Pilot Study of the EncephaLog Smartphone Application for Gait Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. EncephaLog
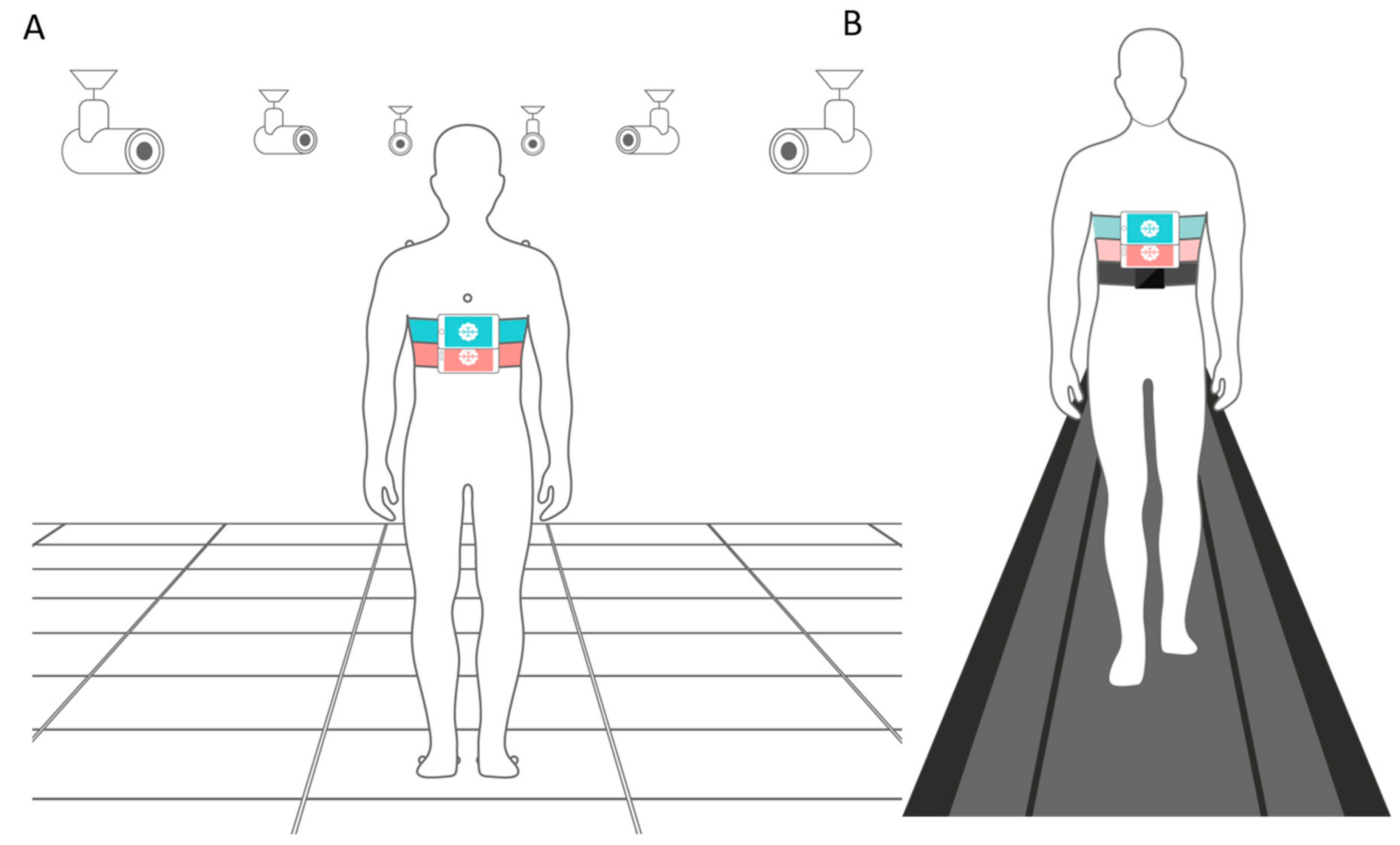
2.2. Motion Capture Cameras (MCC)
2.3. Pressure Mat
2.4. Wearable Sensors
3. Results
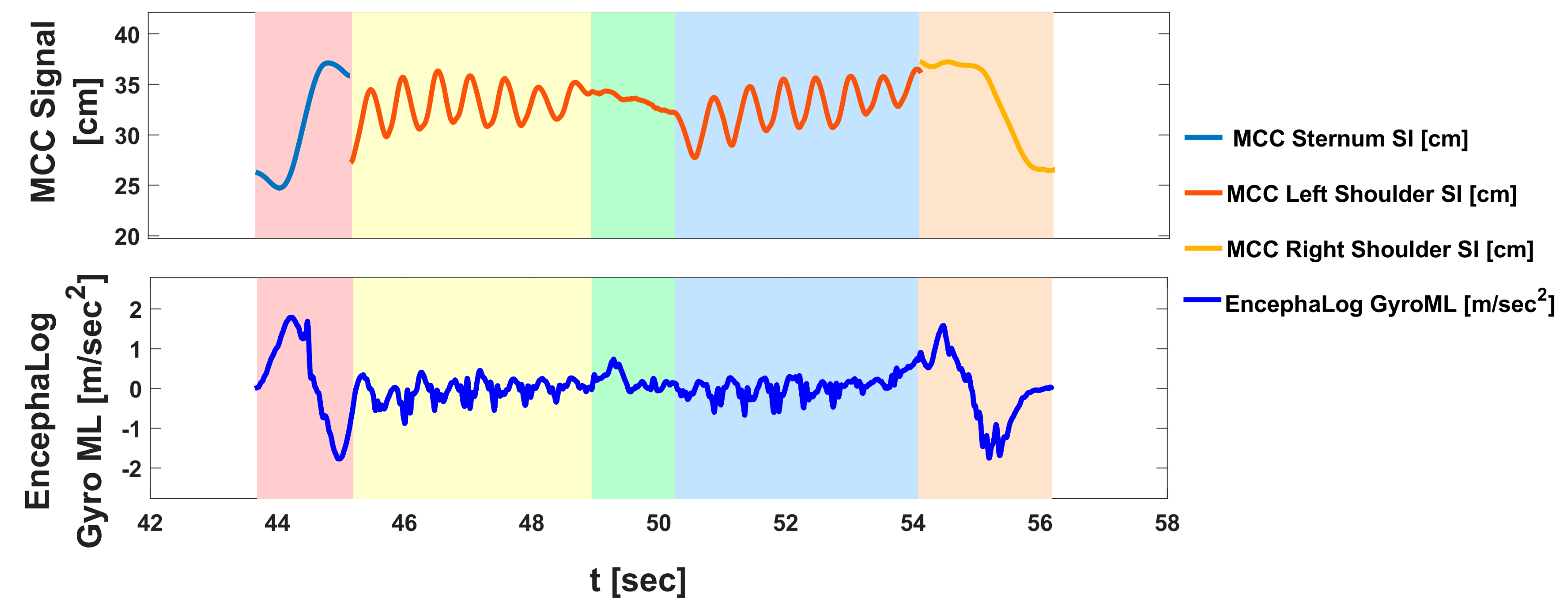
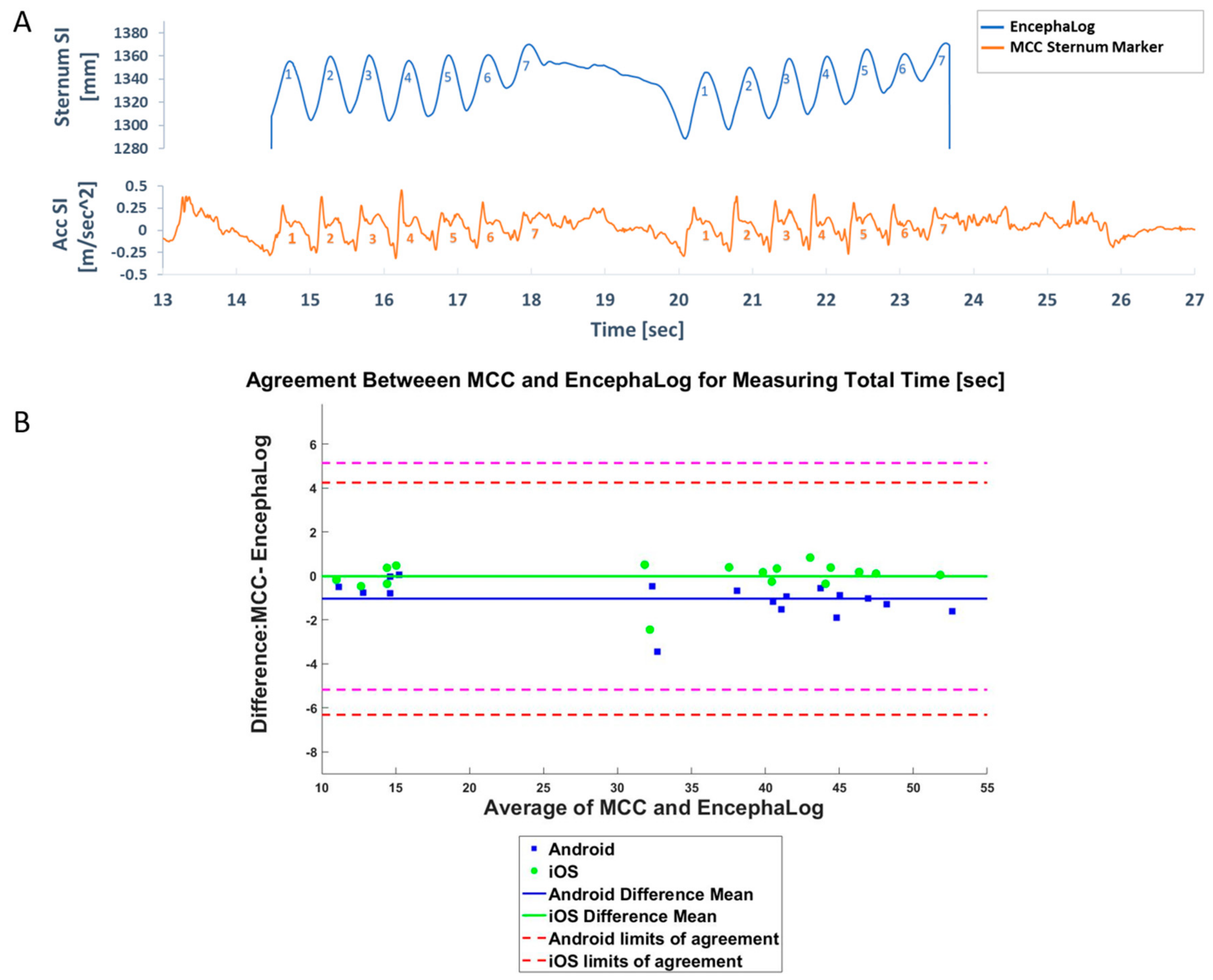
3.1. Experiment 1: EncephaLog vs. MCC
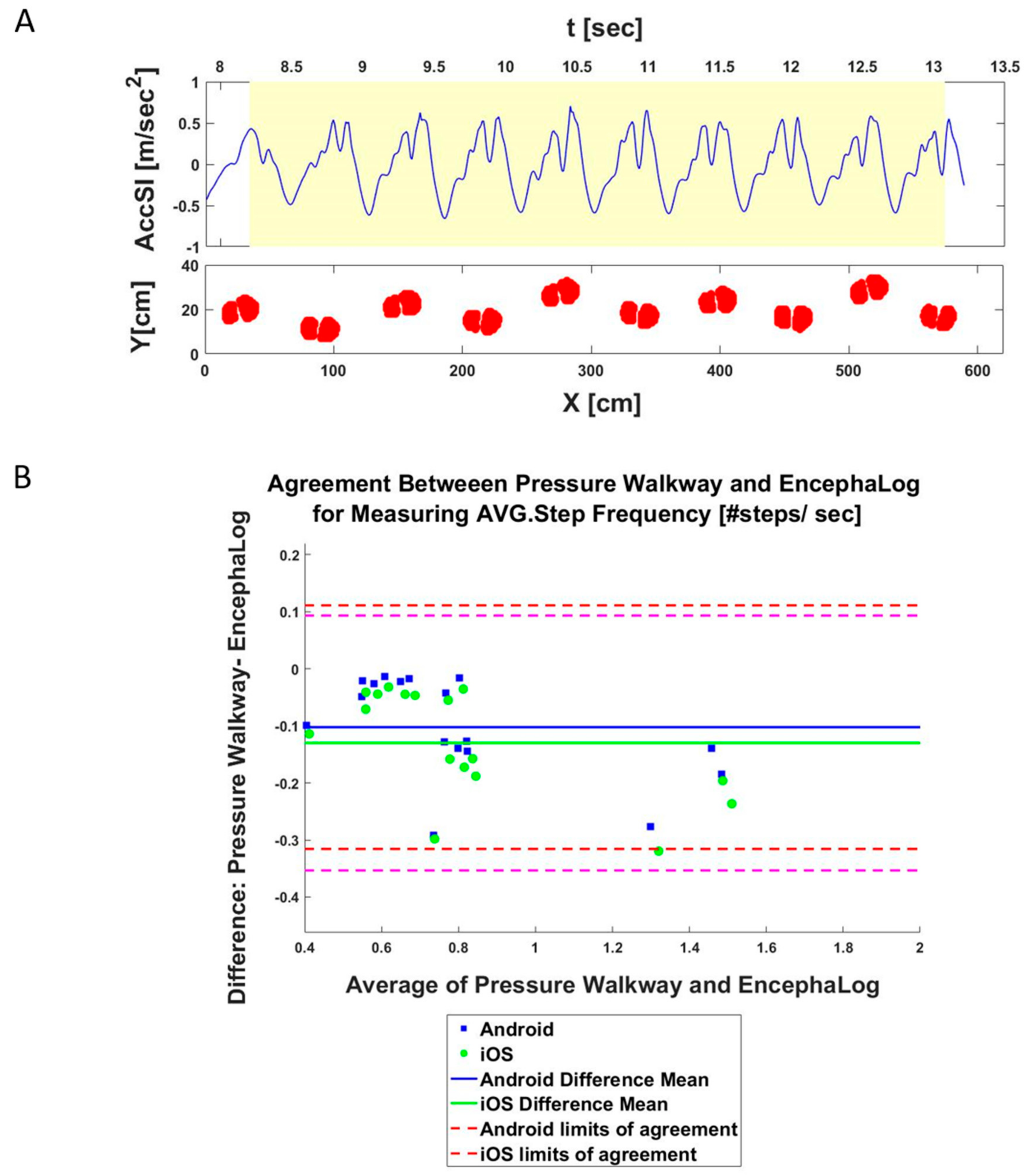
3.2. Experiment 2: EncephaLog vs. Pressure Mat
3.3. Experiment 3: EncephaLog vs. Other Wearable Sensors
4. Discussion
4.1. EncephaLog vs. Other Methods
4.2. EncephaLog Benefits for iTUG
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
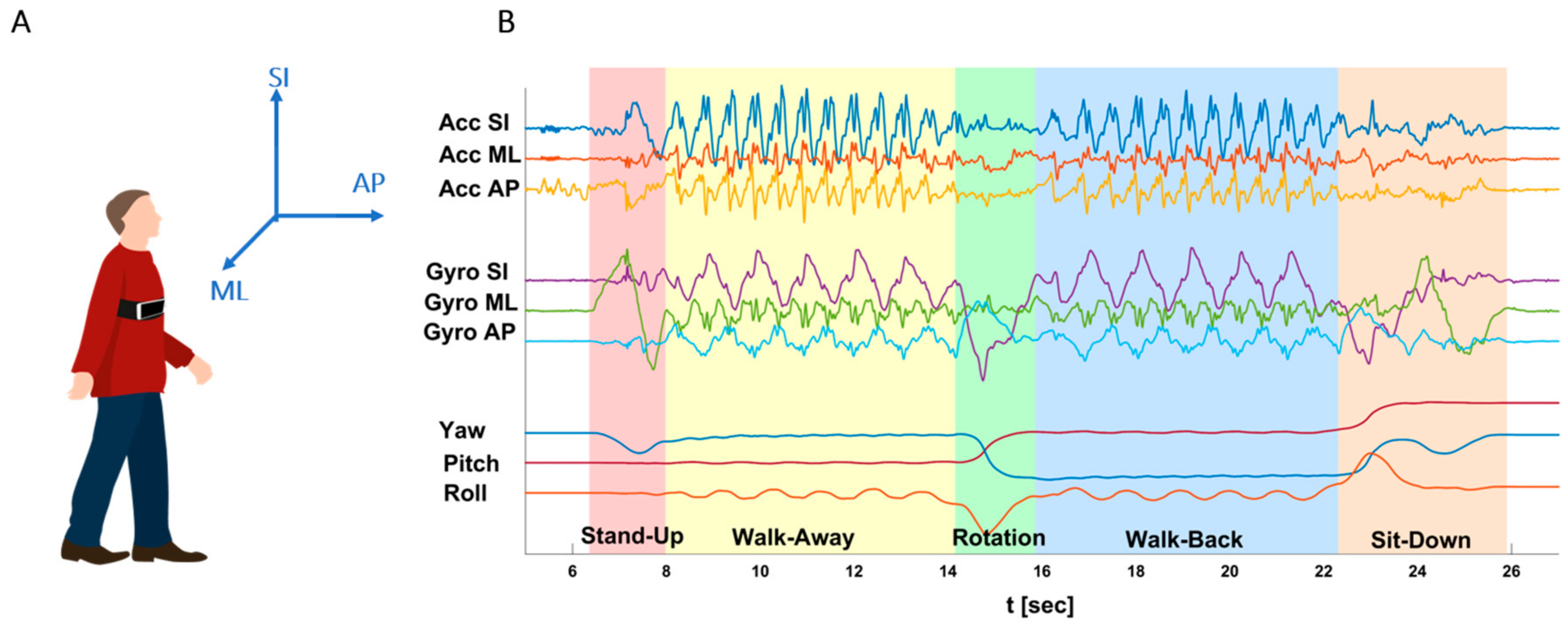
| Completion Time (s) | Completion time of entire TUG sequence. Taken from the "Go" command until the subject re-sits with his/her back leaning on the chair’s backrest, identified by flattening of all signals (mostly identified by the Gyro ML signal). |
| Stand-Up time (s) | Begins when the subject stands up until the first step forward. Mostly identified from the Gyro-ML signal, which dramatically increases during the initial bending forward of the torso during stand-up, and then decreases dramatically at the straightening of the torso and return of the signal to baseline. |
| Walk-Away time (s) | From the first step forward (end of SU phase) until the first rotation step. During this phase, all accelerometers and gyroscopes are characterized by a harmonic, cyclic pattern, while the Yaw, Pitch, and Roll are flat. |
| Rotation Time (s) | When the subject turns around, until the first walking backward step. Clearly evident by a 180 degrees Yaw shift. Gyros SI and AP also change dramatically, while the accelerometers become quite flat, lacking the rhythmic pattern of straight walking. |
| Walk-Back Time (s) | Between rotations – from the first step backward after the end of the Rotation phase, until the last second before the turning part of the Sit-Down phase. Similarly to the WA phase, characterized by harmonic accelerometers and gyroscopes pattern, while the Yaw, Pitch, and Roll are flat. |
| Sit-Down Time [s] | Incudes a turn to orient the body to the chair and sitting down on the chair. Starts with the first turning step, indicating the intention to sit down, until the subject sits "fully" on chair with his/her back leaning on the chair’s backrest. As in the Rotation phase, the Yaw changes dramatically from 180 degrees back towards 0, and the Gyros perform a dramatic change. |
| Walking Steps (#) | Average number of steps during the straight walking phases (WA and WB; excluding steps while rotating). |
| Rotation Steps (#) | Number of steps during the Rotation phase. |
| AVG. Steps Frequency (Cadence) (#/s) | Cadence, i.e. the AVG. number of steps per second, during straight walking (WA and WB). |
| AVG. Steps Length (m) | Average of step length, defined as one heel strike followed by the other heel strike (half a cycle). Taken from straight walking phases (WA and WB) only. |
Motion Capture Cameras (MCC) Data Analysis

References
- Hausdorff, J.M. Gait variability: methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Kerrigan, D.C. Identification of kinetic differences between fallers and nonfallers in the elderly. Am. J. Phys. Med. Rehabil. 1999, 78, 242. [Google Scholar]
- Mortaza, N.; Abu Osman, N.A.; Mehdikhani, N. Are the spatio-temporal parameters of gait capable of distinguishing a faller from a non-faller elderly? Eur. J. Phys. Rehabil. Med. 2014, 50, 677–691. [Google Scholar] [PubMed]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar]
- Stolze, H.; Kuhtz-Buschbeck, J.; Drucke, H.; Johnk, K.; Illert, M.; Deuschl, G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 289–297. [Google Scholar] [CrossRef]
- Singer, C.; Sanchez-Ramos, J.; Weiner, W.J. Gait abnormality in essential tremor. Mov. Disord. 1994, 9, 193–196. [Google Scholar] [CrossRef]
- Yeung, T.S.M.; Wessel, J.; Stratford, P.; Macdermid, J. The Timed Up and Go Test for Use on an Inpatient Orthopaedic Rehabilitation Ward. J. Orthop. Sports Phys. Ther. 2008, 38, 410–417. [Google Scholar] [CrossRef]
- Di Stasi, S.L.; Logerstedt, D.; Gardinier, E.S.; Snyder-Mackler, L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am. J. Sports Med. 2013, 41, 1310–1318. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Koyama, S.; Tanabe, S.; Itoh, N.; Saitoh, E.; Takeda, K.; Hirano, S.; Ohtsuka, K.; Mukaino, M.; Yanohara, R.; Sakurai, H.; et al. Intra- and inter-rater reliability and validity of the tandem gait test for the assessment of dynamic gait balance. Eur. J. Physiother. 2018, 20, 135–140. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2011, Suppl 1, 007. [Google Scholar]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Bihl, T.; Bresciani, J.-P. Automating the Timed Up and Go Test Using a Depth Camera. Sensors 2017, 18. [Google Scholar] [CrossRef]
- Sheridan, P.L.; Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J. Am. Geriatr. Soc. 2003, 51, 1633–1637. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Plotnik, M.; Brozgol, M.; Maidan, I.; Giladi, N.; Gurevich, T.; Hausdorff, J.M. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson’s disease? Med. Eng. Phys. 2010, 32, 119–125. [Google Scholar]
- Blin, O.; Ferrandez, A.M.; Serratrice, G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J. Neurol. Sci. 1990, 98, 91–97. [Google Scholar] [CrossRef]
- Najafi, B.; Aminian, K.; Loew, F.; Blanc, Y.; Robert, P.A. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans. Biomed. Eng. 2002, 49, 843–851. [Google Scholar] [CrossRef]
- Mancini, M.; Carlson-Kuhta, P.; Zampieri, C.; Nutt, J.G.; Chiari, L.; Horak, F.B. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture 2012, 36, 471–476. [Google Scholar] [CrossRef]
- Oberg, T.; Karsznia, A.; Oberg, K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J. Rehabil. Res. Dev. 1993, 30, 210–223. [Google Scholar] [PubMed]
- Whittle, M.W. Clinical gait analysis: A review. Hum. Movement Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Mummolo, C.; Mangialardi, L.; Kim, J.H. Quantifying dynamic characteristics of human walking for comprehensive gait cycle. J. Biomech. Eng. 2013, 135, 91006. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Weiss, A.; Buchman, A.S.; Bennett, D.A.; Giladi, N.; Hausdorff, J.M. Association between performance on Timed Up and Go subtasks and mild cognitive impairment: further insights into the links between cognitive and motor function. J. Am. Geriatr. Soc. 2014, 62, 673–678. [Google Scholar] [CrossRef]
- Jovanov, E.; Frith, K.H.; Madhushri, P.; Hunter, A.; Coffey, S.S.; Milenkovic, A. Chapter 44 - Gender Differences in Mobility of Elderly: Measurements and Interventions to Improve Mobility. In Principles of Gender-Specific Medicine, 3rd ed.; Legato, M.J., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 639–653. ISBN 978-0-12-803506-1. [Google Scholar]
- Dibilio, V.; Nicoletti, A.; Mostile, G.; Toscano, S.; Luca, A.; Raciti, L.; Sciacca, G.; Vasta, R.; Cicero, C.E.; Contrafatto, D.; et al. Dopaminergic and non-dopaminergic gait components assessed by instrumented timed up and go test in Parkinson’s disease. J. Neural. Transm. 2017, 124, 1539–1546. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Assessing mobility at home in people with early Parkinson’s disease using an instrumented Timed Up and Go test. Parkinsonism Relat. Disord. 2011, 17, 277–280. [Google Scholar] [CrossRef]
- Herman, T.; Weiss, A.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. Identifying axial and cognitive correlates in patients with Parkinson’s disease motor subtype using the instrumented Timed Up and Go. Exp. Brain Res. 2014, 232, 713–721. [Google Scholar] [CrossRef]
- Yahalom, G.; Yekutieli, Z.; Israeli-korn, S.D.; Elincx-Benizri, S.; Livneh, V.; Fay-Karmon, T.; Rubel, Y.; Tchelet, K.; Zauberman, J.; Hassin-Baer, S. AppTUG - A Smartphone Application of Instrumented ‘Timed Up and Go’ for Neurological Disorders. EC Neurol. 2018, 10, 689–695. [Google Scholar]
- Wall, J.C.; Bell, C.; Campbell, S.; Davis, J. The Timed Get-up-and-Go test revisited: measurement of the component tasks. J. Rehabil. Res. Dev. 2000, 37, 109–113. [Google Scholar]
- Weiss, A.; Mirelman, A.; Buchman, A.S.; Bennett, D.A.; Hausdorff, J.M. Using a Body-Fixed Sensor to Identify Subclinical Gait Difficulties in Older Adults with IADL Disability: Maximizing the Output of the Timed Up and Go. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Lee, S.W.; Verghese, J.; Holtzer, R.; Mahoney, J.R.; Oh-Park, M. Trunk Sway during Walking among Older Adults: Norms and Correlation with Gait Velocity. Gait Posture 2014, 40, 676–681. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Kim, K.J.; Gimmon, Y.; Millar, J.; Schubert, M.C. Using Inertial Sensors to Quantify Postural Sway and Gait Performance during the Tandem Walking Test. Sensors 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Human Biomechanics | Qualisys. Available online: https://www.qualisys.com/applications/human-biomechanics/ (accessed on 26 August 2019).
- Sprint, G.; Cook, D.; Weeks, D. Towards Automating Clinical Assessments: A Survey of the Timed Up and Go (TUG). IEEE Rev. Biomed. Eng. 2015, 8, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Gait Analysis Software For Medical Device Manufacturers » ProtoKinetics. Available online: https://www.protokinetics.com/medical-device-manufacturers/ (accessed on 13 November 2019).
- Zeno Walkway Gait Analysis System » ProtoKinetics. Available online: https://www.protokinetics.com/zeno-walkway/ (accessed on 13 November 2019).
- Shumway-Cook, A.; Anson, D.; Haller, S. Postural sway biofeedback: its effect on reestablishing stance stability in hemiplegic patients. Arch. Phys. Med. Rehabil. 1988, 69, 395–400. [Google Scholar] [PubMed]
- Hof, A.L. The “extrapolated center of mass” concept suggests a simple control of balance in walking. Hum. Mov. Sci. 2008, 27, 112–125. [Google Scholar] [CrossRef]
- Comprehensive Gait and Balance Analysis - APDM Wearable Technologies. Available online: https://www.apdm.com/mobility/ (accessed on 13 November 2019).
- Danion, F.R.; Varraine, E.; Bonnard, M.; Pailhous, J. Stride variability in human gait: the effect of stride frequency and stride length. Gait Posture 2003, 18, 69–77. [Google Scholar]
- Kooistra, J. Newzoo’s 2018 Global Mobile Market Report: Insights into the World’s 3 Billion Smartphone Users. Available online: https://newzoo.com/insights/articles/newzoos-2018-global-mobile-market-report-insights-into-the-worlds-3-billion-smartphone-users/ (accessed on 13 November 2019).
- Kwon, D.Y.; Gross, M. Combining body sensors and visual sensors for motion training. In Proceedings of the ACM SIGCHI International Conference on Advances in Computer Entertainment Technology - ACE ’05; ACM Press: Valencia, Spain, June 2005; pp. 94–101. [Google Scholar]
- Ansai, J.H.; Farche, A.C.S.; Rossi, P.G.; de Andrade, L.P.; Nakagawa, T.H.; Takahashi, A.C.M. Performance of Different Timed Up and Go Subtasks in Frailty Syndrome. J. Geriatr. Phys. Ther. 2019, 42, 287–293. [Google Scholar] [CrossRef]
- Gouwanda, D.; Senanayake, S.M.N.A. Emerging Trends of Body-Mounted Sensors in Sports and Human Gait Analysis. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering, Kuala Lumpur, Malaysia, June 2008; Abu Osman, N.A., Ibrahim, F., Wan Abas, W.A.B., Abdul Rahman, H.S., Ting, H.-N., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 715–718. [Google Scholar]
- Sankarpandi, S.K.; Baldwin, A.J.; Ray, J.; Mazzà, C. Reliability of inertial sensors in the assessment of patients with vestibular disorders: a feasibility study. BMC Ear Nose Throat Disord. 2017, 17. [Google Scholar] [CrossRef]
- Smulders, K.; Dale, M.L.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat. Disord. 2016, 31, 3–13. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Kim, H.; Yoshida, H.; Suzukawa, M.; Makizako, H.; Yoshida, Y.; Saito, K.; Suzuki, T. Relationship between Age-Associated Changes of Gait and Falls and Life-Space in Elderly People. J. Phys. Ther. Sci. 2010, 22, 419–424. [Google Scholar] [CrossRef]
- O’Brien, K.; Culham, E.; Pickles, B. Balance and Skeletal Alignment in a Group of Elderly Female Fallers and Nonfallers. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52A, B221–B226. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.H. The evolution of clinical gait analysis part l: kinesiological EMG. Gait Posture 2001, 14, 61–70. [Google Scholar] [CrossRef]

| Motion Capture Cameras n = 17 | Pressure Mat n = 15 | Wearable Sensors n = 15 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Agreement Limits | p-Value | Agreement Limits | p-Value | Agreement Limits | p-Value | ||||
| Completion Time (s) | −1.044 | [−6.327,4.238] | 0.833 | - | - | - | −0.858 | [−4.938,3.222] | 0.409 |
| −0.026 | [−5.184,5.132] | 0.996 | - | - | - | 0.039 | [−3.945,4.024] | 0.496 | |
| Stand-Up Time (s) | −0.024 | [−0.903,0854] | 0.855 | - | - | - | −0.051 | [−0.350,0.249] | 0.299 |
| 0.031 | [−0.848,0.910 | 0.812 | - | - | - | −0.003 | [−0.305,0.298] | 0.485 | |
| Walk-Away Time (s) | −0.350 | [−3.568,2.868] | 0.874 | - | - | - | −0.307 | [−2.543,1.928] | 0.424 |
| 0.018 | [−3.144,3.179] | 0.994 | - | - | - | 0.003 | [−2.193,2.198] | 0.424 | |
| Rotation Time (s) | 0.319 | [−0.908,1.545] | 0.481 | - | - | - | −0.082 | [−0.533,0.369] | 0.308 |
| 0.401 | [−0.801,1.603] | 0.369 | - | - | - | 0.005 | [−0.481,0.490] | 0.488 | |
| Walk-Back Time (s) | −0.970 | [−3.706,1.766] | 0.656 | - | - | - | −0.286 | [−2.147,1.574] | 0.429 |
| −0.532 | [−3.146,2.083] | 0.804 | - | - | - | 0.026 | [−1.730,1.781] | 0.499 | |
| Sit-Down Time (s) | −0.014 | [−1.414,1.387] | 0.962 | - | - | - | −0.038 | [−0.899,0.823] | 0.422 |
| 0.061 | [−1.279,1.402] | 0.828 | - | - | - | 0.082 | [−0.678,0.842] | 0.334 | |
| Rotation Steps (#) | 1.912 | [−1.200,5.024] | 0.243 | - | - | - | 0.031 | [−0.911,0.974] | 0.407 |
| 1.912 | [−1.200,5.024] | 0.243 | - | - | - | 0.000 | [−0.943,0.943] | 0.500 | |
| Walking Steps (#) | −2.454 | [−9.771,4.864] | 0.633 | −1.103 | [−6.301,4.096] | 0.637 | −0.092 | [−5.646,5.462] | 0.484 |
| −2.485 | [−9.723,4.753] | 0.628 | −1.035 | [−6.255,4.185] | 0.665 | −0.025 | [−5.431,5.381] | 0.496 | |
| AVG. Steps Frequency (Cadence) (#/s) | −0.102 | [−0.316,0.111] | 0.352 | 0.030 | [−0.026,0.086] | 0.691 | 0.026 | [−0.028,0.079] | 0.367 |
| −0.130 | [−0.354,0.093] | 0.381 | −0.001 | [−0.026,0.086] | 0.958 | −0.003 | [−0.062,0.055] | 0.483 | |
| AVG. Steps Length (m) | −0.015 | [−0.087,0.058] | 0.849 | 0.036 | [−0.049,0.121] | 0.556 | 0.002 | [−0.073,0.077] | 0.488 |
| −0.015 | [−0.086,0.057] | 0.849 | 0.033 | [−0.052,0.117] | 0.596 | −0.002 | [−0.066,0.063] | 0.488 | |
| Motion Capture Cameras n = 17 | Pressure Mat n = 15 | Wearable Sensors n = 15 | |
|---|---|---|---|
| Completion Time (s) | 11.192 | - | 4.959 |
| 7.930 | - | 2.737 | |
| Stand-Up Time (s) | 21.324 | - | 7.957 |
| 20.956 | - | 5.263 | |
| Walk-Away Time (s) | 21.313 | - | 6.381 |
| 16.794 | - | 1.725 | |
| Rotation Time (s) | 34.112 | - | 13.302 |
| 37.617 | - | 6.400 | |
| Walk-Back Time (s) | 32.904 | - | 3.896 |
| 28.860 | - | 3.506 | |
| Sit-Down Time (s) | 22.203 | - | 10.961 |
| 15.734 | - | 8.511 | |
| Rotation Steps (#) | 50.000 | - | 20.000 |
| 50.000 | - | 0.000 | |
| Walking Steps (#) | 40.450 | 22.124 | 18.771 |
| 41.466 | 16.628 | 2.258 | |
| AVG. Steps Frequency (Cadence) (#/s) | 49.564 | 7.204 | 4.996 |
| 50.646 | 3.874 | 2.500 | |
| AVG. Steps Length (m) | 19.586 | 13.308 | 6.627 |
| 17.347 | 14.159 | 2.256 |
| Acc SI(X) | Acc ML(Y) | Acc AP(Z) | Gyro SI(X) | Gyro ML(Y) | Gyro AP(Z) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | NRMSE | R | NRMSE | R | NRMSE | R | NRMSE | R | NRMSE | R | NRMSE | |
| AVG | 0.907 | 0.062 | 0.881 | 0.13 | 0.568 | 0.271 | 0.861 | 0.071 | 0.787 | 0.068 | 0.875 | 0.069 |
| STD | 0.14 | 0.049 | 0.121 | 0.082 | 0.097 | 0.058 | 0.225 | 0.07 | 0.243 | 0.046 | 0.208 | 0.069 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchelet, K.; Stark-Inbar, A.; Yekutieli, Z. Pilot Study of the EncephaLog Smartphone Application for Gait Analysis. Sensors 2019, 19, 5179. https://doi.org/10.3390/s19235179
Tchelet K, Stark-Inbar A, Yekutieli Z. Pilot Study of the EncephaLog Smartphone Application for Gait Analysis. Sensors. 2019; 19(23):5179. https://doi.org/10.3390/s19235179
Chicago/Turabian StyleTchelet, Keren, Alit Stark-Inbar, and Ziv Yekutieli. 2019. "Pilot Study of the EncephaLog Smartphone Application for Gait Analysis" Sensors 19, no. 23: 5179. https://doi.org/10.3390/s19235179
APA StyleTchelet, K., Stark-Inbar, A., & Yekutieli, Z. (2019). Pilot Study of the EncephaLog Smartphone Application for Gait Analysis. Sensors, 19(23), 5179. https://doi.org/10.3390/s19235179






