Development of Human Serum Albumin Selective Fluorescent Probe Using Thieno[3,2-b]pyridine-5(4H)-one Fluorophore Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of KF Derivatives (Thieno[3,2-b]pyridine-5(4H)-one Derivatives)
2.3. Fluorescence-Based High-Throughput Screening of KF Derivatives against Major Plasma Proteins
2.4. Comparison of Fluorophores as Human Serum Albumin (HSA) Probe Candidates in Terms of Selectivity
2.5. The Effects of pH and DMSO on the HSA-Sensing Behavior of 4
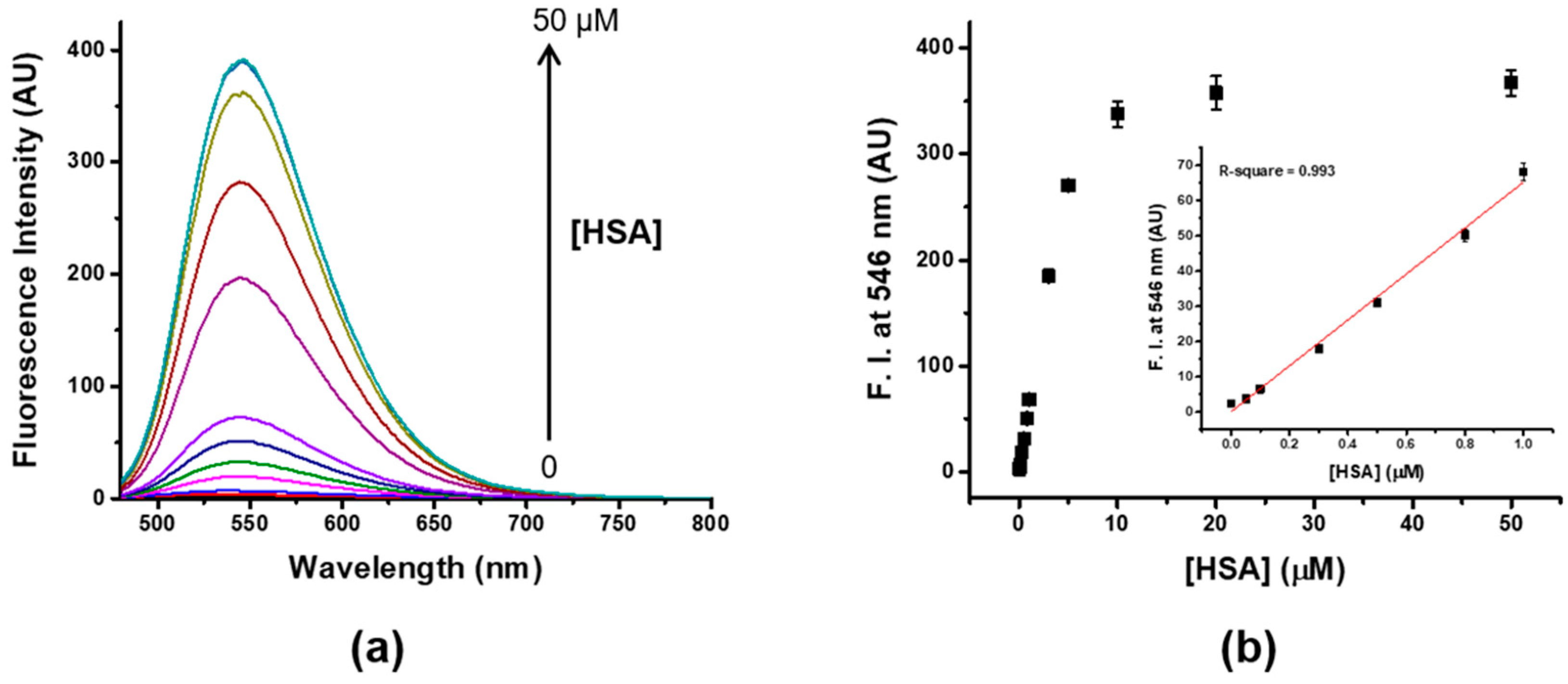
2.6. Determination of the Limit of Detection (LOD)
2.7. Determination of Quantum Yield of 4
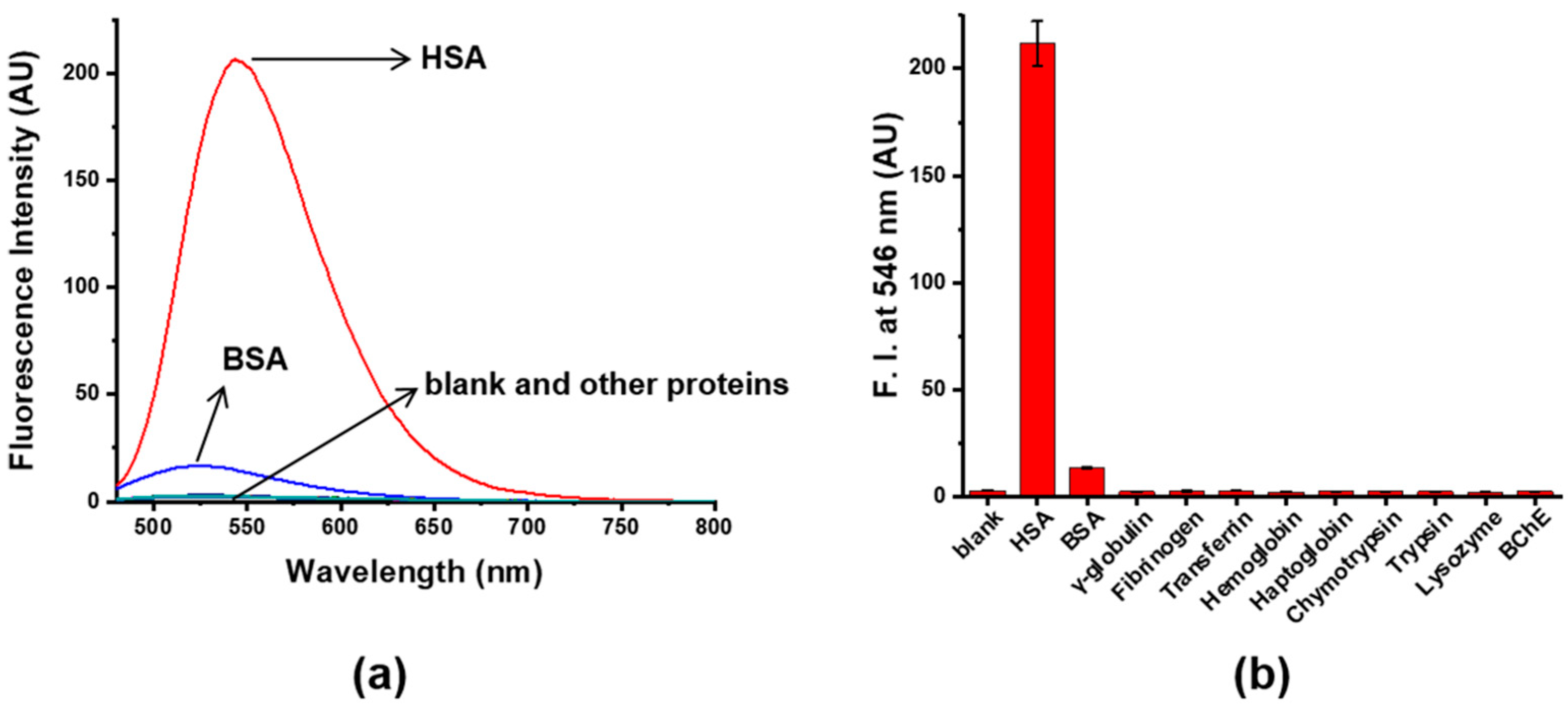
2.8. Selectivity Test of 4 toward HSA over Plasma Proteins
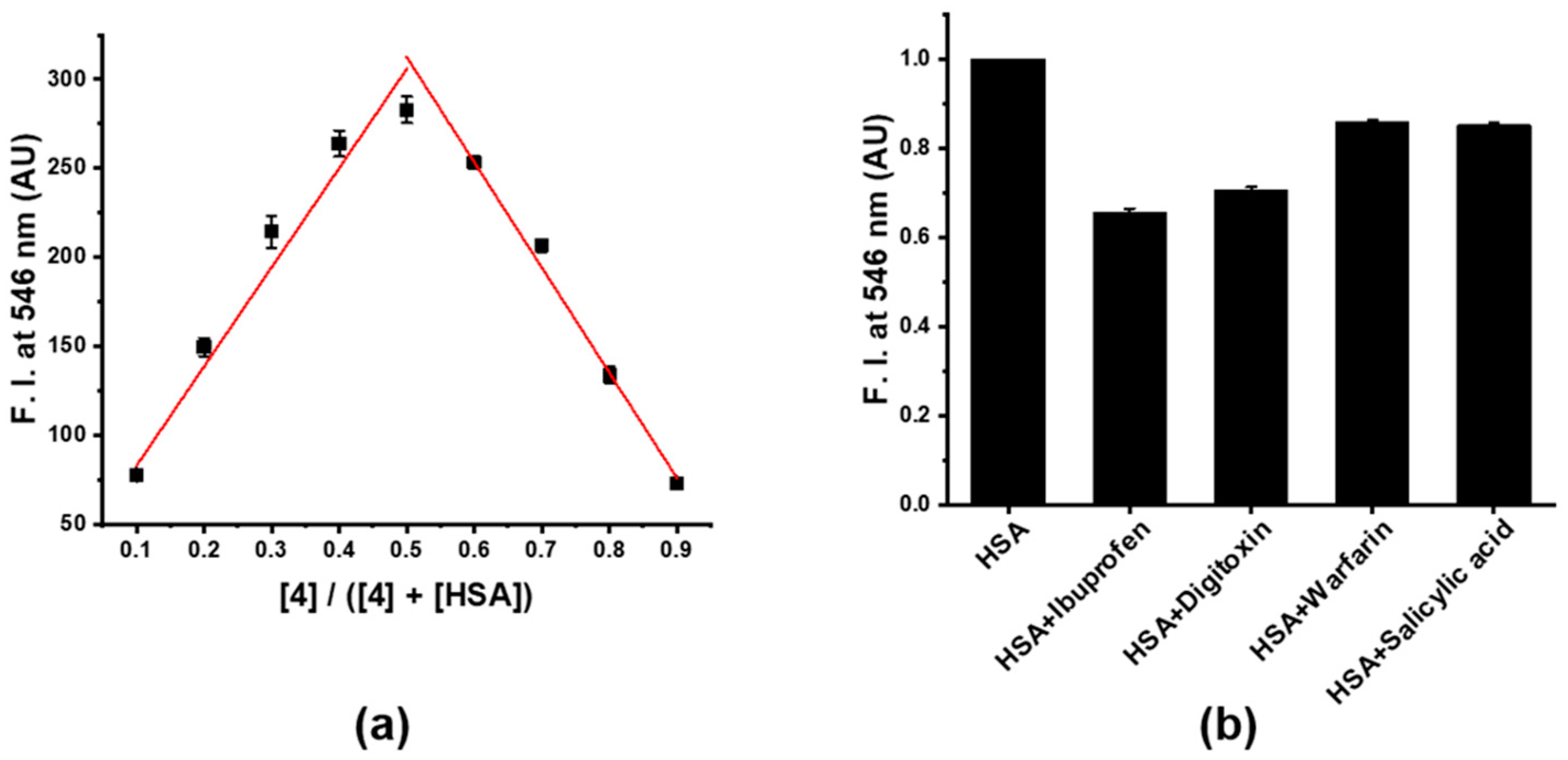
2.9. Job’s Plot Analysis
2.10. Determination of the Dissociation Constant (Kd) of HSA and 4
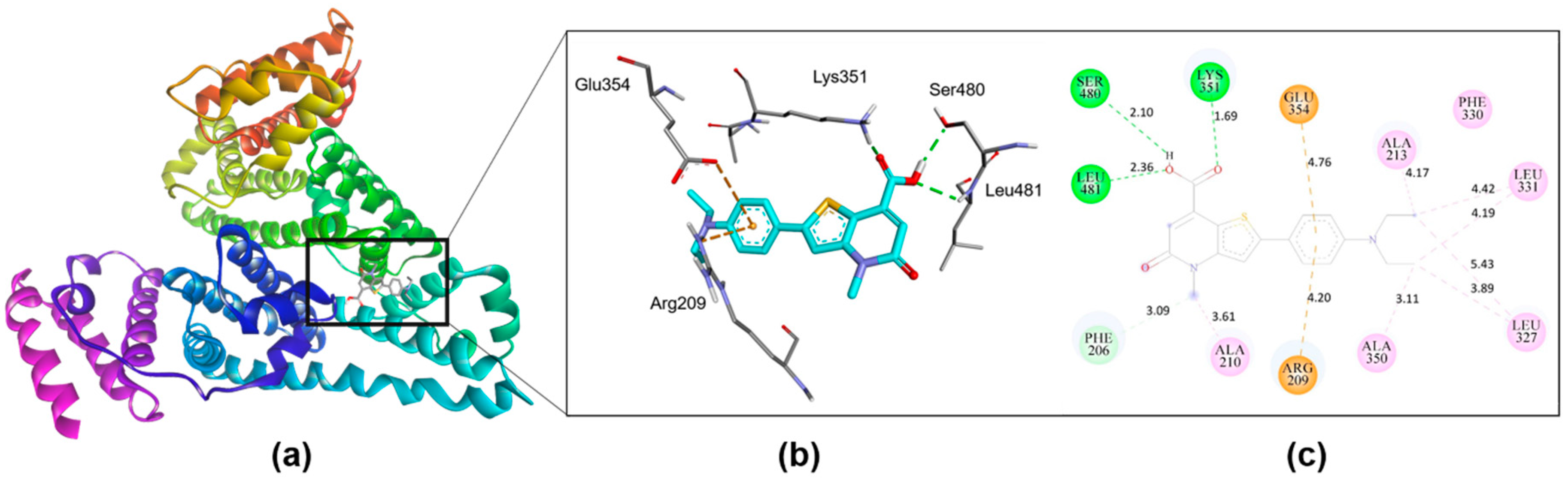
2.11. Assignment of the Binding Site of 4 on HSA
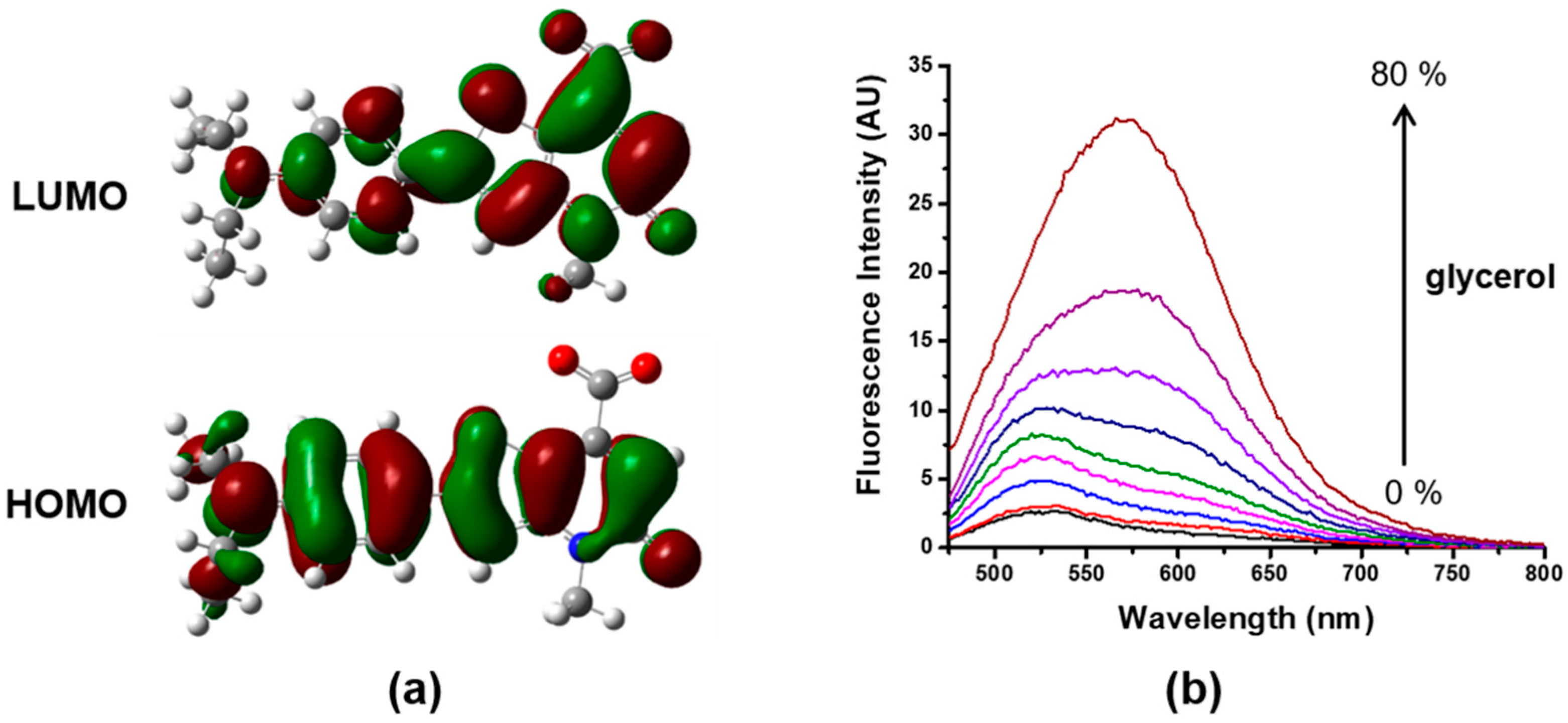
2.12. Identification of the Sensing Mechanism of 4 toward HSA
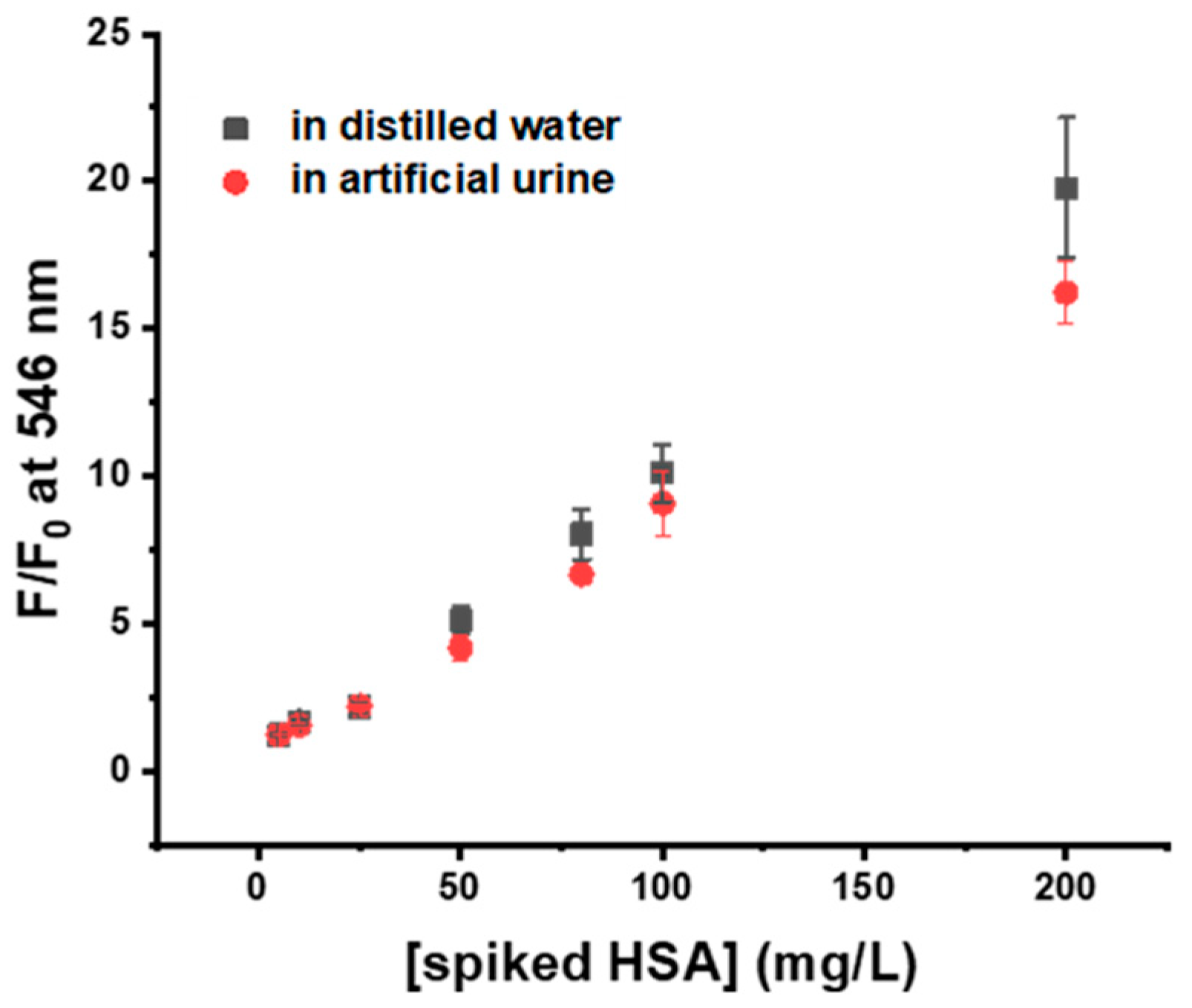
2.13. Quantitative Detection of HSA in Artificial Urine
3. Results and Discussion
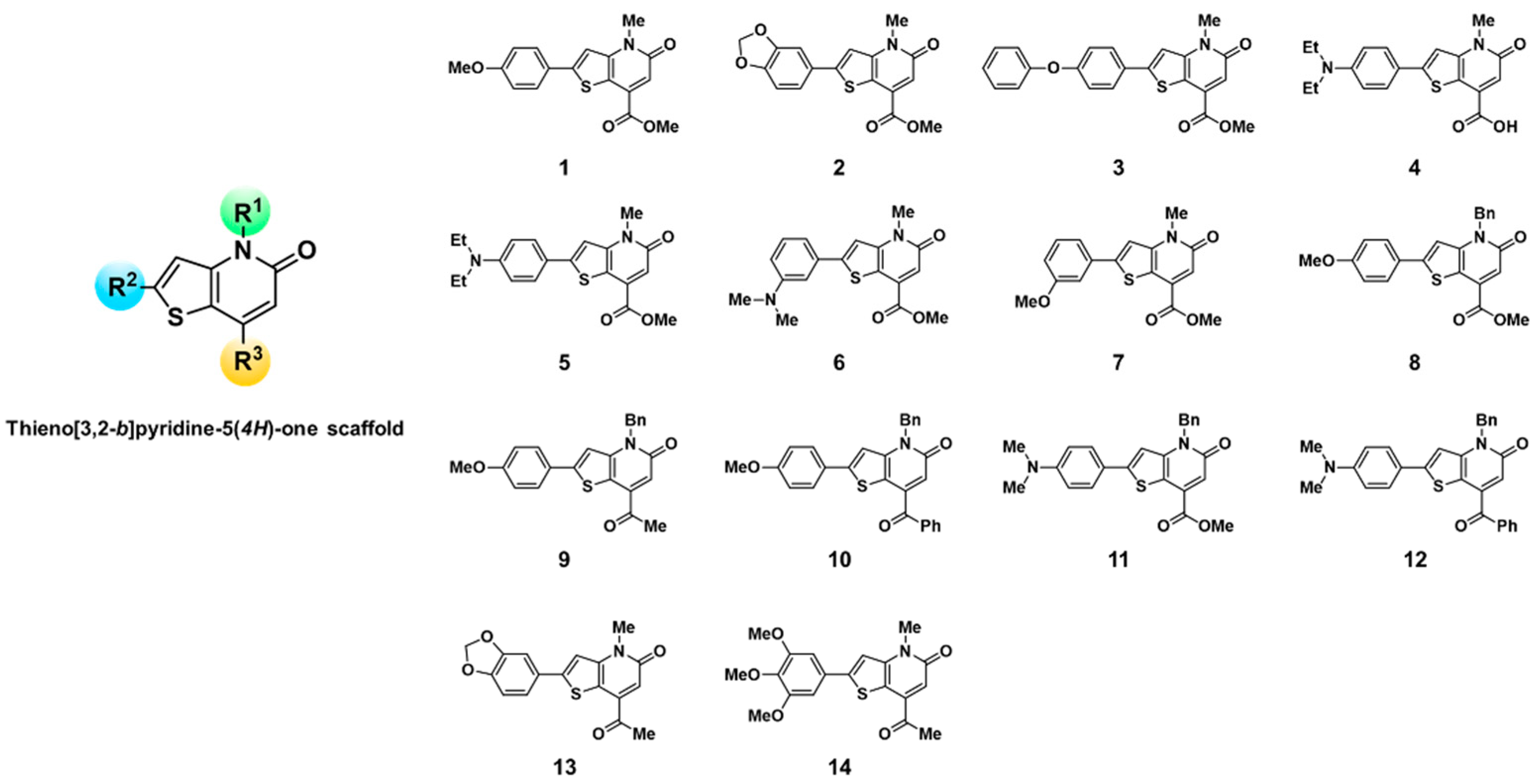
3.1. Fluorescence-Based High-Throughput Screening of KF Derivatives against Major Plasma Proteins
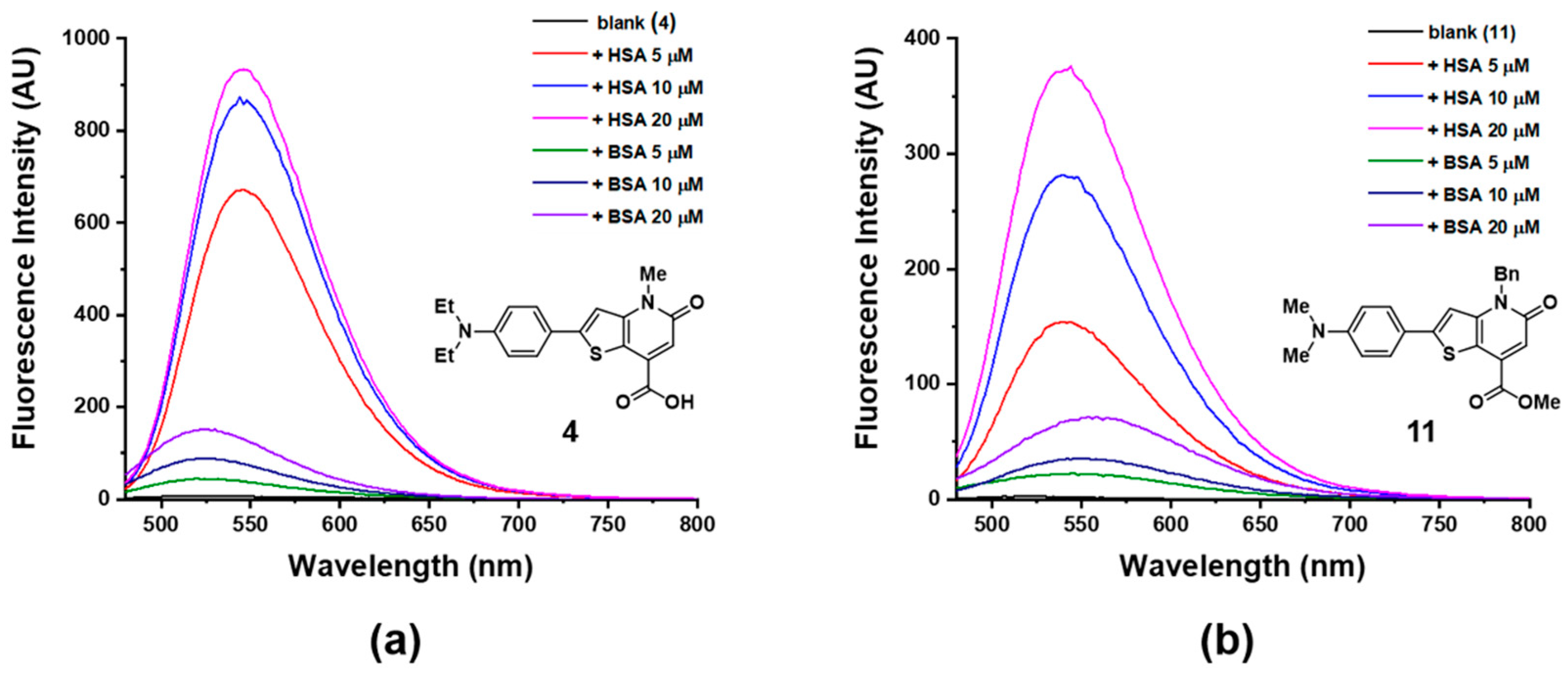
3.2. Comparison of Two Candidates in Absorption and Fluorescence Response toward HSA
3.3. Sensitivity and Selectivity of Probe 4 to HSA
3.4. Binding Properties of Probe 4 on HSA
3.5. Sensing Mechanism of Probe 4
3.6. Application of Probe 4 in Artificial Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berde, C.B.; Hudson, B.S.; Simoni, R.D.; Sklar, L.A. Human Serum Albumin. Spectroscopic studies of binding and proximity relationships for fatty acids and bilirubin. J. Biol. Chem. 1979, 254, 391–400. [Google Scholar] [PubMed]
- Müller, W.E.; Wollert, U. Human Serum Albumin as a ‘Silent Receptor’ for Drugs and Endogenous Substances. Pharmacology 1979, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical Aspects of the Ligand-Binding and Enzymatic Properties of Human Serum Albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical Properties and Therapeutic Potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Stanyon, H.F.; Viles, J.H. Human Serum Albumin Can Regulate Amyloid-β Peptide Fiber Growth in the Brain Interstitium. Implications for Alzheimer disease. J. Biol. Chem. 2012, 287, 28163–28168. [Google Scholar] [CrossRef]
- Doweiko, J.P.; Nompleggi, D.J. The Role of Albumin in Human Physiology and Pathophysiology, Part III: Albumin and Disease States. J. Parenter. Enter. Nutr. 1991, 15, 476–483. [Google Scholar] [CrossRef]
- Gatta, A.; Verardo, A.; Bolognesi, M. Hypoalbuminemia. Intern. Emerg. Med. 2012, 7, S193–S199. [Google Scholar] [CrossRef]
- Viberti, G.C.; Hill, R.D.; Jarrett, R.J.; Argyropoulos, A.; Mahmud, U.; Keen, H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982, 319, 1430–1432. [Google Scholar] [CrossRef]
- Rowe, D.J.F.; Dawnay, A.; Watts, G.F. Microalbuminuria in diabetes mellitus: Review and recommendations for the measurement of albumin in urine. Ann. Clin. Biochem. 1990, 27, 297–312. [Google Scholar] [CrossRef]
- Doumas, B.T.; Peters, T., Jr. Serum and urine albumin: A progress report on their measurement and clinical significance. Clin. Chim. Acta 1997, 258, 3–20. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hill, P.G. The measurement of albumin in serum and plasma. Ann. Clin. Biochem. 1985, 22, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1997, 258, 21–30. [Google Scholar] [CrossRef]
- Liang, H.; Scott, M.K.; Murry, D.J.; Sowinski, K.M. Determination of albumin and myoglobin in dialysate and ultrafiltrate samples by high-performance size-exclusion chromatography. J. Chromatogr. B 2001, 754, 141–151. [Google Scholar] [CrossRef]
- Seegmiller, J.C.; Barnidge, D.R.; Burns, B.E.; Larson, T.S.; Lieske, J.C.; Kumar, R. Quantification of Urinary Albumin by Using Protein Cleavage and LC-MS/MS. Clin. Chem. 2009, 55, 1100–1107. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Chang, Y.T.; Liu, K.L.; Chang, H.Y.; Yew, T.R. Electrical impedimetric biosensors for liver function detection. Biosens. Bioelectron. 2011, 28, 368–372. [Google Scholar] [CrossRef]
- Tu, M.C.; Chang, Y.T.; Kang, Y.T.; Chang, H.Y.; Chang, P.; Yew, T.R. A quantum dot-based optical immunosensor for human serum albumin detection. Biosens. Bioelectron. 2012, 34, 286–290. [Google Scholar] [CrossRef]
- Caballero, D.; Martinez, E.; Bausells, J.; Errachid, A.; Samitier, J. Impedimetric immunosensor for human serum albumin detection on a direct aldehyde-functionalized silicon nitride surface. Anal. Chim. Acta 2012, 720, 43–48. [Google Scholar] [CrossRef]
- Wang, R.E.; Tian, L.; Chang, Y.H. A Homogeneous Fluorescent Sensor for Human Serum Albumin. J. Pharm. Biomed. Anal. 2012, 63, 165–169. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Yang, W. Gold Nanoparticle-Based Facile Detection of Human Serum Albumin and Its Application as an INHIBIT Logic Gate. ACS Appl. Mater. Interfaces 2015, 7, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Lee, J.W.; Ahn, Y.H.; Chang, Y.T. Combinatorial Dapoxyl Dye Library and its Application to Site Selective Probe for Human Serum Albumin. J. Comb. Chem. 2007, 9, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Feng, C.; Yu, Y.; Liu, J.; Lam, J.W.Y.; Luo, K.Q.; Tang, B.Z. Quantitation, Visualization, and Monitoring of Conformational Transitions of Human Serum Albumin by a Tetraphenylethene Derivative with Aggregation-Induced Emission Characteristics. Anal. Chem. 2010, 82, 7035–7043. [Google Scholar] [CrossRef] [PubMed]
- Er, J.C.; Vendrell, M.; Tang, M.K.; Zhai, D.; Chang, Y.T. Fluorescent Dye Cocktail for Multiplex Drug-Site Mapping on Human Serum Albumin. ACS Comb. Sci. 2013, 15, 452–457. [Google Scholar] [CrossRef]
- Galpothdeniya, W.I.S.; Das, S.; De Rooy, S.L.; Regmi, B.P.; Hamdan, S.; Warner, I.M. Fluorescein-based ionic liquid sensor for label-free detection of serum albumins. RSC Adv. 2014, 4, 17533–17540. [Google Scholar] [CrossRef]
- Fan, J.; Sun, W.; Wang, Z.; Peng, X.; Li, Y.; Cao, J. A fluorescent probe for site I binding and sensitive discrimination of HSA from BSA. Chem. Commun. 2014, 50, 9573–9576. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Yu, W.T.; Hou, T.C.; Liu, T.K.; Huang, C.L.; Chen, I.C.; Tan, K.T. A selective and sensitive fluorescent albumin probe for the determination of urinary albumin. Chem. Commun. 2014, 50, 11507–11510. [Google Scholar] [CrossRef]
- Anees, P.; Sreejith, S.; Ajayaghosh, A. Self-Assembled Near-Infrared Dye Nanoparticles as a Selective Protein Sensor by Activation of a Dormant Fluorophore. J. Am. Chem. Soc. 2014, 136, 13233–13239. [Google Scholar] [CrossRef]
- Li, W.; Chen, D.; Wang, H.; Luo, S.; Dong, L.; Zhang, Y.; Shi, J.; Tong, B.; Dong, Y. Quantitation of Albumin in Serum Using “Turn-on” Fluorescent Probe with Aggregation-Enhanced Emission Characteristics. ACS Appl. Mater. Interfaces 2015, 7, 26094–26100. [Google Scholar] [CrossRef]
- Fan, X.; He, Q.; Sun, S.; Li, H.; Pei, Y.; Xu, Y. Nanoparticles self-assembled from multiple interactions: A novel near-infrared fluorescent sensor for the detection of serum albumin in human sera and turn-on live-cell imaging. Chem. Commun. 2016, 52, 1178–1181. [Google Scholar] [CrossRef]
- Dey, G.; Gaur, P.; Giri, R.; Ghosh, S. Optical signaling in biofluids: A nondenaturing photostable molecular probe for serum albumins. Chem. Commun. 2016, 52, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Feng, L.; Xu, L.; Li, Y.; Wang, D.D.; Hou, J.; Zhou, K.; Jin, Q.; Ge, G.B.; Cui, J.N.; et al. A rapid-response fluorescent probe for the sensitive and selective detection of human albumin in plasma and cell culture supernatants. Chem. Commun. 2016, 52, 6064–6067. [Google Scholar] [CrossRef]
- Wang, Y.R.; Feng, L.; Xu, L.; Hou, J.; Jin, Q.; Zhou, N.; Lin, Y.; Cui, J.N.; Ge, G.B. An ultrasensitive and conformation sensitive fluorescent probe for sensing human albumin in complex biological samples. Sens. Actuator B Chem. 2017, 245, 923–931. [Google Scholar] [CrossRef]
- Singh, P.; Mittal, L.S.; Kaur, S.; Kaur, S.; Bhargava, G.; Kumar, S. Self-assembled small molecule based fluorescent detection of serum albumin proteins: Clinical detection and cell imaging. Sens. Actuator B Chem. 2018, 255, 478–489. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhu, X.; Pan, Z.; Zhang, X.; Wang, J.; Fu, N. Self-assembled nanosensor based on squaraine dye for specific recognition and detection of human serum albumin. Sens. Actuator B Chem. 2018, 255, 977–985. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, B.; Zhu, K.; Huang, Y.; Pan, C.; Wang, B.; Wang, L. An environment-sensitive fluorescent probe for quantification of human serum albumin: Design, sensing mechanism, and its application in clinical diagnosis of hypoalbuminemia. Dyes Pigments 2018, 152, 60–66. [Google Scholar] [CrossRef]
- Choudhury, R.; Patel, S.R.; Ghosh, A. Selective detection of human serum albumin by near infrared emissive fluorophores: Insights into structure-property relationship. J. Photochem. Photobiol. A Chem. 2019, 376, 100–107. [Google Scholar] [CrossRef]
- Sung, D.-B.; Mun, B.; Park, S.; Lee, H.-S.; Lee, J.; Lee, Y.-J.; Shin, H.J.; Lee, J.S. Synthesis, Molecular Engineering, and Photophysical Properties of Fluorescent Thieno[3,2-b]pyridine-5(4H)-ones. J. Org. Chem. 2019, 84, 379–391. [Google Scholar] [CrossRef]
- Huang, C.Y. Determination of Binding Stoichiometry by the Continuous Variation Method: The Job Plot. Methods Enzymol. 1982, 87, 509–525. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Visualizer; BIOVIA: San Diego, CA, USA, 2017. [Google Scholar]
- Brooks, T.; Keevil, C.W. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Han, K.L. The sensing mechanism studies of the fluorescent probes with electronically excited state calculations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1351–e1367. [Google Scholar] [CrossRef]
- Cao, C.; Liu, X.; Qiao, Q.; Zhao, M.; Yin, W.; Mao, D.; Zhang, H.; Xu, Z. A twisted-intramolecular-charge-transfer (TICT) based ratiometric fluorescent thermometer with a mega-Stokes shift and a positive temperature coefficient. Chem. Commun. 2014, 50, 15811–15814. [Google Scholar] [CrossRef]
- Lou, Z.; Yang, S.; Li, P.; Zhou, P.; Han, K. Experimental and theoretical study on the sensing mechanism of a fluorescence probe for hypochloric acid: A Se⋯N nonbonding interaction modulated twisting process. Phys. Chem. Chem. Phys. 2014, 16, 3749–3756. [Google Scholar] [CrossRef]
- Reja, S.I.; Khan, I.A.; Bhalla, V.; Kumar, M. A TICT based NIR-fluorescent probe for human serum albumin: A pre-clinical diagnosis in blood serum. Chem. Commun. 2016, 52, 1182–1185. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Feng, Y.; Ning, P.; Yu, H.; Zhu, M.; Xi, X.; Guo, Q.; Meng, X. A TICT based two-photon fluorescent probe for cysteine and homocysteine in living cells. Sens. Actuator B Chem. 2016, 231, 285–292. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Lu, S.; Cheng, S.; Du, J.; Gao, Q.; Shen, P.; Luo, H.; Liu, Y.; Yang, C. Red-emitting benzo[e]indolium probes for HSA based on the TICT characteristics. J. Lumin. 2017, 192, 478–485. [Google Scholar] [CrossRef]
- Samanta, S.; Halder, S.; Das, G. Twisted-Intramolecular-Charge-Transfer-Based Turn-On Fluorogenic Nanoprobe for Real-Time Detection of Serum Albumin in Physiological Conditions. Anal. Chem. 2018, 90, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11–24. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Sung, D.-B.; Kang, S.; Parameswaran, S.; Choi, J.-H.; Lee, J.S.; Han, M.S. Development of Human Serum Albumin Selective Fluorescent Probe Using Thieno[3,2-b]pyridine-5(4H)-one Fluorophore Derivatives. Sensors 2019, 19, 5298. https://doi.org/10.3390/s19235298
Lee S, Sung D-B, Kang S, Parameswaran S, Choi J-H, Lee JS, Han MS. Development of Human Serum Albumin Selective Fluorescent Probe Using Thieno[3,2-b]pyridine-5(4H)-one Fluorophore Derivatives. Sensors. 2019; 19(23):5298. https://doi.org/10.3390/s19235298
Chicago/Turabian StyleLee, Suji, Dan-Bi Sung, Seungyoon Kang, Saravanan Parameswaran, Jun-Ho Choi, Jong Seok Lee, and Min Su Han. 2019. "Development of Human Serum Albumin Selective Fluorescent Probe Using Thieno[3,2-b]pyridine-5(4H)-one Fluorophore Derivatives" Sensors 19, no. 23: 5298. https://doi.org/10.3390/s19235298
APA StyleLee, S., Sung, D.-B., Kang, S., Parameswaran, S., Choi, J.-H., Lee, J. S., & Han, M. S. (2019). Development of Human Serum Albumin Selective Fluorescent Probe Using Thieno[3,2-b]pyridine-5(4H)-one Fluorophore Derivatives. Sensors, 19(23), 5298. https://doi.org/10.3390/s19235298






