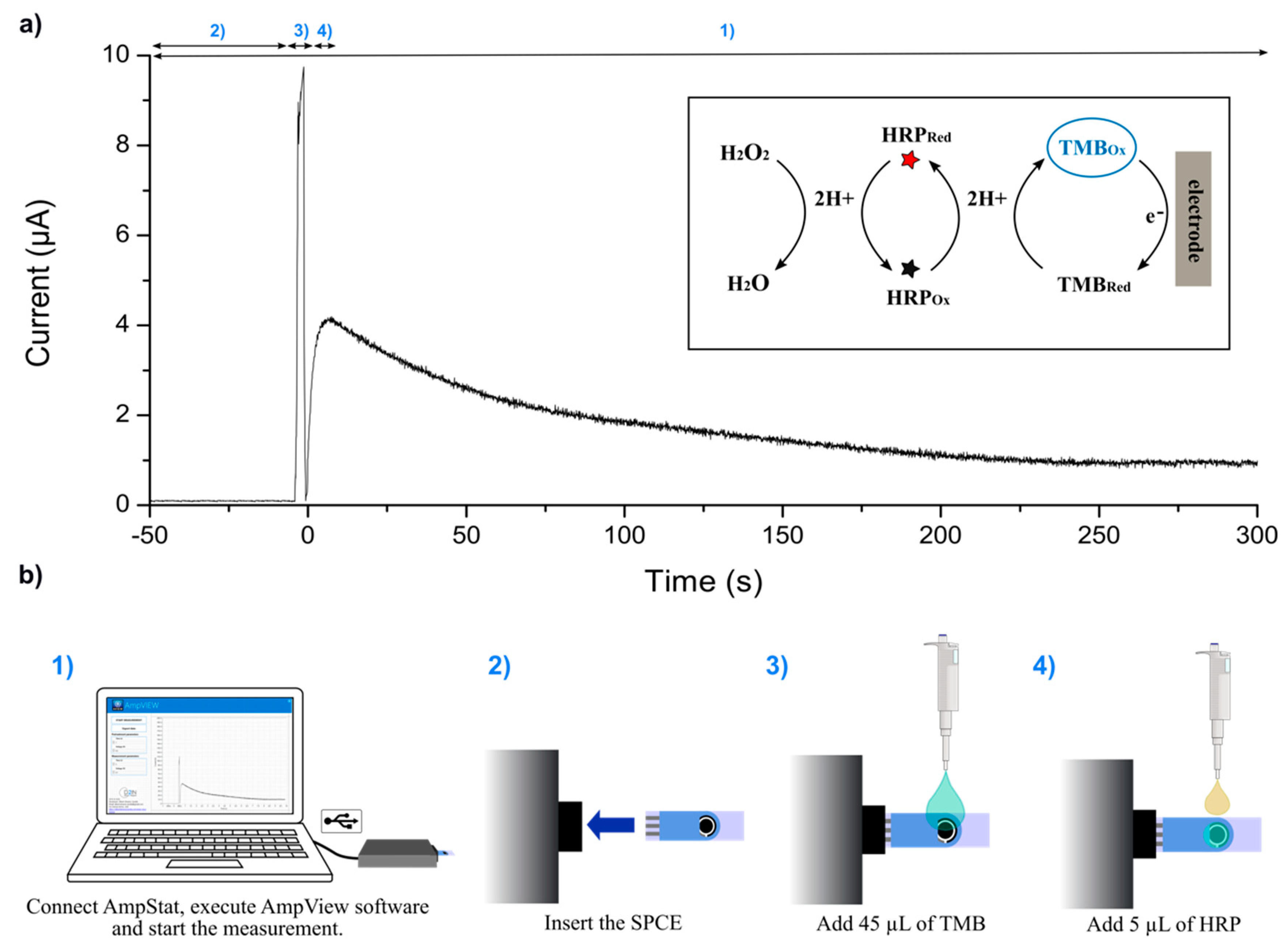
2.2.1. Electrochemical HRP Detection Procedure
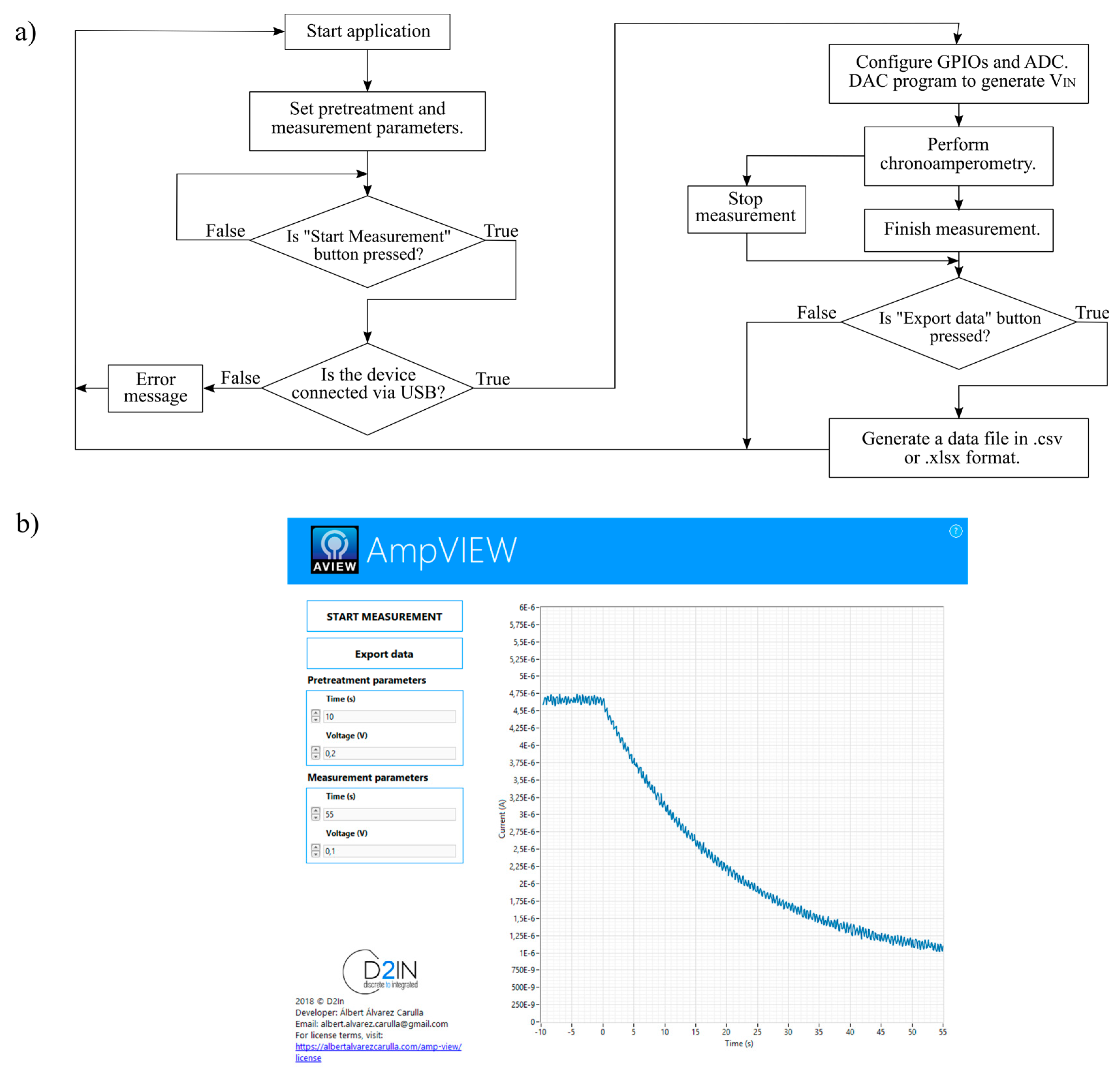
2.2.2. Data Analysis
3. Results and Discussion
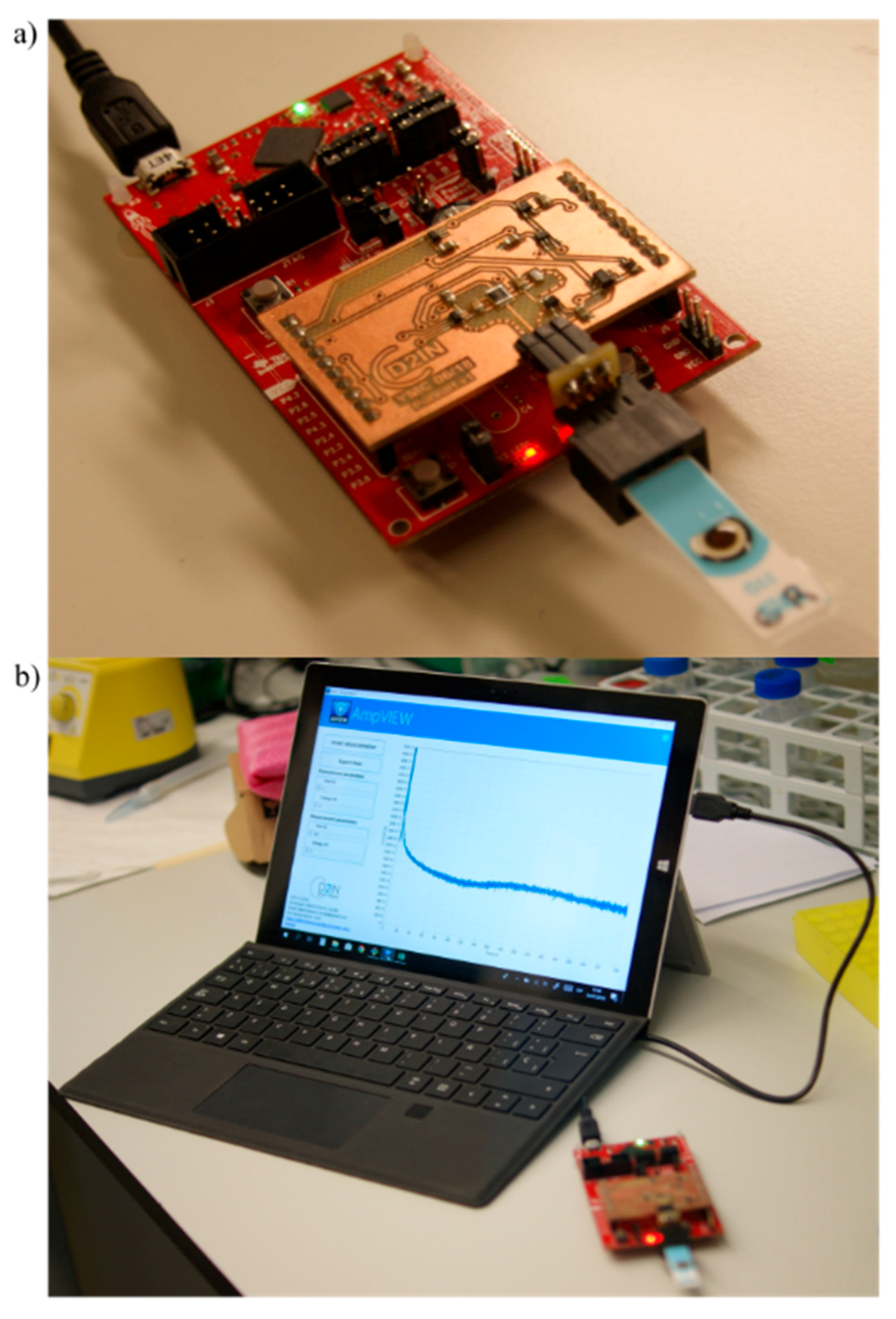
3.1. Test and Calibration of the Electronic Platform
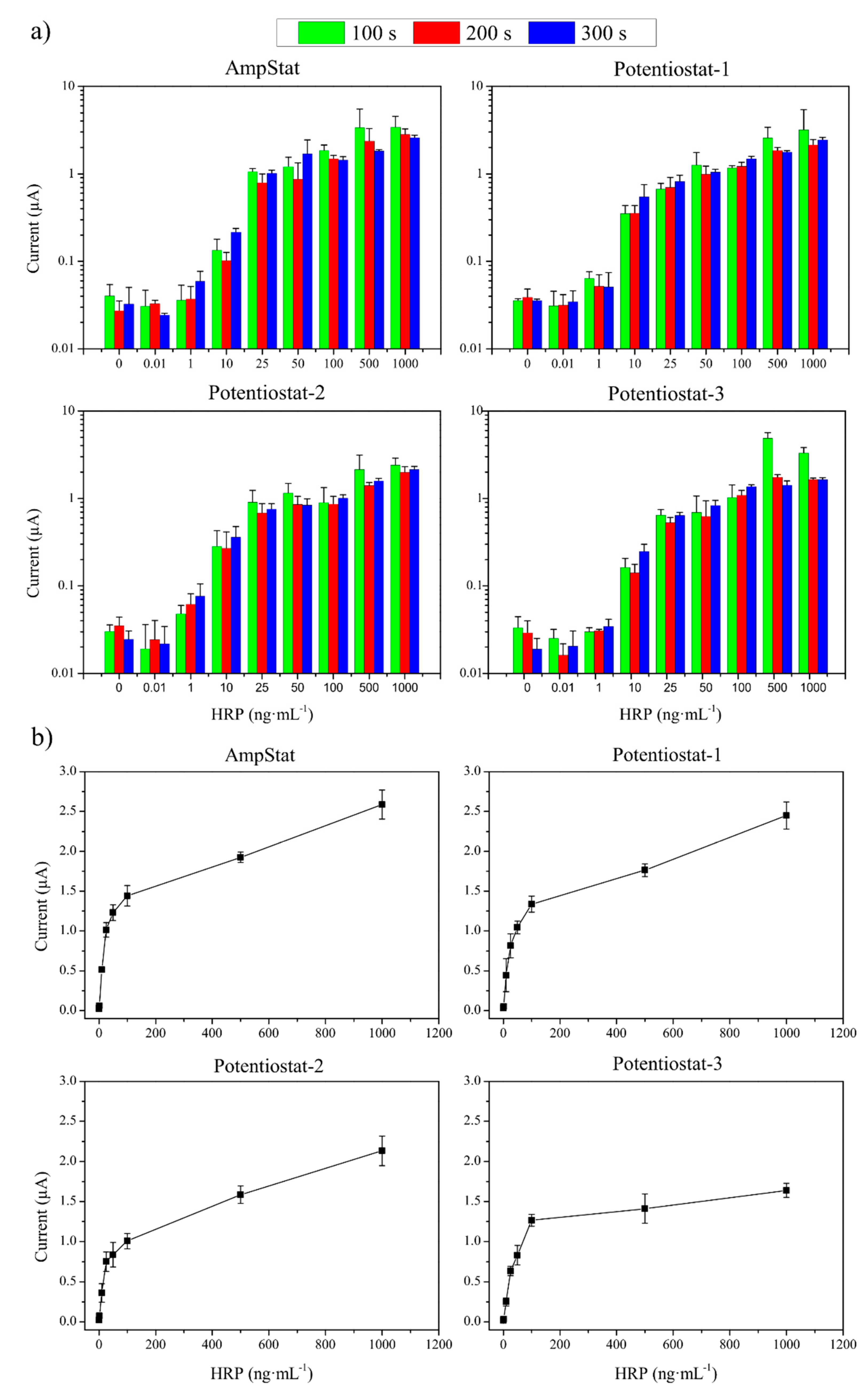
3.2. System Verification by HRP Electrochemical Detection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R. Point-of-Care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St John, A.; Price, C.P. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Lillehoj, P.B.; Huang, M.C.; Truong, N.; Ho, C.M. Rapid electrochemical detection on a mobile phone. Lab Chip 2013, 13, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Si, Y.; Wang, H.; Liu, G. A Portable Electrochemical Detection System based on Graphene/Ionic Liquid Modified Screen-printed Electrode for the Detection of Cadmium in Soil by Square Wave Anodic Stripping Voltammetry. Int. J. Electrochem. Sci. 2016, 11, 54–64. [Google Scholar]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical paper-based peptide nucleic acid biosensor for detecting human papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [Google Scholar] [CrossRef]
- Bettazzi, F.; Voccia, D.; Martellini, T.; Cincinelli, A.; Palchetti, I. Different enzyme-based strategies for the development of disposable electrochemical biosensors: Application to environmental pollutant monitoring. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–3. [Google Scholar]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Zuo, L.; Islam, S.K.; Mahbub, I.; Quaiyum, F. A low-power 1-V potentiostat for glucose sensors. IEEE Trans. Circuits Syst. II Express Briefs 2015, 62, 204–208. [Google Scholar] [CrossRef]
- Colomer-Farrarons, J.; Miribel-Català, P.L. A CMOS Self-Powered Front-End Architecture for Subcutaneous Event-Detector Devices: Three-Electrodes Amperometric Biosensor Approach; Springer: Dordrecht, The Netherlands, 2011; ISBN 9789400706859. [Google Scholar]
- Steinberg, M.D. A micropower amperometric potentiostat. Sens. Actuators B Chem. 2004, 97, 284–289. [Google Scholar] [CrossRef]
- Montes-Cebrián, Y.; del Torno-de Román, L.; Álvarez-Carulla, A.; Colomer-Farrarons, J.; Minteer, S.D.; Sabaté, N.; Miribel-Català, P.L.; Esquivel, J.P. ‘Plug-and-Power’ Point-of-Care diagnostics: A novel approach for self-powered electronic reader-based portable analytical devices. Biosens. Bioelectron. 2018, 118, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.D.M.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, 10, e0140349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, J.S.; Shriver-Lake, L.C.; Zabetakis, D.; Stenger, D.A.; Trammell, S.A. A simple and inexpensive electrochemical assay for the identification of nitrogen containing explosives in the field. Sensors 2017, 17, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafín, V.; Martínez-García, G.; Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Determination of progesterone in saliva using an electrochemical immunosensor and a COTS-based portable potentiostat. Anal. Chim. Acta 2019, 1049, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Otero, T.F. A cots-based portable system to conduct accurate substance concentration measurements. Sensors 2018, 18, 539. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Lee, J.; Shin, S.; Tae Kim, Y. Development of a telemetric, miniaturized electrochemical amperometric analyzer. Sensors 2017, 17, 2416. [Google Scholar] [CrossRef] [Green Version]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef]
- Muñoz-Martínez, A.I.; Peña, O.I.G.; Colomer-Farrarons, J.; Rodríguez-Delgado, J.M.; Ávila-Ortega, A.; Dieck-Assad, G. Electrochemical instrumentation of an embedded potentiostat system (Eps) for a programmable-system-on-a-chip. Sensors 2018, 18, 4490. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.C.; Yao, C.; Ag, V.; Hall, D.A. An efficient power harvesting mobile phone-based electrochemical biosensor for point-of-care health monitoring. Sens. Actuators B Chem. 2016, 235, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Aymerich, J.; Márquez, A.; Terés, L.; Muñoz-Berbel, X.; Jiménez, C.; Domínguez, C.; Serra-Graells, F.; Dei, M. Cost-effective smartphone-based reconfigurable electrochemical instrument for alcohol determination in whole blood samples. Biosens. Bioelectron. 2018, 117, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Montes-Cebrián, Y.; Álvarez-Carulla, A.; Colomer-Farrarons, J.; Puig-Vidal, M.; Miribel-Català, P.L. Self-Powered Portable Electronic Reader for Point-of-Care Amperometric Measurements. Sensors 2019, 19, 3715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, A.C.; Hall, D.A. Point-of-Care Smartphone-based Electrochemical Biosensing. Electroanalysis 2019, 31, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Chen, P.; Ma, Z.; Li, S.; Gao, X.; Wu, R.; Pan, L.; Shi, Y. Near-Field Communication Sensors. Sensors 2019, 19, 3947. [Google Scholar] [CrossRef] [Green Version]
- Krainer, F.W.; Glieder, A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1611–1625. [Google Scholar] [CrossRef] [Green Version]
- Khanmohammadi, M.; Dastjerdi, M.B.; Ai, A.; Ahmadi, A.; Godarzi, A.; Rahimi, A.; Ai, J. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater. Sci. 2018, 6, 1286–1298. [Google Scholar] [CrossRef]
- Hong, T.; Liu, W.; Li, M.; Chen, C. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences. Anal. Chim. Acta 2019, 1067, 31–47. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, M.D.; Kassal, P.; Kereković, I.; Steinberg, I.M. A wireless potentiostat for mobile chemical sensing and biosensing. Talanta 2015, 143, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Ramirez, T.; Torres, C.C.; Rosero, A.C.; Espinoza-Montero, P. Low cost potentiostat: Criteria and considerations for its design and construction. In Proceedings of the Proceedings of the 2016 IEEE ANDESCON, Arequipa, Peru, 19–21 October 2016; pp. 1–4. [Google Scholar]

| AmpStat | Potentiostat-1 | Potentiostat-2 | Poteniostat-3 | ||
|---|---|---|---|---|---|
| LOD | |||||
| Current (µA) | 0.05 | 0.05 | 0.04 | 0.04 | |
| Concentration (ng·mL−1) | 0.83 | 0.52 | 0.56 | 1.27 | |
| LOQ | |||||
| Current (µA) | 0.07 | 0.08 | 0.07 | 0.06 | |
| Concentration (ng·mL−1) | 1.52 | 1.16 | 1.61 | 2.89 | |
| Sensitivity (ng·mL−1·µA−1) | 0.0328 | 0.0326 | 0.0305 | 0.0219 | |
| Weight (g) | 41 | 1600 | 480 | 5433 | |
| Dimensions (cm) | 10.5 × 5.8 × 2.5 | 22.2 × 20.5 × 7.5 | 13.2 × 10.0 × 3.6 | 360.7 × 233.7 × 116.9 | |
| Cost (USD) | 85 | 11,013 | 4087 | 16,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Cebrián, Y.; Álvarez-Carulla, A.; Ruiz-Vega, G.; Colomer-Farrarons, J.; Puig-Vidal, M.; Baldrich, E.; Miribel-Català, P.L. Competitive USB-Powered Hand-Held Potentiostat for POC Applications: An HRP Detection Case. Sensors 2019, 19, 5388. https://doi.org/10.3390/s19245388
Montes-Cebrián Y, Álvarez-Carulla A, Ruiz-Vega G, Colomer-Farrarons J, Puig-Vidal M, Baldrich E, Miribel-Català PL. Competitive USB-Powered Hand-Held Potentiostat for POC Applications: An HRP Detection Case. Sensors. 2019; 19(24):5388. https://doi.org/10.3390/s19245388
Chicago/Turabian StyleMontes-Cebrián, Yaiza, Albert Álvarez-Carulla, Gisela Ruiz-Vega, Jordi Colomer-Farrarons, Manel Puig-Vidal, Eva Baldrich, and Pere Ll. Miribel-Català. 2019. "Competitive USB-Powered Hand-Held Potentiostat for POC Applications: An HRP Detection Case" Sensors 19, no. 24: 5388. https://doi.org/10.3390/s19245388
APA StyleMontes-Cebrián, Y., Álvarez-Carulla, A., Ruiz-Vega, G., Colomer-Farrarons, J., Puig-Vidal, M., Baldrich, E., & Miribel-Català, P. L. (2019). Competitive USB-Powered Hand-Held Potentiostat for POC Applications: An HRP Detection Case. Sensors, 19(24), 5388. https://doi.org/10.3390/s19245388





