An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
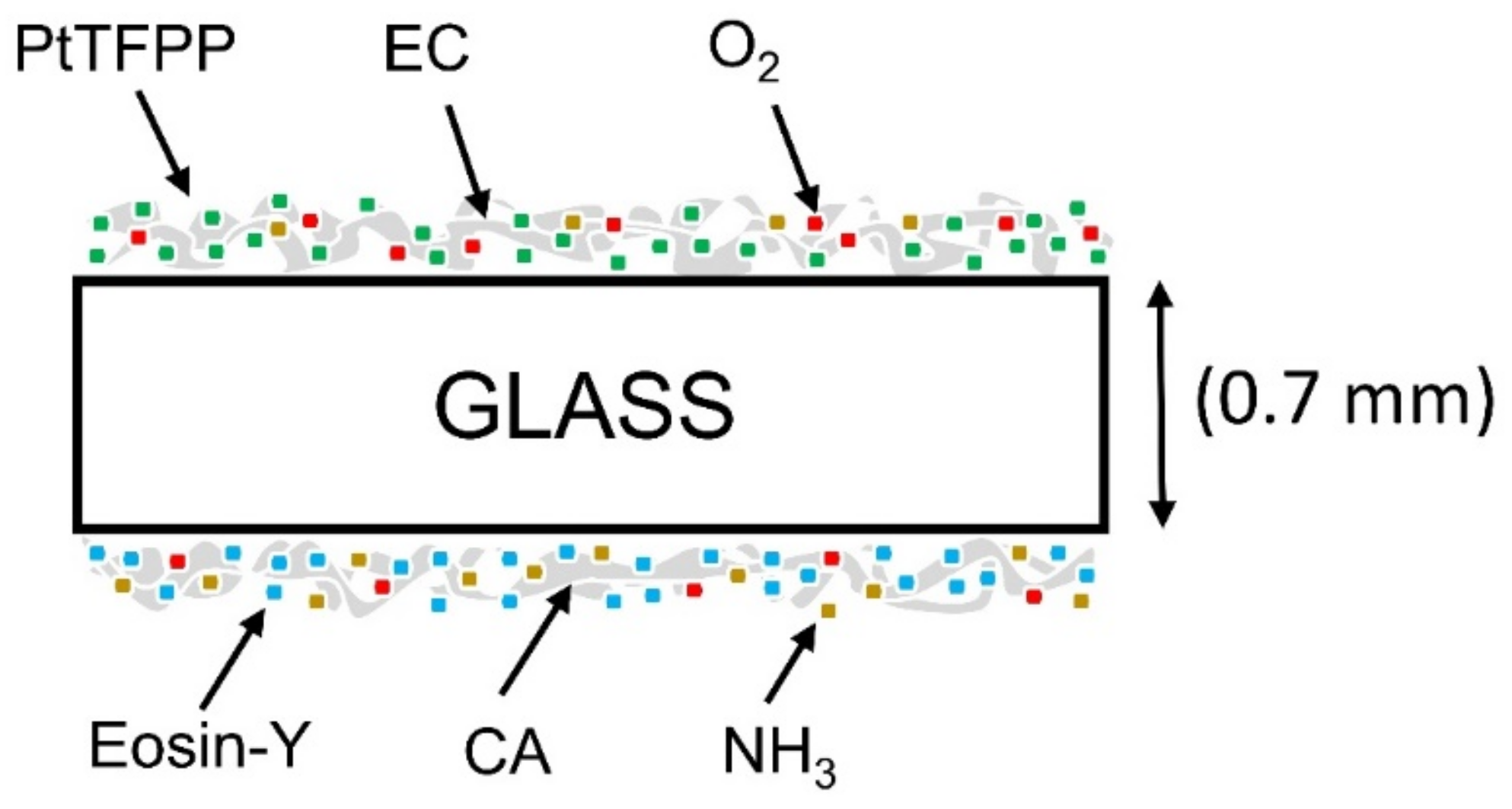
2.2.1. Preparation of Oxygen and Ammonia Sensing Material
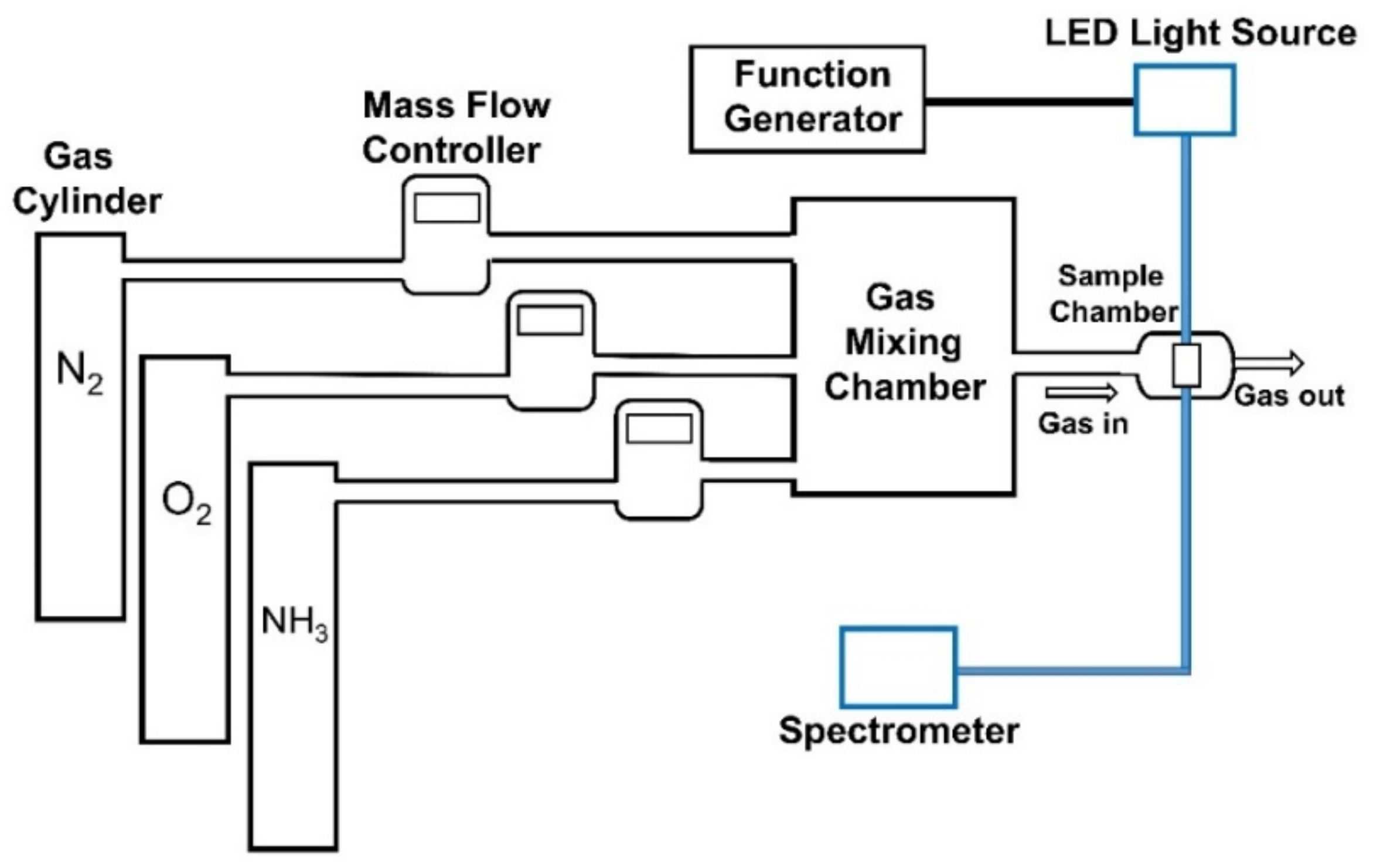
2.2.2. Instrumentation
3. Basic Theory
4. Results and Discussion
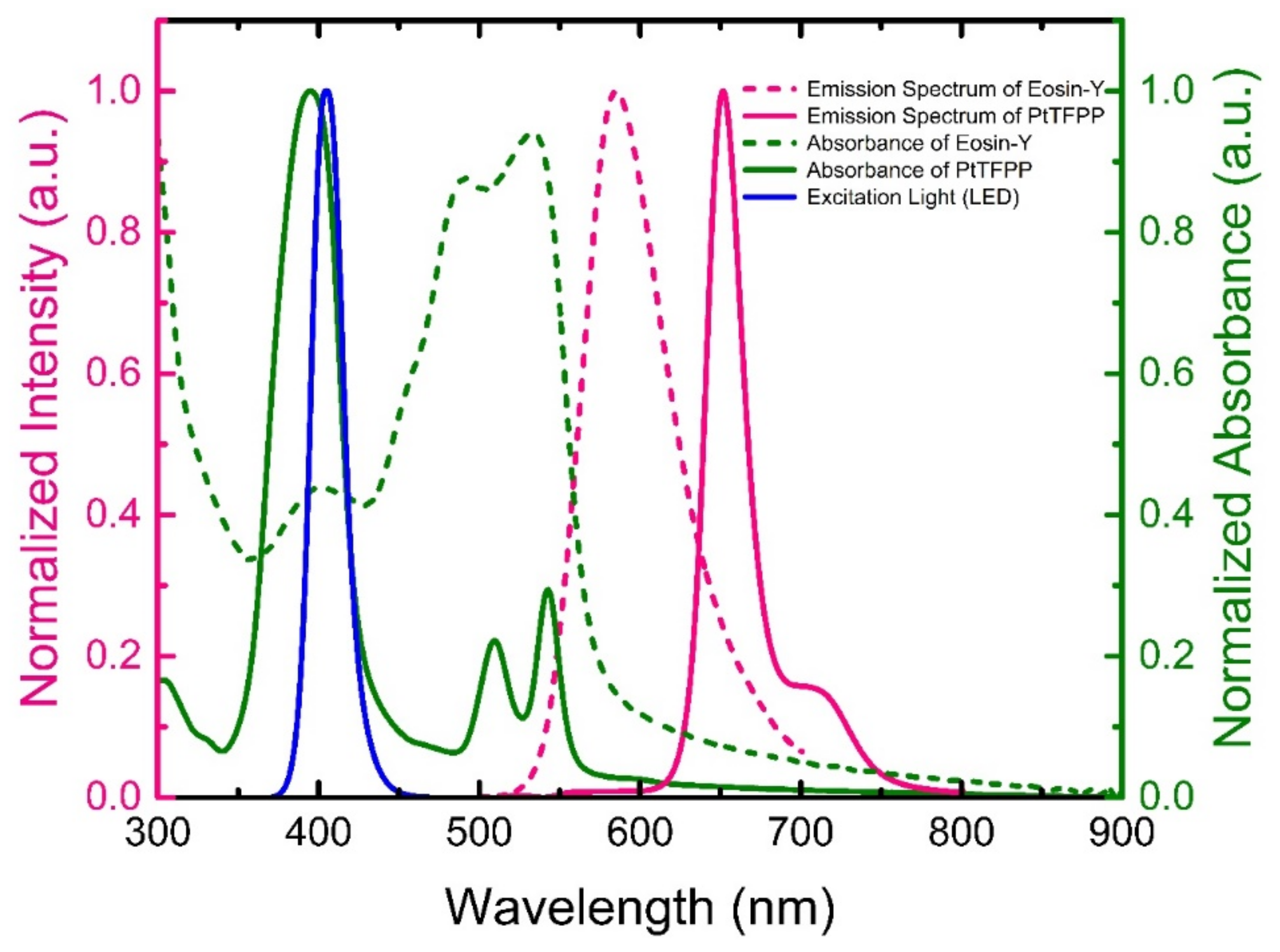
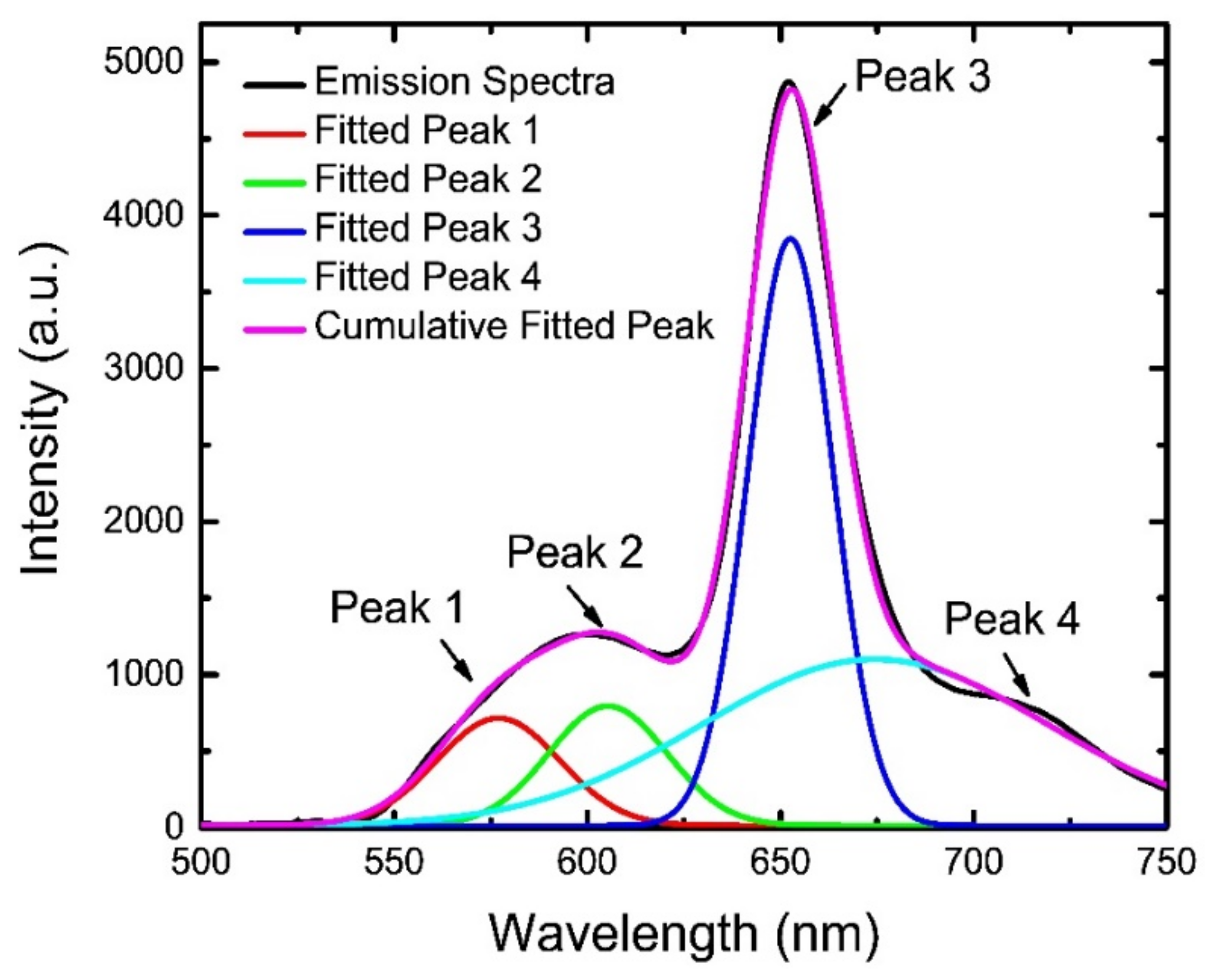
4.1. Optical Characterizations of the Sensing Materials
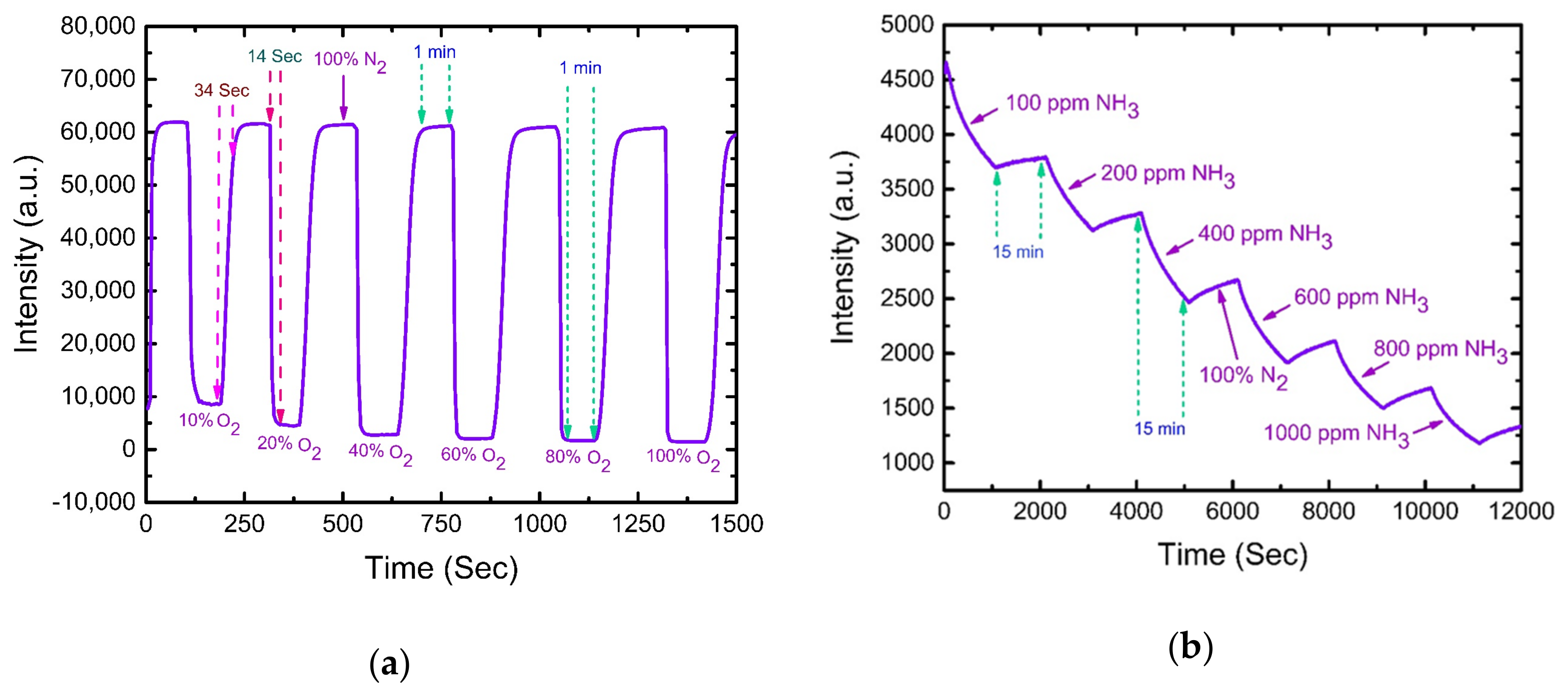
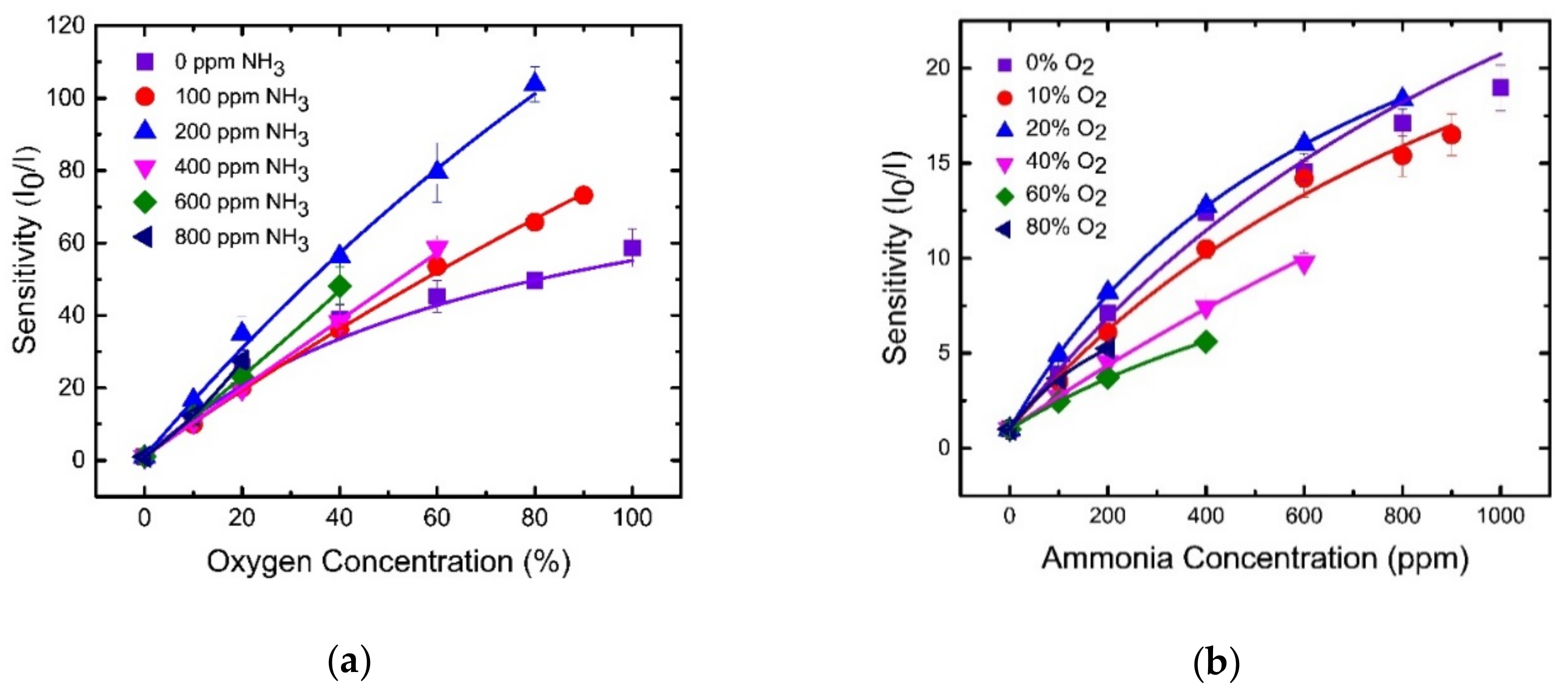
4.2. O2 Sensing Properties of Optical Dual Sensor
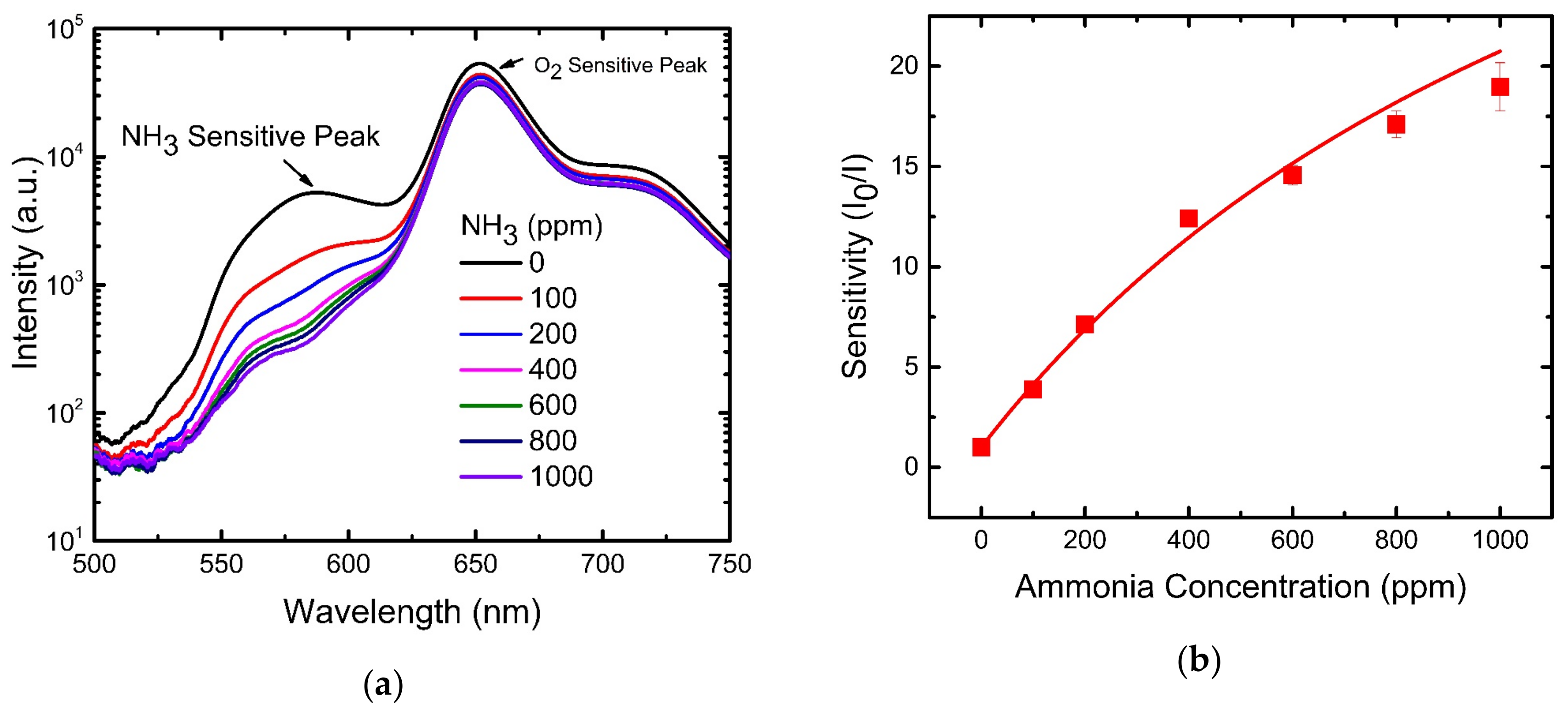
4.3. NH3 Sensing Properties of Optical Dual Sensor
4.4. Photostability of Optical Dual Sensor
4.5. Response Time of Optical Dual Sensor
4.6. Selectivity of Optical Dual Sensor
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Amao, Y.; Miyashita, T.; Okura, I. Platinum tetrakis (pentafluorophenyl) porphyrin immobilized in polytrifluoroethylmethacrylate film as a photostable optical oxygen detection material. J. Fluor. Chem. 2001, 107, 101–106. [Google Scholar] [CrossRef]
- Chu, C.S.; Syu, J.J. Optical sensor for dual sensing of oxygen and carbon dioxide based on sensing films coated on filter paper. Appl. Opt. 2017, 56, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.S.; Chu, C.S.; Lo, Y.L. Highly sensitive optical fiber oxygen sensor using Pt(II) complex embedded in sol-gel matrices. Sens. Actuators B Chem. 2006, 119, 701–707. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. High-performance fiber-optic oxygen sensors based on fluorinated xerogels doped with Pt(II) complexes. Sens. Actuators B Chem. 2007, 124, 376–382. [Google Scholar] [CrossRef]
- Basu, B.J. Optical oxygen sensing based on luminescence quenching of platinum porphyrin dyes doped in ormosil coatings. Sens. Actuators B Chem. 2007, 123, 568–577. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L.; Sung, T.W. Enhanced oxygen sensing properties of Pt(II) complex and dye entrapped core-shell silica nanoparticles embedded in sol-gel matrix. Talanta 2010, 82, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Lo, Y.L. Highly sensitive and linear calibration optical fiber oxygen sensor based on Pt(II) complex embedded in sol-gel matrix. Sens. Actuators B Chem. 2011, 155, 53–57. [Google Scholar] [CrossRef]
- Elosua, C.; de Acha, N.; Hernaez, M.; Matias, I.R.; Arregui, F.J. Layer-by-Layer assembly of a water-insoluble platinum complex for optical fiber oxygen sensors. Sens. Actuators B Chem. 2015, 207, 683–689. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–667. [Google Scholar] [CrossRef]
- Damink, S.W.M.O.; Deutz, N.E.P.; Dejong, C.H.C.; Soeter, P.B.; Jalan, R. Interorgan ammonia metabolism in liver failure. Neurochem. Int. 2002, 41, 177–188. [Google Scholar] [CrossRef]
- Cohen, B.I. The significance of ammonia/gamma-aminobutyric acid (GABA) ratio for normality and liver disorders. Med. Hyphothess 2002, 59, 757–758. [Google Scholar] [CrossRef]
- Ryer-Powder, J.E. Health effects of ammonia. Plant/Oper. Prog. 1991, 10, 228–232. [Google Scholar] [CrossRef]
- Takagai, Y.; Nojiri, Y.; Takase, T.; Hinze, W.L.; Butsugan, M.; Igarashi, S. “Turn-on” fluorescent polymeric microparticle sensors for the determination of ammonia and amines in the vapor state. Analyst 2010, 135, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.A.; Baron, M.G.; Narayanaswamy, R. Optical ammonia sensing films based on an immobilized metalloporphyrin. Anal. Commun. 1996, 33, 393–396. [Google Scholar] [CrossRef]
- Sung, T.W.; Lo, Y.L. Ammonia vapor sensor based on CdSe/SiO2 core-shell nanoparticles embedded in sol-gel matrix. Sens. Actuators B Chem. 2013, 188, 702–708. [Google Scholar] [CrossRef]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sanchez-Valencia, J.R.; Barranco, A.; Gonzalez-Elip, A.R.; Pedrosa, J.M. Optical gas sensing of ammonia and amines based on protonated porphyrin/TiO2 composite thin film. Sensors 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.R.; Yang, X.H.; Yuan, L.B.; Wang, L.L.; Zhao, E.M.; Tian, F.J.; Liu, Y.X. Gaseous ammonia fluorescence probe based on cellulose acetate modified microstructured optical fiber. Opt. Commun. 2011, 284, 4810–4814. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Carraway, E.R.; Demas, J.N.; DeGraff, B.A. Luminescence quenching mechanism for microheterogeneous systems. Anal. Chem. 1991, 63, 332–336. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biring, S.; Sadhu, A.S.; Deb, M. An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia. Sensors 2019, 19, 5124. https://doi.org/10.3390/s19235124
Biring S, Sadhu AS, Deb M. An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia. Sensors. 2019; 19(23):5124. https://doi.org/10.3390/s19235124
Chicago/Turabian StyleBiring, Sajal, Annada Sankar Sadhu, and Moumita Deb. 2019. "An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia" Sensors 19, no. 23: 5124. https://doi.org/10.3390/s19235124
APA StyleBiring, S., Sadhu, A. S., & Deb, M. (2019). An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia. Sensors, 19(23), 5124. https://doi.org/10.3390/s19235124




