Sensors for Positron Emission Tomography Applications
Abstract
1. Introduction
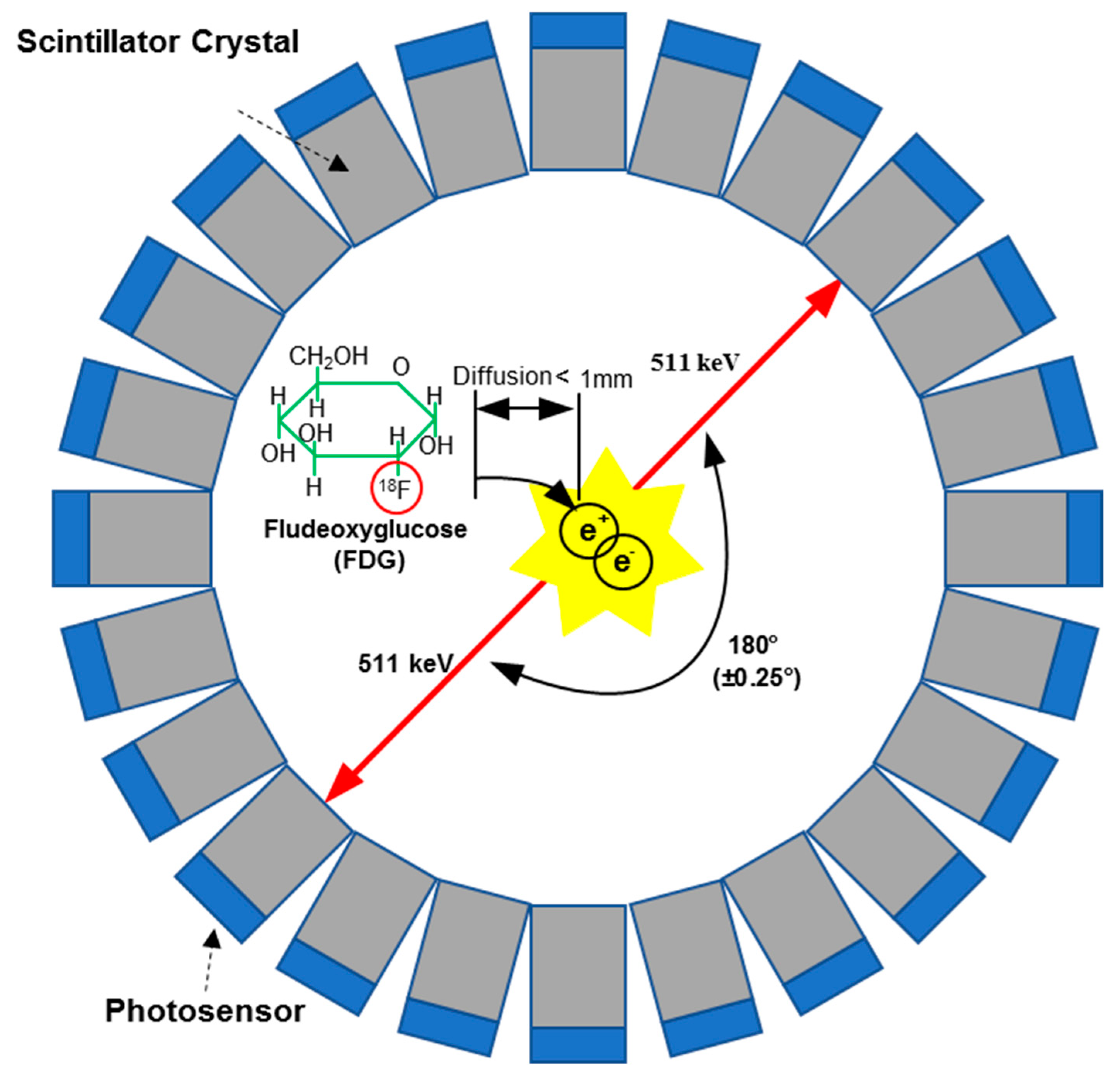
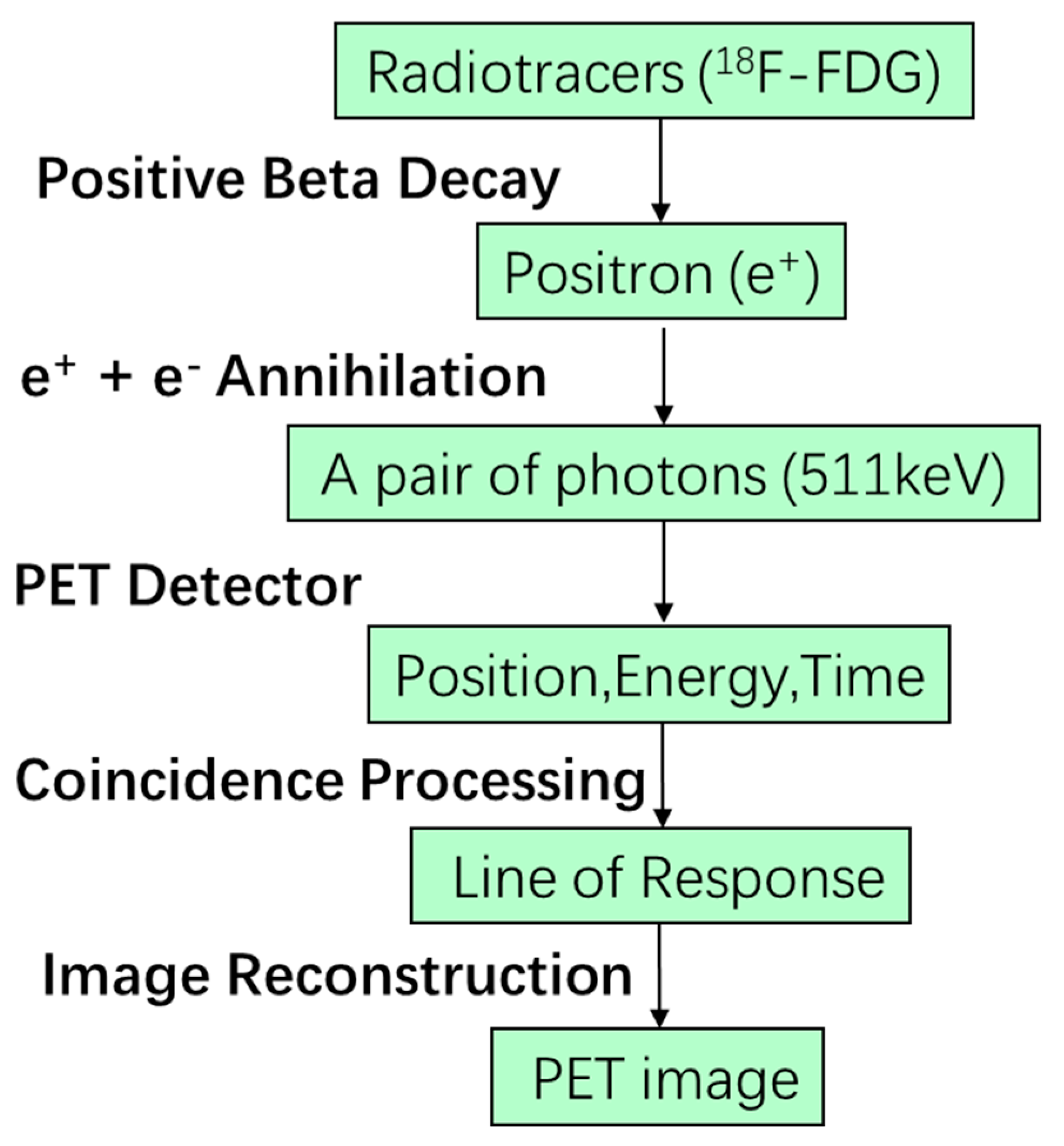
2. Positron Emission Tomography
2.1. Physics of Positron Emission Tomography
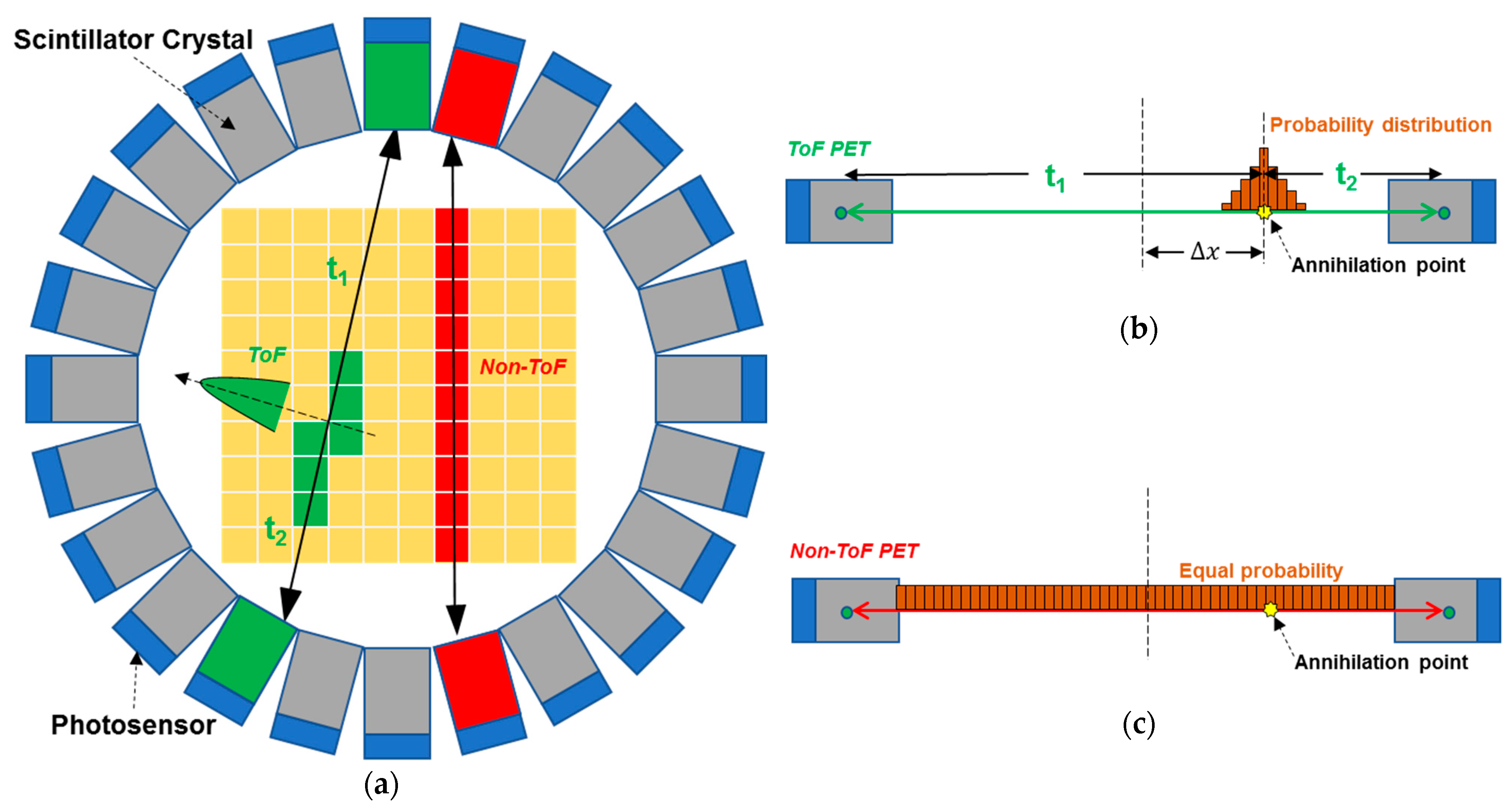
2.2. Time-of-Flight (ToF) PET
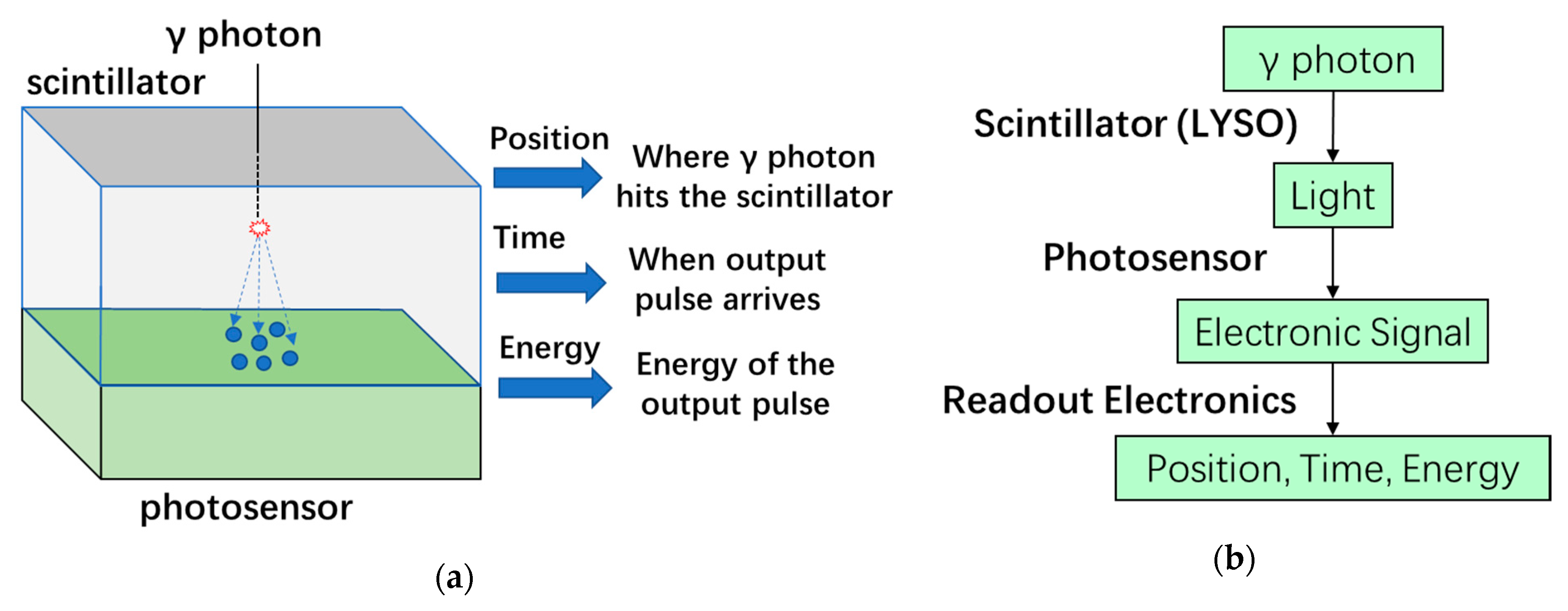
2.3. PET Detector
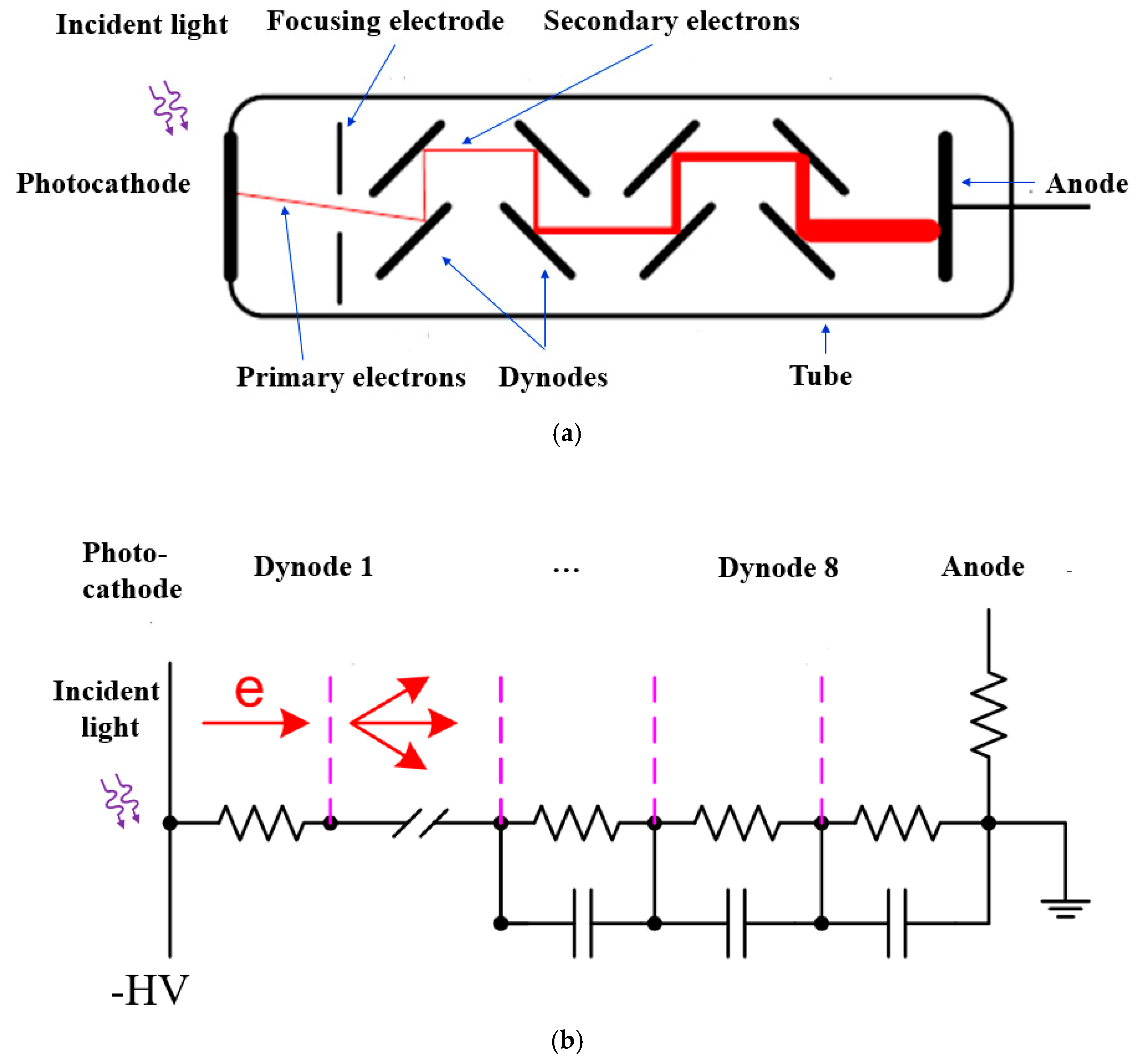
3. Photomultiplier Tubes (PMTs)
3.1. Operating Principles of PMTs
3.2. Key Performance Parameters
3.3. PET Applications
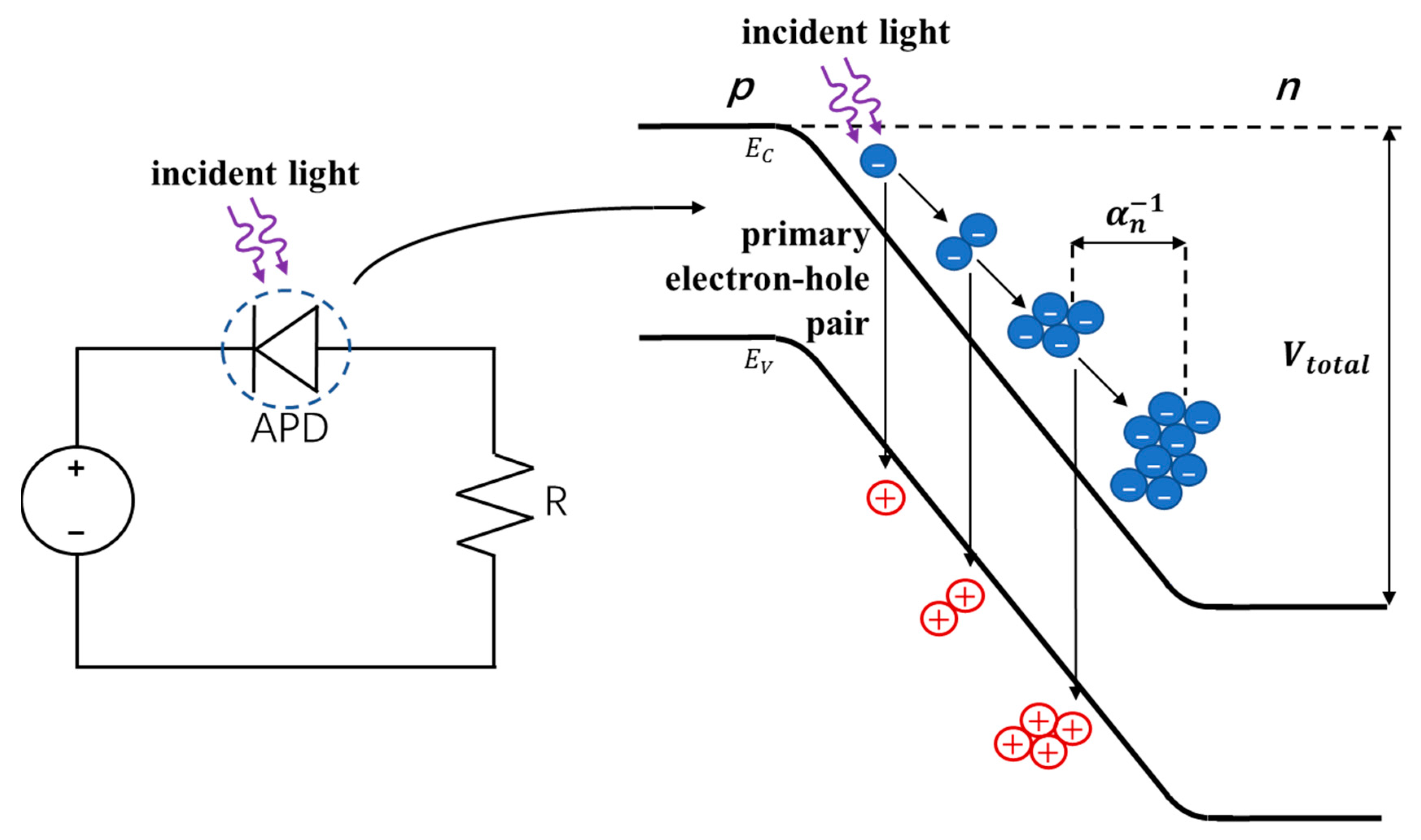
4. Avalanche Photodiodes (APDs)
4.1. Operating Principles of APDs
4.2. Key Performance Parameters of APDs
4.3. PET Applications
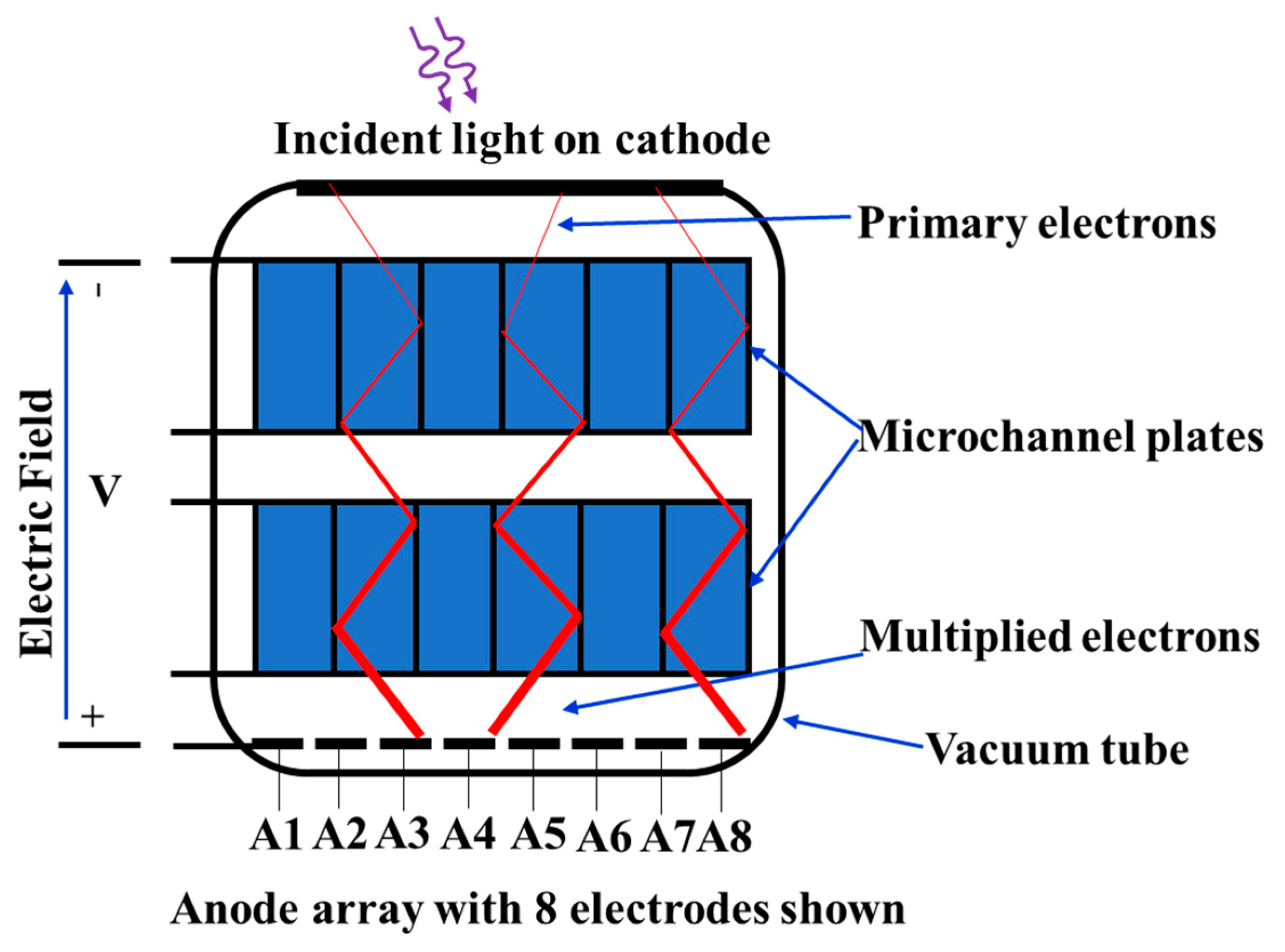
5. Silicon Photomultipliers (SiPMs)
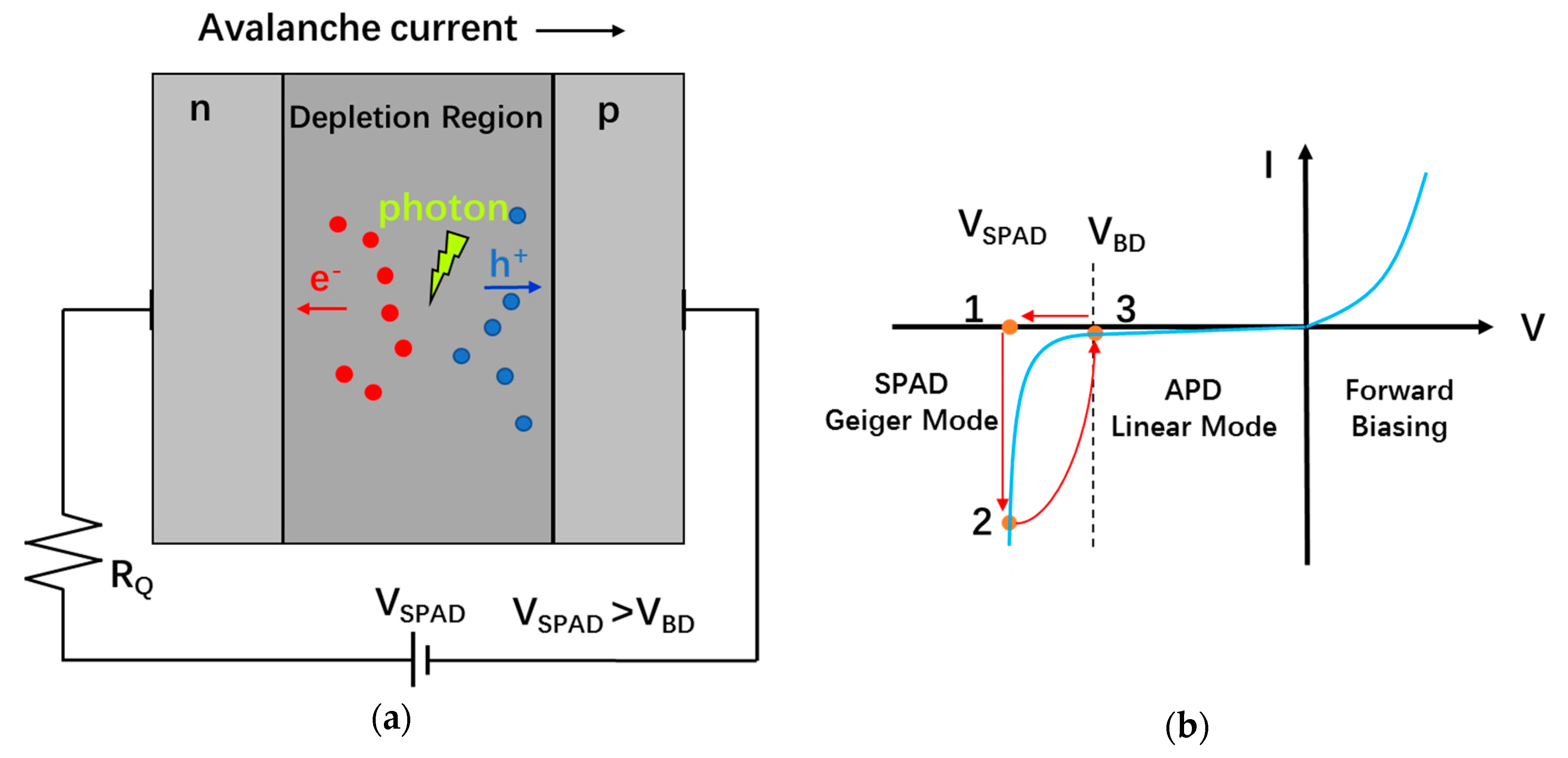
5.1. Operating Principles of SPADs
5.2. Key Performance Parameters of SPADs
5.3. SPAD Design Considerations
5.3.1. p-n Junction
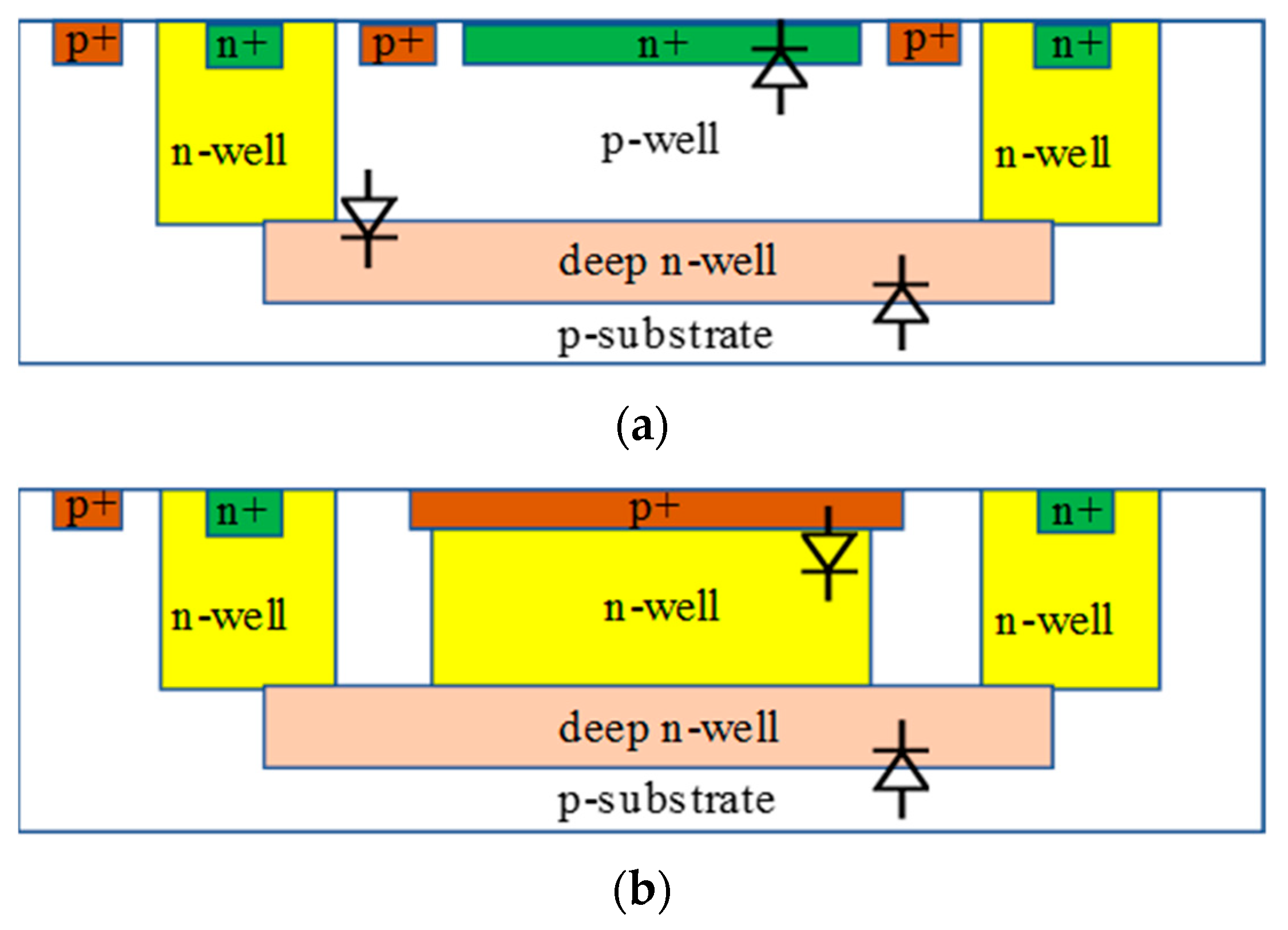
5.3.2. Guard Ring (GR)
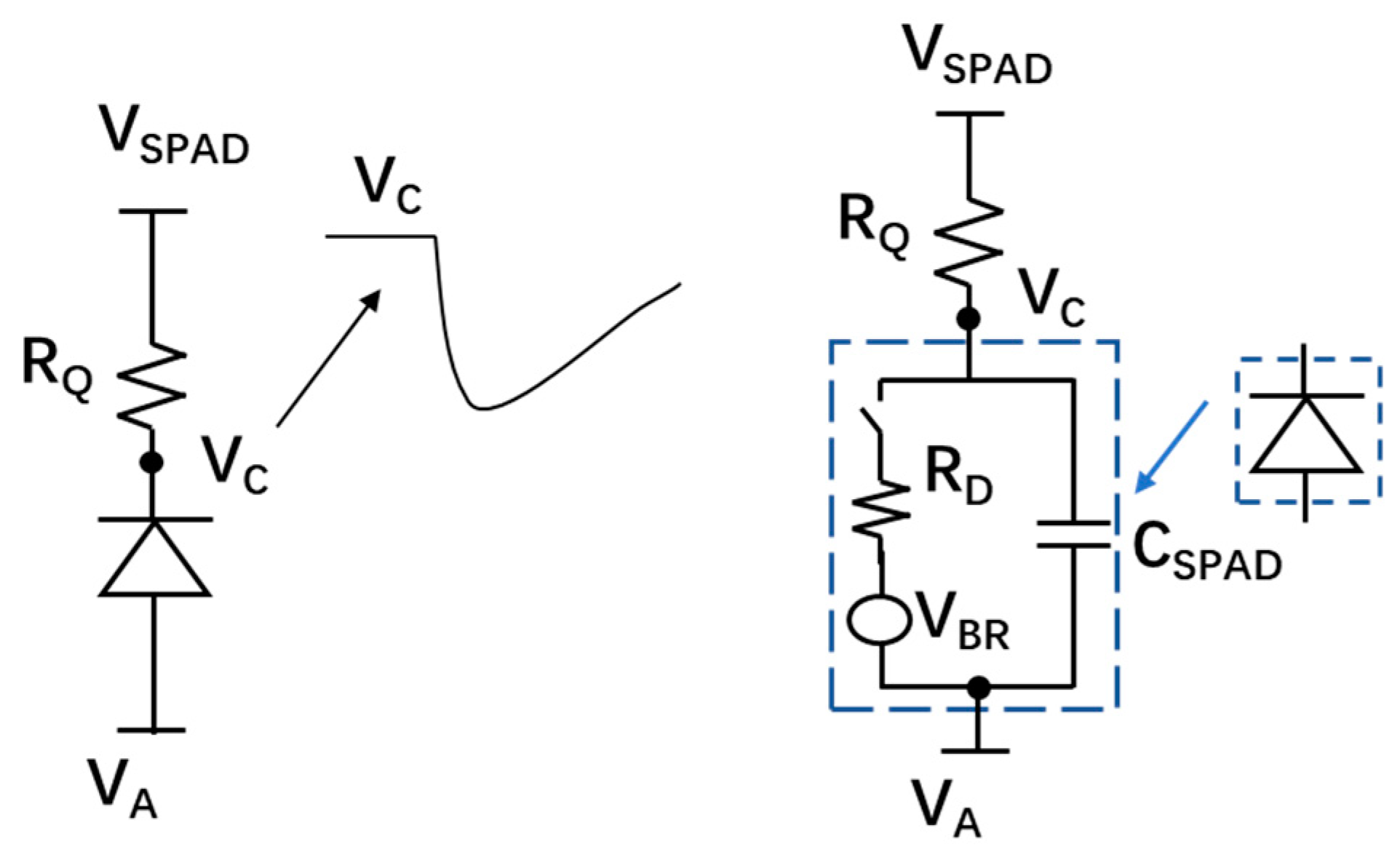
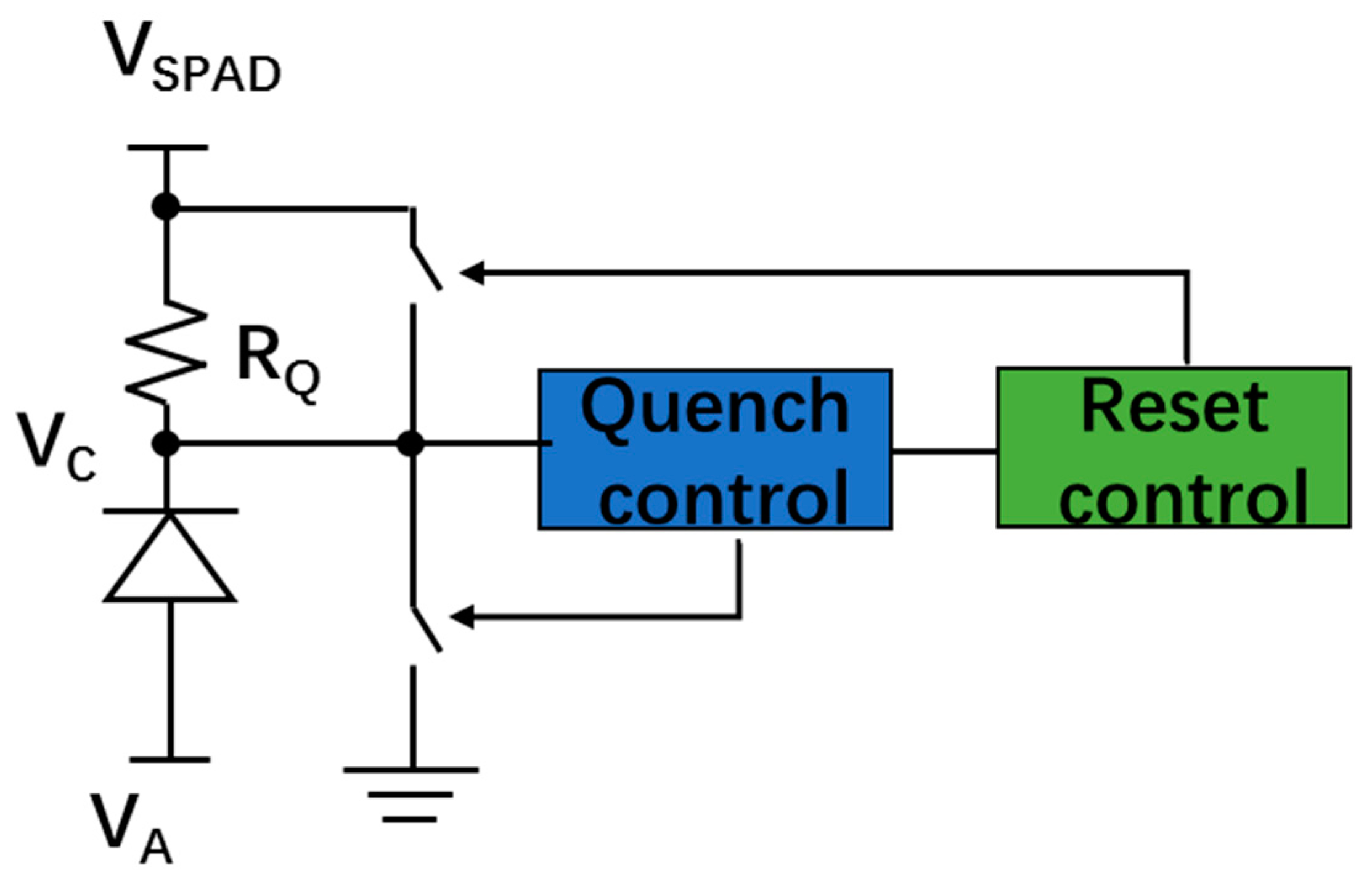
5.3.3. Quench and Reset Circuit
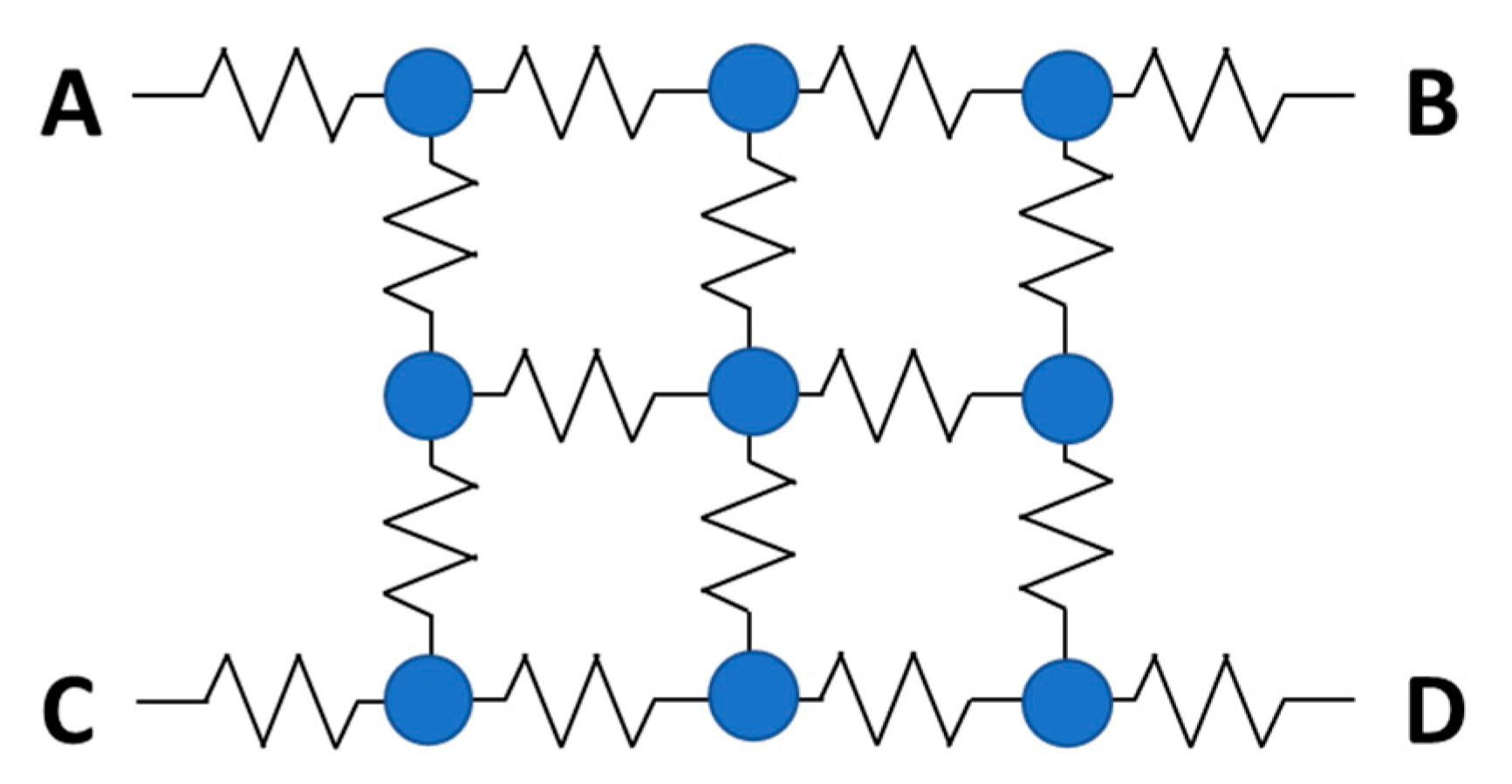
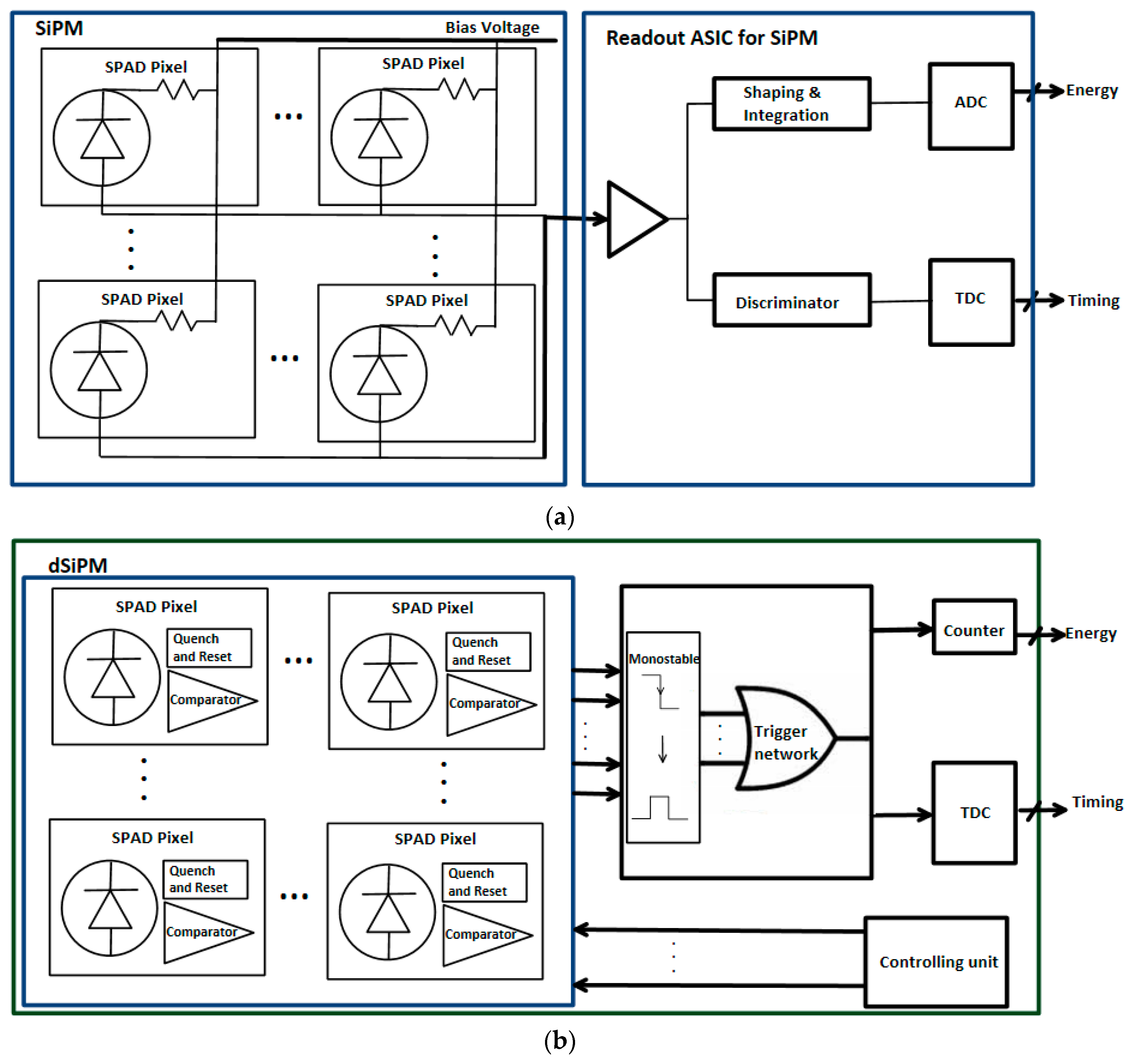
5.4. Analog and Digital SiPMs
| Reference, Year | Technology (nm) | Junction | VBR (V) | Active Area (µm) | PDE (%) | Median DCR (Hz) | DT (ns) | After Pulse (%) | Time Resolution (ps) |
|---|---|---|---|---|---|---|---|---|---|
| [64], 2017 | CIS 65 (BSI) a | – | – | - (Square) | 21.9 @ 660 nm Vex = 4.4 V | – | 8 | 0.08 @ Vex = 4.4 V DT = 8 ns | 95 @ Vex = 4.4 V (700 nm) |
| [105], 2012 | CIS 90 | p-well/DNW | 14.9 | 6.4 (Circular) | 44 @ 690 nm Vex = 3.5 V | ~100 @ Low Excess Voltage | 8 | 0.375 @ Vex = 0.36 V DT = 15 ns | 82 @ Vex = 0.36 V (470 nm) 53 @ Vex = 1.36 V (470 nm) 51 @ Vex = 2.36 V (470 nm) |
| [60], 2009 | CIS 130 | p-well/DNW | 14.4 | 8 (Circular) | 28 @ 500 nm Vex = 1.4 V | 25 @ Vex = 1.4 V 20 °C | – | – | – |
| [106], 2011 | CMOS 150 | p-well/Niso | 23.1 | 10 (Circular) | 31 @ 470 nm Vex = 5 V | ~230 @ Vex = 5 V 25 °C | 30 | 2.1 @ Vex = 5 V DT = 30 ns | 170 @ Vex = 5 V (470 nm) |
| [106], 2011 | CMOS 150 | p+/n-well | 16.1 | 10 (Circular) | 26 @ 470 nm Vex = 3.5 V | ~160 @ Vex = 3.5 V 25 °C | 30 | 1.3 @ Vex = 3.5 V DT = 30 ns | 60 @ Vex = 3.5 V (470 nm) |
| [65], 2017 | CMOS 150 | p+/n-well | 18.01 | 10 (Square) | 31 @ 450 nm Vex = 5 V | 39 @ Vex = 3 V | 0.85 @ Vex = 3 V DT = 150 ns | 52 @ Vex = 4 V (468 nm) 42 @ Vex = 4 V (831 nm) | |
| [61], 2018 | CMOS HV 180 | p+/shallow n-well | 16.8 | 12.08 (Square) | 55 @ 480 nm Vex = 4 V b | 28 @ Vex = 1 V 217 @ Vex = 4 V | – | – | 260 @ Vex = 4 V (640 nm) |
| [107], 2010 | CMOS HV 180 | p+/DNW | 20.3 | 8 (Octagonal) | 20 @ 470 nm Vex = 3.5 V | 180 @ Vex = 3.5 V | 6 | 0 @ DT = 6 ns | 80 @ Vex = 3.5 V (470 nm) |
| [108], 2014 | CMOS 180 | p-well/ DNW | 23.5 | 12 | >40 @ 440–620 nm Vex = 10 V b | 17 @ Vex = 2 V 1.45k @ Vex = 10 V | 300 | 0.3 @ Vex = 10 V DT = 300 ns | 70 @ Vex = 10 V (405 nm) 86 @ Vex = 10 V (637 nm) |
| [109], 2015 | CMOS 180 | p+/n-well/DNW | 14.64 | 12 (Circular) | >40 @ 440–580 nm Vex = 4 V b | 31 @ Vex = 1 V 1.8k @ Vex = 4 V 25 °C | 300 | 0.2 @ Vex = 4 V DT = 300 ns | 95 @ Vex = 4 V (405 nm) 141 @ Vex = 4 V (637 nm) |
| [110], 2016 | CMOS 180 | p-well- epi-BN (Shallow PW) | 36.5 | – | 27.8 @ 490 nm Vex = 4 V b | 2k @ Vex = 4 V 25 °C c | – | 0.34 @ Vex = 4 V DT = 300 ns | 427 @ Vex = 2 V (637 nm) 223 @ Vex = 4 V (637 nm) 243 @ Vex = 2 V (405 nm) 141 @ Vex = 4 V (405 nm) |
| [110], 2016 | CMOS 180 | p-well- epi-BN | 25.46 | – | >40 @ 460–600 nm Vex = 11 V b | 40 @ Vex = 4 V 25 °C c | – | 7.2 @ Vex = 11 V DT = 300 ns | 139.5 @ Vex = 3 V (637 nm) 100.8 @ Vex = 11 V (637 nm) 133 @ Vex = 3 V (405 nm) 97.2 @ Vex = 11 V (405 nm) |
| [111], 2013 | CMOS 350 | – | 25 | 20 | 28 @ 570 nm Vex = 6 V d | 25 @ Vex = 6 V Room Temp | 20 | 1.3 @ Vex = 6 V DT = 20 ns | – |
| [112], 2009 | CMOS 350 | p+/DNW | 27.5–28.4 | – | – | 4k @ Vex = 4 V Room Temp e | – | 4.5 @ Vex = 5 V DT = 500 ns | – |
5.5. PET Applications
5.5.1. SiPMs
5.5.2. dSiPMs
- Trigger scheme 1 = ST1 ∨ ST2 ∨ ST3 ∨ ST4,
- Trigger scheme 2 = [(ST1 ∨ ST2) ∧ (ST3 ∨ ST4)] ∨ [(ST1 ∨ ST4) ∧ (ST2 ∨ ST3)],
- Trigger scheme 3 = (ST1 ∨ ST2) ∧ (ST3 ∨ ST4),
- Trigger scheme 4 = ST1 ∧ ST2 ∧ ST3 ∧ ST4,
6. Cadmium Zinc Telluride (CZT) Detectors
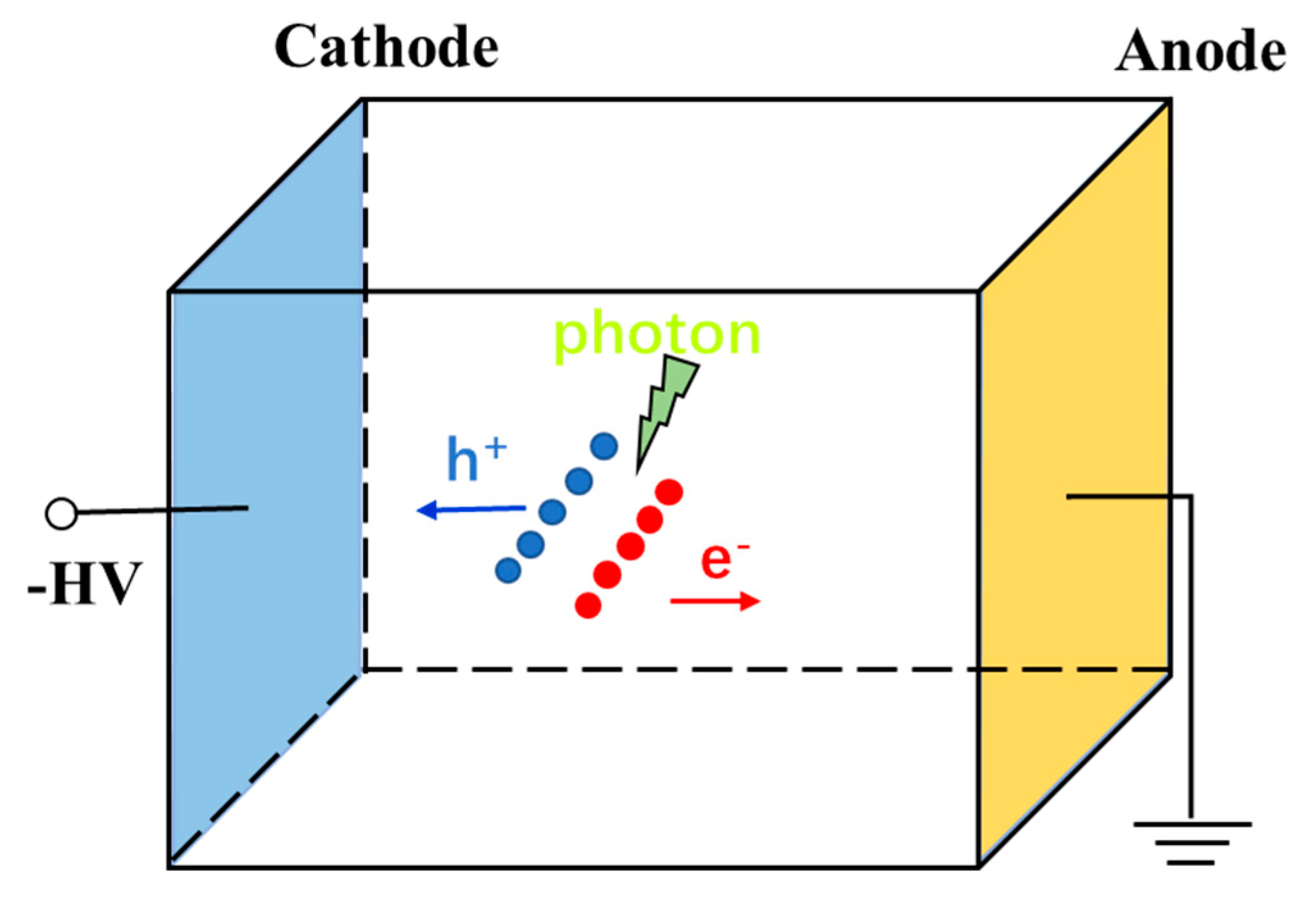
6.1. Operating Principles of CZT
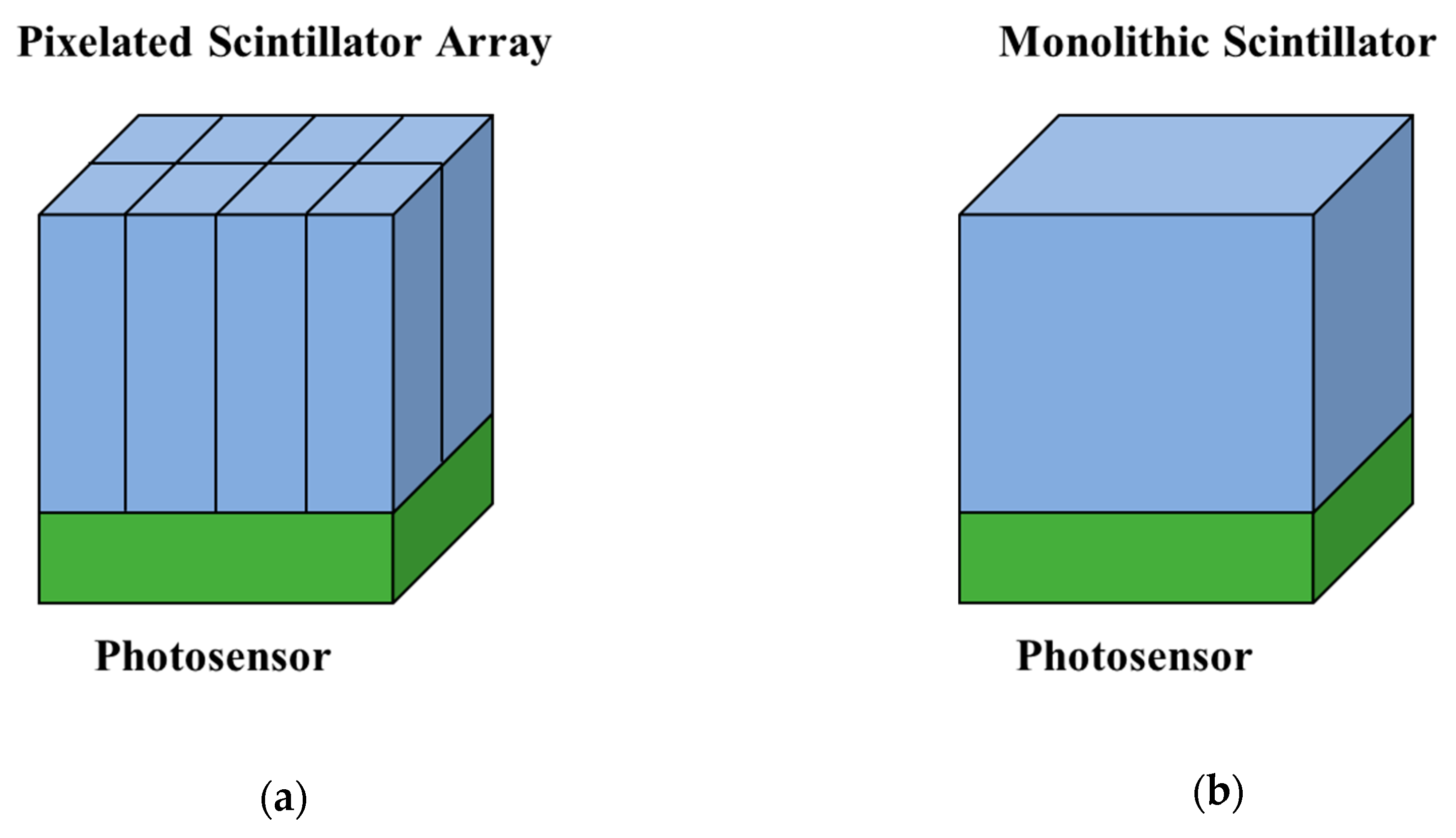
6.2. CZT Detector for PET Applications
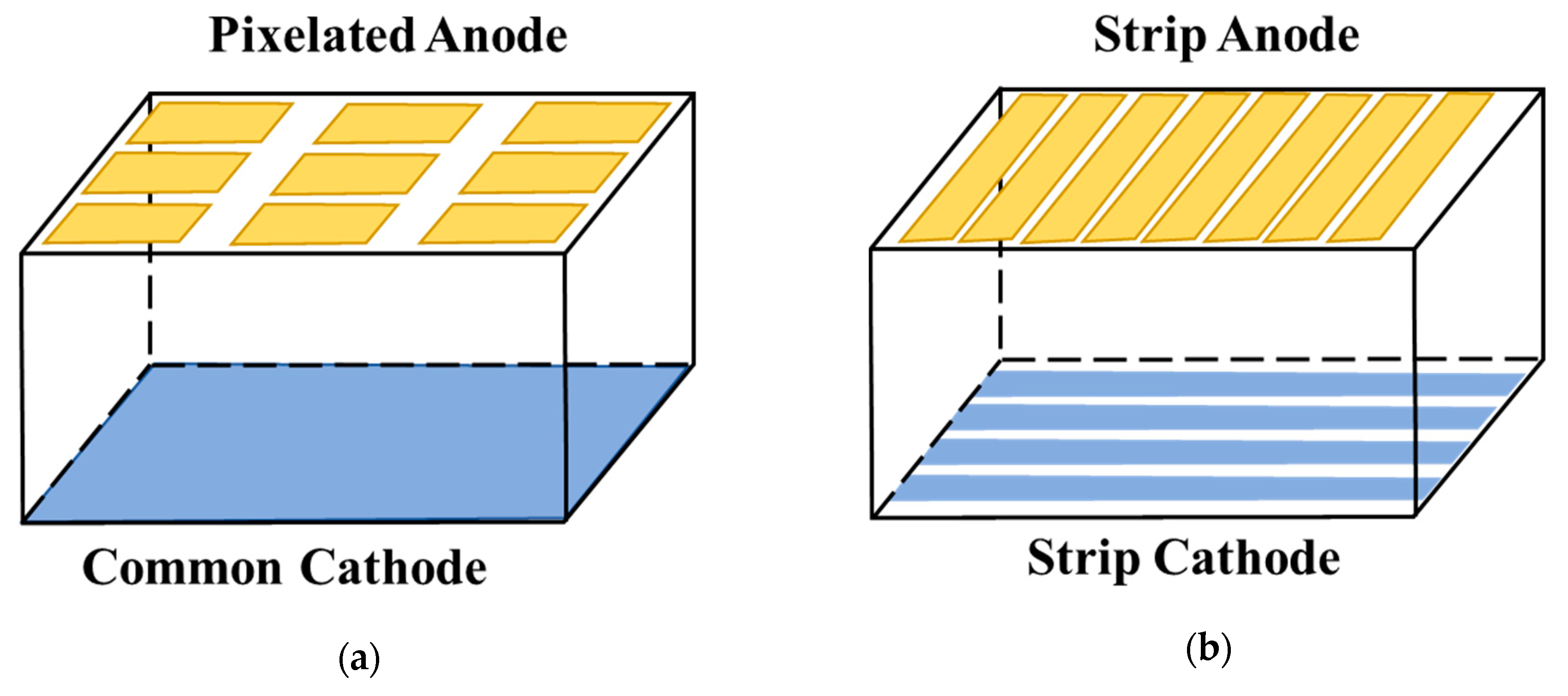
6.2.1. Pixelated Electrodes
6.2.2. Cross-Strip Electrodes
7. State-of-the-Art Systems
7.1. PET/CT Systems
| Reference | [176,177] | [178,179] | [180,181] | [183] |
|---|---|---|---|---|
| Manufacturer | Philips | GE | Siemens | Canon |
| Model Name | Vereos Digital PET/CT | Discovery MI PET/CT | Biograph Vision PET/CT | Celesteion™ PUREViSION Edition PET/CT |
| Scintillator Material | LYSO | LBS | LSO | LBS |
| Scintillator Size (mm3) | 4 × 4 × 19 | 4.0 × 5.3 × 25 | 3.2 × 3.2 × 20 | 4 × 4 (Length Unknown) |
| Sensor | dSiPM | SiPM | SiPM | PMT |
| Spatial Resolution (mm) | 4.1 | 4.2 | 3.6 | 5.1 |
| Timing Resolution (ps) | 325 | 385 | 214 | 394 |
| Energy Resolution (%) | 11.1 | 9.4 | 9 | 11.2 |
7.2. PET/MRI Systems
8. Research Challenges and Conclusions
8.1. Research Challenges for SiPMs
8.1.1. Improving the PDE of CMOS SiPMs
8.1.2. Lowering the Dark Noise of SiPM Pixels (SPADs)
8.1.3. Reducing the Timing Jitter
8.2. Research Challenges for CZT Detectors
8.2.1. Improving the Timing Performance
8.2.2. Processing Small Amplitude Readout Signals
8.2.3. Managing a Large Number of Electrodes
8.3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Giger, M.L. Machine Learning in Medical Imaging. J. Am. Coll. Radiol. 2018, 15, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rohren, E.M.; Turkington, T.G.; Coleman, R.E. Clinical Applications of PET in Oncology. Radiology 2004, 231, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Kadir, A.; Almkvist, O.; Forsberg, A.; Wall, A.; Engler, H.; Långström, B.; Nordberg, A. Dynamic Changes in PET Amyloid and FDG Imaging at Different Stages of Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 198.e1–198.e14. [Google Scholar] [CrossRef] [PubMed]
- Keng, F.Y. Clinical Applications of Positron Emission Tomography in Cardiology: A Review. Ann. Acad. Med. Singap. 2004, 33, 175–182. [Google Scholar]
- Nader, M.A.; Czoty, P.W. Brain Imaging in Nonhuman Primates: Insights into Drug Addiction. ILAR J. 2008, 49, 89–102. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.S.; Im, K.C.; Kim, S.J.; Kim, S.-Y.; Lee, D.S.; Moon, D.H. Performance Measurement of the microPET Focus 120 Scanner. J. Nucl. Med. 2007, 48, 1527–1535. [Google Scholar] [CrossRef]
- Sato, K.; Shidahara, M.; Watabe, H.; Watanuki, S.; Ishikawa, Y.; Arakawa, Y.; Nai, Y.; Furumoto, S.; Tashiro, M.; Shoji, T.; et al. Performance Evaluation of the Small-animal PET Scanner ClairvivoPET Using NEMA NU 4-2008 Standards. Phys. Med. Biol. 2016, 61, 696–711. [Google Scholar] [CrossRef]
- Prasad, R.; Ratib, O.; Zaidi, H. Performance Evaluation of the FLEX Triumph X-PET Scanner Using the National Electrical Manufacturers Association NU-4 Standards. J. Nucl. Med. 2010, 51, 1608–1615. [Google Scholar] [CrossRef]
- Zhang, H.; Bao, Q.; Vu, N.T.; Silverman, R.W.; Taschereau, R.; Berry-Pusey, B.N.; Douraghy, A.; Rannou, F.R.; Stout, D.B.; Chatziioannou, A.F. Performance Evaluation of PETbox: A Low Cost Bench Top Preclinical PET Scanner. Mol. Imaging Biol. 2011, 13, 949–961. [Google Scholar] [CrossRef][Green Version]
- Herrmann, K.; Dahlbom, M.; Nathanson, D.; Wei, L.; Radu, C.; Chatziioannou, A.; Czernin, J. Evaluation of the Genisys4, a Bench-Top Preclinical PET Scanner. J. Nucl. Med. 2013, 54, 1162–1167. [Google Scholar] [CrossRef][Green Version]
- Ingenuity TF PET/MR system. Available online: https://www.philips.ca/healthcare/product/HC882380/ingenuity-tf-petmr-system/documentation (accessed on 20 March 2019).
- Zaidi, H.; Ojha, N.; Morich, M.; Griesmer, J.; Hu, Z.; Maniawski, P.; Ratib, O.; Izquierdo-Garcia, D.; Fayad, Z.A.; Shao, L. Design and Performance Evaluation of A Whole-Body Ingenuity TF PET-MRI System. Phys. Med. Biol. 2011, 56, 3091–3106. [Google Scholar] [CrossRef] [PubMed]
- SIGNATM PET/MR. Available online: https://www.gehealthcare.com/en/products/magnetic-resonance-imaging/3-0t/signa-pet-mr (accessed on 20 March 2019).
- Biograph mMR. Available online: https://www.siemens-healthineers.com/molecular-imaging/pet-mr/mi-biograph-mmr (accessed on 20 March 2019).
- Boss, A.; Weiger, M.; Wiesinger, F. Future Image Acquisition Trends for PET/MRI. Semin. Nucl. Med. 2015, 45, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Colletti, P.M. Competitive Advantage of PET/MRI. Eur. J. Radiol. 2014, 83, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Torigian, D.A.; Zaidi, H.; Kwee, T.C.; Saboury, B.; Udupa, J.K.; Cho, Z.-H.; Alavi, A. PET/MR Imaging: Technical Aspects and Potential Clinical Applications. Radiology 2013, 267, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.B. Basics of PET Imaging: Physics, Chemistry, and Regulations, 3rd ed.; Springer International Publishing: Cleveland, OH, USA, 2015. [Google Scholar]
- Braga, L.H.C.; Gasparini, L.; Grant, L.; Henderson, R.K.; Massari, N.; Perenzoni, M.; Stoppa, D.; Walker, R. A Fully Digital 8 x 16 SiPM Array for PET Applications With Per-Pixel TDCs and Real-Time Energy Output. IEEE J. Solid-State Circuits 2014, 49, 301–314. [Google Scholar] [CrossRef]
- Iniewski, K. Electronics for Radiation Detection; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine; Elsevier/Saunders: Amsterdam, The Netherlands, 2012; ISBN 9781416051985. [Google Scholar]
- Cheng, Z.; Deen, M.J.; Peng, H. A Low-Power Gateable Vernier Ring Oscillator Time-to-Digital Converter for Biomedical Imaging Applications. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 445–454. [Google Scholar] [CrossRef]
- Peng, H.; Levin, C.S. Recent Development in PET Instrumentation. Curr. Pharm. Biotechnol. 2010, 11, 555–571. [Google Scholar] [CrossRef]
- Budinger, T.F. Time-of-flight Positron Emission Tomography: Status Relative to Conventional PET. J. Nucl. Med. 1983, 24, 73–78. [Google Scholar]
- Karp, J.S. Time-of-Flight PET. Available online: http://snmmi.files.cms-plus.com/docs/PETCENews_Fall06.pdf (accessed on 4 March 2019).
- Becker, W. Advanced Time-Correlated Single Photon Counting Techniques; Castleman, A.W., Toennies, J.P., Zinth, W., Eds.; Springer Series in Chemical Physics; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2005; Volume 81, ISBN 978-3-540-26047-9. [Google Scholar]
- Spanoudaki, V.C.; Levin, C.S. Photo-Detectors for Time of Flight Positron Emission Tomography (ToF-PET). Sensors 2010, 10, 10484–10505. [Google Scholar] [CrossRef]
- Hamamatsu Photonics, K.K. Photomultiplier Tubes—Basics and Applications. Available online: https://www.hamamatsu.com/resources/pdf/etd/PMT_handbook_v3aE.pdf (accessed on 14 November 2019).
- Moriya, T.; Omura, T.; Watanabe, M.; Yamashita, T. Development of a Position-Sensitive Detector for TOF-PET. IEEE Trans. Nucl. Sci. 2008, 55, 2455–2459. [Google Scholar] [CrossRef]
- Ito, M.; Lee, J.P.; Lee, J.S. Timing Performance Study of New Fast PMTs With LYSO for Time-of-Flight PET. IEEE Trans. Nucl. Sci. 2013, 60, 30–37. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Ramirez, R.; An, S.; Wang, C.; Liu, S.; Dong, Y.; Baghaei, H.; Wong, W.-H. Design and Development of a Gapless Ring With Modular PMT-quadrant-sharing Detector (PQS) for a Time-of-flight PET Camera. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), IEEE, Seoul, Korea, 27 October–2 November 2013; pp. 1–5. [Google Scholar]
- An, S.; Li, H.; Liu, S.; Rocio, R.; Zhang, Y.; Wang, C.; Baghaei, H.; Wong, W.-H. A First Study on the Timing Performance of PMT-Quadrant-Sharing LYSO Detector Array for Time-of-flight PET. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; pp. 3593–3596. [Google Scholar]
- Wong, W.-H.; Li, H.; Zhang, Y.; Ramirez, R.; An, S.; Wang, C.; Liu, S.; Dong, Y.; Baghaei, H. A High-Resolution Time-of-Flight Clinical PET Detection System Using a Gapless PMT-Quadrant-Sharing Method. IEEE Trans. Nucl. Sci. 2015, 62, 2067–2074. [Google Scholar] [CrossRef]
- Inadama, N.; Murayama, H.; Nishikido, F.; Ohi, J.; Yamaya, T. Improvement of a PET Detector Performance by Setting Reflectors in Parallel With PMT Face. IEEE Trans. Nucl. Sci. 2019, 66, 497–505. [Google Scholar] [CrossRef]
- Lee, J.P.; Ito, M.; Lee, J.S. Evaluation of a Fast Photomultiplier Tube for Time-of-flight PET. Biomed. Eng. Lett. 2011, 1, 174–179. [Google Scholar] [CrossRef]
- Ng, K. Complete Guide to Semiconductor Devices; McGraw-Hill: New York, NY, USA, 1999; ISBN 978-0-07-035860-7. [Google Scholar]
- Hamamatsu Si APD (Avalanche Photodiode) Selection Guide. Available online: https://www.hamamatsu.com/resources/pdf/ssd/si_apd_kapd0001e.pdf (accessed on 3 June 2019).
- Grazioso, R.; Aykac, M.; Casey, M.E.; Givens, G.; Schmand, M. APD Performance in Light Sharing PET Applications. IEEE Trans. Nucl. Sci. 2005, 52, 1413–1416. [Google Scholar] [CrossRef]
- Nassalski, A.; Moszynski, M.; Syntfeld-Kzuch, A.; Swiderski, L.; Szczesniak, T.; Wolski, D.; Batsch, T. Application of Hamamatsu S8550 APD Array to the Common PET/CT Detector. IEEE Trans. Nucl. Sci. 2008, 55, 2460–2464. [Google Scholar] [CrossRef]
- Bergeron, M.; Cadorette, J.; Beaudoin, J.-F.; Rousseau, J.A.; Dumoulin, M.; Lepage, M.D.; Robert, G.; Selivanov, V.; Tetrault, M.-A.; Viscogliosi, N.; et al. Performance Evaluation of the LabPETTM APD-based Digital PET Scanner. In Proceedings of the 2007 IEEE Nuclear Science Symposium Conference Record, Honolulu, HI, USA, 26 October–3 November 2007; pp. 4185–4191. [Google Scholar]
- Bergeron, M.; Thibaudeau, C.; Cadorette, J.; Tetrault, M.-A.; Pepin, C.M.; Clerk-Lamalice, J.; Loignon-Houle, F.; Davies, M.; Dautet, H.; Deschamps, P.; et al. LabPET II, an APD-based Detector Module with PET and Counting CT Imaging Capabilities. IEEE Trans. Nucl. Sci. 2015, 62, 756–765. [Google Scholar] [CrossRef]
- Vandenbroucke, A.; Reynolds, P.D.; Lau, F.W.; Innes, D.R.; Freese, D.L.; Hsu, D.F.C. First Measurements of a 512 PSAPD Prototype of a Sub-mm Resolution Clinical PET Camera. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), IEEE, Seoul, Korea, 27 October–2 November 2013; pp. 1–4. [Google Scholar]
- Sarasola, I.; Rato Mendes, P.; Garcia de Acilu, P.; Canadas, M.; Vela, O.; Cela, J.M.; Oller, J.C.; Nunez, L.; Willmott, C.; Perez, J.M. PET Demonstrator for a Human Brain Scanner Based on Monolithic Detector Blocks. IEEE Trans. Nucl. Sci. 2011, 58, 2190–2197. [Google Scholar] [CrossRef]
- Berard, P.; Bergeron, M.; Pepin, C.M.; Cadorette, J.; Tetrault, M.-A.; Viscogliosi, N.; Fontaine, R.; Dautet, H.; Davies, M.; Deschamps, P.; et al. LabPET II, a Novel 64-channel APD-based PET Detector Module with Individual Pixel Readout Achieving Submillimetric Spatial Resolution. In Proceedings of the 2008 IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 5457–5462. [Google Scholar]
- Vandenbroucke, A.; Foudray, A.M.K.; Olcott, P.D.; Levin, C.S. Performance Characterization of a New High Resolution PET Scintillation Detector. Phys. Med. Biol. 2010, 55, 5895–5911. [Google Scholar] [CrossRef]
- Freese, D.L.; Vandenbroucke, A.; Innes, D.; Lau, F.W.Y.; Hsu, D.F.C.; Reynolds, P.D.; Levin, C.S. Analyzing the Stability of 256 APDs Through Leakage Current and Temperature Monitoring in a 1 mm3 Resolution Clinical PET System. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), Seoul, Korea, 27 October–2 November 2013; pp. 1–3. [Google Scholar]
- Garcia de Acilu, P.; Mendes, P.R.; Canadas, M.; Sarasola, I.; Cuerdo, R.; Romero, L.; Willmott, C. Evaluation of APD and SiPM Matrices as Sensors for Monolithic PET Detector Blocks. In Proceedings of the 2011 IEEE Nuclear Science Symposium Conference Record, Valencia, Spain, 23–29 October 2011; pp. 3300–3304. [Google Scholar]
- Palubiak, D.; El-Desouki, M.M.; Marinov, O.; Deen, M.J.; Fang, Q. High-Speed, Single-Photon Avalanche-Photodiode Imager for Biomedical Applications. IEEE Sens. J. 2011, 11, 2401–2412. [Google Scholar] [CrossRef]
- Gallivanoni, A.; Rech, I.; Ghioni, M. Progress in Quenching Circuits for Single Photon Avalanche Diodes. IEEE Trans. Nucl. Sci. 2010, 57, 3815–3826. [Google Scholar] [CrossRef]
- Mita, R.; Palumbo, G. High-Speed and Compact Quenching Circuit for Single-Photon Avalanche Diodes. IEEE Trans. Instrum. Meas. 2008, 57, 543–547. [Google Scholar] [CrossRef]
- Ma, C.L.F.; Deen, M.J.; Tarof, L.E.; Yu, J.C.H. Temperature dependence of breakdown voltages in separate absorption, grading, charge, and multiplication InP/InGaAs avalanche photodiodes. IEEE Trans. Electron Devices 1995, 42, 810–818. [Google Scholar] [CrossRef]
- Hall, R. Temperature Coefficient of the Breakdown Voltage of Silicon P-N Junctions. Int. J. Electron. 1967, 22, 513–519. [Google Scholar] [CrossRef]
- Faramarzpour, N.; Deen, M.J.; Shirani, S.; Fang, Q. Fully Integrated Single Photon Avalanche Diode Detector in Standard CMOS 0.18-μm Technology. IEEE Trans. Electron Devices 2008, 55, 760–767. [Google Scholar] [CrossRef]
- Palubiak, D.P.; Li, Z.; Deen, M.J. Afterpulsing Characteristics of Free-Running and Time-Gated Single-Photon Avalanche Diodes in 130-nm CMOS. IEEE Trans. Electron Devices 2015, 62, 3727–3733. [Google Scholar] [CrossRef]
- Cheng, Z.; Palubiak, D.; Zheng, X.; Deen, M.J.; Peng, H. Impact of Silicide Layer on Single Photon Avalanche Diodes in a 130 nm CMOS Process. J. Phys. D Appl. Phys. 2016, 49, 345105. [Google Scholar] [CrossRef]
- Leitner, T.; Feiningstein, A.; Turchetta, R.; Coath, R.; Chick, S.; Visokolov, G.; Savuskan, V.; Javitt, M.; Gal, L.; Brouk, I.; et al. Measurements and Simulations of Low Dark Count Rate Single Photon Avalanche Diode Device in a Low Voltage 180-nm CMOS Image Sensor Technology. IEEE Trans. Electron Devices 2013, 60, 1982–1988. [Google Scholar] [CrossRef]
- Richardson, J.A.; Webster, E.A.G.; Grant, L.A.; Henderson, R.K. Scaleable Single-Photon Avalanche Diode Structures in Nanometer CMOS Technology. IEEE Trans. Electron Devices 2011, 58, 2028–2035. [Google Scholar] [CrossRef]
- Finkelstein, H.; Hsu, M.J.; Zlatanovic, S.; Esener, S. Performance Trade-offs in Single-photon Avalanche Diode Miniaturization. Rev. Sci. Instrum. 2007, 78, 103103. [Google Scholar] [CrossRef]
- Kindt, W.J. Geiger Mode Avalanche Photodiode Arrays; Delft University Press: Delft, The Netherlands, 1999. [Google Scholar]
- 6Richardson, J.A.; Grant, L.A.; Henderson, R.K. Low Dark Count Single-photon Avalanche Diode Structure Compatible with Standard Nanometer Scale CMOS Technology. IEEE Photonics Technol. Lett. 2009, 21, 1020–1022. [Google Scholar] [CrossRef]
- Accarino, C.; Mohammed, A.R.; Shah, Y.D.; Maneuski, D.; Mitra, S.; Buttar, C.; Cumming, D.R.S. Low Noise and High Photodetection Probability SPAD in 180 nm Standard CMOS Technology. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–4. [Google Scholar]
- Cova, S.; Lacaita, A.; Ripamonti, G. Trapping Phenomena in Avalanche Photodiodes on Nanosecond Scale. IEEE Electron Device Lett. 1991, 12, 685–687. [Google Scholar] [CrossRef]
- Kindt, W.J.; Van Zeijl, H.W. Modelling and Fabrication of Geiger Mode Avalanche Photodiodes. IEEE Trans. Nucl. Sci. 1998, 45, 715–719. [Google Scholar] [CrossRef]
- Lindner, S.; Pellegrini, S.; Henrion, Y.; Rae, B.; Wolf, M.; Charbon, E. A High-PDE, Backside-Illuminated SPAD in 65/40-nm 3D IC CMOS Pixel With Cascoded Passive Quenching and Active Recharge. IEEE Electron Device Lett. 2017, 38, 1547–1550. [Google Scholar] [CrossRef]
- Xu, H.; Pancheri, L.; Betta, G.-F.D.; Stoppa, D. Design and Characterization of a p+/n-well SPAD Array in 150 nm CMOS Process. Opt. Express 2017, 25, 12765. [Google Scholar] [CrossRef]
- Bonanno, G.; Finocchiaro, P.; Pappalardo, A.; Billotta, S.; Cosentino, L.; Belluso, M.; Di Mauro, S.; Occhipinti, G. Precision Measurements of Photon Detection Efficiency for SiPM Detectors. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectromrters Detect. Assoc. Equip. 2009, 610, 93–97. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Jamal Deen, M.; Tarof, L.E.; Clark, W. A simplified approach to time-domain modeling of avalanche photodiodes. IEEE J. Quantum Electron. 1998, 34, 691–699. [Google Scholar] [CrossRef]
- Spinelli, A.; Lacaita, A.L. Physics and Numerical Simulation of Single Photon Avalanche Diodes. IEEE Trans. Electron Devices 1997, 44, 1931–1943. [Google Scholar] [CrossRef]
- Ardeshirpour, Y.; Deen, M.J.; Shirani, S. 2-D CMOS Based Image Sensor System for Fluorescent Detection. In Proceedings of the Canadian Conference on Electrical and Computer Engineering 2004 (IEEE Cat. No. 04CH37513), Niagara Falls, Ontario, Canda, 2–5 May 2004; pp. 1441–1444. [Google Scholar]
- Finkelstein, H.; Hsu, M.J.; Esener, S.C. Dual-junction Single-photon Avalanche Diode. Electron. Lett. 2007, 43, 1228. [Google Scholar] [CrossRef]
- Henderson, R.K.; Webster, E.A.G.; Grant, L.A. A Dual-Junction Single-Photon Avalanche Diode in 130-nm CMOS Technology. IEEE Electron Device Lett. 2013, 34, 429–431. [Google Scholar] [CrossRef]
- Finkelstein, H.; Hsu, M.J.; Esener, S.C. STI-Bounded Single-Photon Avalanche Diode in a Deep-Submicrometer CMOS Technology. IEEE Electron Device Lett. 2006, 27, 887–889. [Google Scholar] [CrossRef]
- Gersbach, M.; Richardson, J.; Mazaleyrat, E.; Hardillier, S.; Niclass, C.; Henderson, R.; Grant, L.; Charbon, E. A Low-noise Single-photon Detector Implemented in a 130 nm CMOS Imaging Process. Solid-State Electron. 2009, 53, 803–808. [Google Scholar] [CrossRef]
- Field, R.M.; Lary, J.; Cohn, J.; Paninski, L.; Shepard, K.L. A low-noise, single-photon avalanche diode in standard 0.13 μm complementary metal-oxide-semiconductor process. Appl. Phys. Lett. 2010, 97, 211111. [Google Scholar] [CrossRef]
- Niclass, C.; Gersbach, M.; Henderson, R.; Grant, L.; Charbon, E. A 130-nm CMOS single-photon avalanche diode. In Proceedings of the Optoelectronic Devices: Physics, Fabrication, and Application IV, Boston, MA, USA, 9–12 September 2007; Volume 6766, p. 676606. [Google Scholar]
- Pavia, J.M.; Scandini, M.; Lindner, S.; Wolf, M.; Charbon, E. A 1x400 Backside-Illuminated SPAD Sensor With 49.7 ps Resolution, 30 pJ/Sample TDCs Fabricated in 3D CMOS Technology for Near-Infrared Optical Tomography. IEEE J. Solid-State Circuits 2015, 50, 2406–2418. [Google Scholar] [CrossRef]
- Charbon, E.; Yoon, H.-J.; Maruyama, Y. A Geiger Mode APD Fabricated in Standard 65nm CMOS Technology. In Proceedings of the 2013 IEEE International Electron Devices Meeting, Washington, DC, USA, 9–11 December 2013. [Google Scholar]
- Braga, L.H.C.; Pancheri, L.; Gasparini, L.; Perenzoni, M.; Walker, R.; Henderson, R.K.; Stoppa, D. A CMOS mini-SiPM Detector with in-pixel Data Compression for PET Applications. In Proceedings of the 2011 IEEE Nuclear Science Symposium Conference Record, Valencia, Spain, 23–29 October 2011; pp. 548–552. [Google Scholar]
- Zappa, F.; Tisa, S.; Gulinatti, A.; Gallivanoni, A.; Cova, S. Complete Single-photon Counting and Timing Module in a Microchip. Opt. Lett. 2005, 30, 1327. [Google Scholar] [CrossRef]
- Mosconi, D.; Stoppa, D.; Pancheri, L.; Gonzo, L.; Simoni, A. CMOS Single-Photon Avalanche Diode Array for Time-Resolved Fluorescence Detection. In Proceedings of the 2006 32nd European Solid-State Circuits Conference, Montreux, Switerland, 19–21 September 2006; pp. 564–567. [Google Scholar]
- Cheng, Z.; Zheng, X.; Palubiak, D.; Deen, M.J.; Peng, H. A Comprehensive and Accurate Analytical SPAD Model for Circuit Simulation. IEEE Trans. Electron Devices 2016, 63, 1940–1948. [Google Scholar] [CrossRef]
- Dalla Mora, A.; Tosi, A.; Tisa, S.; Zappa, F. Single-Photon Avalanche Diode Model for Circuit Simulations. IEEE Photonics Technol. Lett. 2007, 19, 1922–1924. [Google Scholar] [CrossRef]
- Zappa, F.; Tosi, A.; Mora, A.D.; Tisa, S. SPICE Modeling of Single Photon Avalanche Diodes. Sens. Actuators A Phys. 2009, 153, 197–204. [Google Scholar] [CrossRef]
- Cova, S.; Ghioni, M.; Lacaita, A.; Samori, C.; Zappa, F. Avalanche Photodiodes and Quenching Circuits for Single-photon Detection. Appl. Opt. 1996, 35, 1956–1976. [Google Scholar] [CrossRef]
- Savuskan, V.; Javitt, M.; Visokolov, G.; Brouk, I.; Nemirovsky, Y. Selecting Single Photon Avalanche Diode (SPAD) Passive-Quenching Resistance: An Approach. IEEE Sens. J. 2013, 13, 2322–2328. [Google Scholar] [CrossRef]
- Veerappan, C.; Richardson, J.; Walker, R.; Li, D.-U.; Fishburn, M.W.; Maruyama, Y.; Stoppa, D.; Borghetti, F.; Gersbach, M.; Henderson, R.K.; et al. A 160×128 Single-photon Image Sensor with on-pixel 55ps 10b Time-to-digital Converter. In Proceedings of the 2011 IEEE International Solid-State Circuits Conference, San Francisco, CA, USA, 20–24 February 2011; pp. 312–314. [Google Scholar]
- Niclass, C.; Gersbach, M.; Henderson, R.; Grant, L.; Charbon, E. A single photon avalanche diode implemented in 130-nm CMOS technology. IEEE J. Sel. Top. Quantum Electron. 2007, 13, 863–869. [Google Scholar] [CrossRef]
- Neri, L.; Tudisco, S.; Musumeci, F.; Scordino, A.; Fallica, G.; Mazzillo, M.; Zimbone, M. Note: Dead Time Causes and Correction Method for Single Photon Avalanche Diode Devices. Rev. Sci. Instrum. 2010, 81, 086102. [Google Scholar] [CrossRef] [PubMed]
- Rochas, A.; Besse, P.-A.; Popovic, R.S. Actively Recharged Single Photon Counting Avalanche Photodiode Integrated in an Industrial CMOS Process. Sens. Actuators A Phys. 2004, 110, 124–129. [Google Scholar] [CrossRef]
- Tisa, S.; Tosi, A.; Zappa, F. Fully-integrated CMOS Single Photon Counter. Opt. Express 2007, 15, 2873–2887. [Google Scholar] [CrossRef]
- Li, Z.; Deen, M.J. Towards a Portable Raman Spectrometer Using a Concave Grating and a Time-gated CMOS SPAD. Opt. Express 2014, 22, 18736. [Google Scholar] [CrossRef]
- Acconcia, G.; Labanca, I.; Rech, I.; Gulinatti, A.; Ghioni, M. Note: Fully integrated active quenching circuit achieving 100 MHz count rate with custom technology single photon avalanche diodes. Rev. Sci. Instrum. 2017, 88, 026103. [Google Scholar] [CrossRef]
- Lee, M.-J.; Choi, W.-Y. Performance Optimization and Improvement of Silicon Avalanche Photodetectors in Standard CMOS Technology. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1–13. [Google Scholar] [CrossRef]
- Palubiak, D.P.; Deen, M.J. CMOS SPADs: Design Issues and Research Challenges for Detectors, Circuits, and Arrays. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 409–426. [Google Scholar] [CrossRef]
- Mazzillo, M.; Nagy, F.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Piana, A.; Fallica, G.; Coffa, S. Silicon photomultipliers development at STMicroelectronics; Sobolewski, R., Fiurásek, J., Eds.; SPIE: Prague, Czech Republic, 2013; Volume 8773, p. 877302. [Google Scholar]
- Lewellen, T.K. Recent Developments in PET Detector Technology. Phys. Med. Biol. 2008, 53, R287–R317. [Google Scholar] [CrossRef]
- Bieniosek, M.F.; Olcott, P.D.; Levin, C.S. Time Resolution Performance of an Electro-optical-coupled PET Detector for Time-of-flight PET/MRI. In Proceedings of the 2011 IEEE Nuclear Science Symposium Conference Record, Valencia, Spain, 23–29 October 2011; pp. 2531–2533. [Google Scholar]
- Kwon, S.I.; Lee, J.S.; Yoon, H.S.; Ito, M.; Ko, G.B.; Choi, J.Y.; Lee, S.-H.; Song, I.C.; Jeong, J.M.; Lee, D.S.; et al. Development of Small-Animal PET Prototype Using Silicon Photomultiplier (SiPM): Initial Results of Phantom and Animal Imaging Studies. J. Nucl. Med. 2011, 52, 572–579. [Google Scholar] [CrossRef]
- Yoon, H.S.; Ko, G.B.; Kwon, S.I.; Lee, C.M.; Ito, M.; Chan Song, I.; Lee, D.S.; Hong, S.J.; Lee, J.S. Initial results of simultaneous PET/MRI experiments with an MRI-compatible silicon photomultiplier PET scanner. J. Nucl. Med. 2012, 53, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.W.; Bieniosek, M.F.; Levin, C.S. Highly multiplexed signal readout for a time-of-flight positron emission tomography detector based on silicon photomultipliers. J. Med. Imaging 2017, 4, 011012. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.J.; Choi, Y.; Hu, W.; Yan, J.; Ho Jung, J. Development of capacitive multiplexing circuit for SiPM-based time-of-flight (TOF) PET detector. Phys. Med. Biol. 2017, 62, N120–N133. [Google Scholar] [CrossRef] [PubMed]
- El-Desouki, M.; Deen, M.J.; Fang, Q.; Liu, L.; Tse, F.; Armstrong, D. CMOS image sensors for high speed applications. Sensors 2009, 9, 430–444. [Google Scholar] [CrossRef]
- Cheng, Z.; Zheng, X.; Deen, M.J.; Peng, H. Recent Developments and Design Challenges of High-Performance Ring Oscillator CMOS Time-to-Digital Converters. IEEE Trans. Electron Devices 2016, 63, 235–251. [Google Scholar] [CrossRef]
- Frach, T.; Prescher, G.; Degenhardt, C.; Gruyter, R.; Schmitz, A.; Ballizany, R. The Digital Silicon Photomultiplier—Principle of Operation and Intrinsic Detector Performance. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; pp. 1959–1965. [Google Scholar]
- Webster, E.A.G.; Richardson, J.A.; Grant, L.A.; Renshaw, D.; Henderson, R.K. A Single-Photon Avalanche Diode in 90-nm CMOS Imaging Technology With 44% Photon Detection Efficiency at 690 nm. IEEE Electron Device Lett. 2012, 33, 694–696. [Google Scholar] [CrossRef]
- Pancheri, L.; Stoppa, D. Low-noise single Photon Avalanche Diodes in 0.15 μm CMOS technology. In Proceedings of the 2011 European Solid-State Device Research Conference (ESSDERC), Helsinki, Finland, 12–16 September 2011; pp. 179–182. [Google Scholar]
- Niclass, C.; Soga, M. A miniature Actively Recharged Single-photon Detector Free of Afterpulsing Effects with 6 ns Dead Time in a 0.18 μm CMOS Technology. In Proceedings of the Electron Devices Meet. (IEDM), 2010 IEEE Int, San Francisco, CA, USA, 6–8 December 2010. [Google Scholar]
- Veerappan, C.; Charbon, E. A Substrate Isolated CMOS SPAD Enabling Wide Spectral Response and Low Electrical Crosstalk. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 299–305. [Google Scholar] [CrossRef]
- Veerappan, C.; Charbon, E. CMOS SPAD Based on Photo-Carrier Diffusion Achieving PDP >40% from 440 to 580 nm at 4 v Excess Bias. IEEE Photonics Technol. Lett. 2015, 27, 2445–2448. [Google Scholar] [CrossRef]
- Veerappan, C.; Charbon, E. A Low Dark Count p-i-n Diode Based SPAD in CMOS Technology. IEEE Trans. Electron Devices 2016, 63, 65–71. [Google Scholar] [CrossRef]
- Bronzi, D.; Tisa, S.; Villa, F.; Bellisai, S.; Alberto, T.; Zappa, F. Fast Sensing and Quenching of CMOS SPADs for Minimal Afterpulsing Effects. IEEE Photonics Technol. Lett. 2013, 25, 776–779. [Google Scholar] [CrossRef]
- Stoppa, D.; Mosconi, D.; Pancheri, L.; Lorenzo, G. Single-Photon Avalanche Diode CMOS Sensor forTime-Resolved Fluorescence Measurements. IEEE Sens. J. 2009, 9, 1084–1091. [Google Scholar] [CrossRef]
- González, A.J.; Majewski, S.; Sánchez, F.; Aussenhofer, S.; Aguilar, A.; Conde, P.; Hernández, L.; Vidal, L.F.; Pani, R.; Bettiol, M.; et al. The MINDView brain PET detector, feasibility study based on SiPM arrays. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectromrters Detect. Assoc. Equip. 2016, 818, 82–90. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Gonzalez-Montoro, A.; Vidal, L.F.; Barbera, J.; Aussenhofer, S.; Hernandez, L.; Moliner, L.; Sanchez, F.; Correcher, C.; Pincay, E.J.; et al. Initial Results of the MINDView PET Insert Inside the 3T mMR. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 343–351. [Google Scholar] [CrossRef]
- Camarlinghi, N.; Belcari, N.; Cerello, P.; Pennazio, F.; Sportelli, G.; Zaccaro, E.; Del Guerra, A. Evaluation of Algorithms for Photon Depth of Interaction Estimation for the TRIMAGE PET Component. IEEE Trans. Nucl. Sci. 2016, 63, 70–74. [Google Scholar] [CrossRef]
- Pro, T.; Ferri, A.; Gola, A.; Serra, N.; Tarolli, A.; Zorzi, N.; Piemonte, C. New developments of near-UV SiPMs at FBK. IEEE Trans. Nucl. Sci. 2013, 60, 2247–2253. [Google Scholar] [CrossRef]
- Ahmad, S.; Fleury, J.; De La Taille, C.; Seguin-Moreau, N.; Dulucq, F.; Martin-Chassard, G.; Callier, S.; Thienpont, D.; Raux, L. Triroc: A Multi-Channel SiPM Read-Out ASIC for PET/PET-ToF Application. IEEE Trans. Nucl. Sci. 2015, 62, 664–668. [Google Scholar] [CrossRef]
- Sportelli, G.; Ahmad, S.; Belcari, N.; Bisogni, M.G.; Camarlinghi, N.; Di Pasquale, A.; Dussoni, S.; Fleury, J.; Morrocchi, M.; Zaccaro, E.; et al. The TRIMAGE PET Data Acquisition System: Initial Results. IEEE Trans. Radiat. Plasma Med. Sci. 2016, 1, 168–177. [Google Scholar] [CrossRef]
- Belcari, N.; Bisogni, M.G.; Camarlinghi, N.; Carra, P.; Cerello, P.; Morrocchi, M.; Patera, A.; Sportelli, G.; Del Guerra, A. Design and Detector Performance of the PET Component of the TRIMAGE PET/MR/EEG Scanner. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 292–301. [Google Scholar] [CrossRef]
- Cherry, S.R.; Badawi, R.D.; Karp, J.S.; Moses, W.W.; Price, P.; Jones, T. Total-body imaging: Transforming the role of positron emission tomography. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Total-Body PET Scanner. Available online: https://explorer.ucdavis.edu/about-explorer (accessed on 5 October 2019).
- Badawi, R.; Liu, W.; Berg, E.; Lv, Y.; Xu, T.; An, S.; Dong, Y.; Zhang, X.; Judenhofer, M.; Qi, J.; et al. Progress on the EXPLORER project: Towards a total body PET scanner for human imaging. J. Nucl. Med. 2018, 59, 223. [Google Scholar]
- uEXPLORER|Ultra-High-Resolution Digital PET/CT with 194 cm Axial FOV. Available online: https://usa.united-imaging.com/product/uexplorer/ (accessed on 5 October 2019).
- Total-Body PET/CT Scanner Granted FDA Approval. Available online: https://www.healthimaging.com/topics/advanced-visualization/total-body-petct-scanner-granted-fda-approval (accessed on 5 October 2019).
- González, A.J.; Aguilar, A.; Conde, P.; Hernández, L.; Moliner, L.; Vidal, L.F.; Sánchez, F.; Sánchez, S.; Correcher, C.; Molinos, C.; et al. A PET Design Based on SiPM and Monolithic LYSO Crystals: Performance Evaluation. IEEE Trans. Nucl. Sci. 2016, 63, 2471–2477. [Google Scholar] [CrossRef]
- Petiroc 2A. Available online: https://www.weeroc.com/products/sipm-read-out/petiroc-2a (accessed on 5 October 2019).
- PETsys TOFPET2 ASIC. Available online: https://www.petsyselectronics.com/web/public/products/1 (accessed on 5 October 2019).
- Fischer, P.; Peric, I.; Ritzert, M.; Koniczek, M. Fast self triggered multi channel readout ASIC for time- and energy measurement. IEEE Trans. Nucl. Sci. 2009, 56, 1153–1158. [Google Scholar] [CrossRef]
- Sacco, I.; Fischer, P.; Ritzert, M. PETA4: A multi-channel TDC/ADC ASIC for SiPM readout. J. Instrum. 2013, 8. [Google Scholar] [CrossRef]
- Piemonte, C.; Gola, A.; Tarolli, A.; Fisher, P.; Ritzert, M.; Schulz, V.; Solf, T. Performance of FBK SiPMs coupled to PETA3 read-out ASIC for PET application. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 718, 345–346. [Google Scholar] [CrossRef]
- Schug, D.; Gebhardt, P.; Weissler, B.; Gross-Weege, N.; Dey, T.; Schulz, V. Measurements with a PET Coincidence Setup Based on the PETA5 ASIC and FBK RGB-HD SiPMs. In Proceedings of the 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference, NSS/MIC 2017-Conference Proceedings, Atlanta, GA, USA, 21–28 October 2017. [Google Scholar]
- Schug, D.; Nadig, V.; Weissler, B.; Gebhardt, P.; Schulz, V. Initial Measurements with the PETsys TOFPET2 ASIC Evaluation Kit and a Characterization of the ASIC TDC. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 444–453. [Google Scholar] [CrossRef]
- Tabacchini, V.; Westerwoudt, V.; Borghi, G.; Seifert, S.; Schaart, D.R. Probabilities of triggering and validation in a digital silicon photomultiplier. J. Instrum. 2014, 9. [Google Scholar] [CrossRef]
- Frach, T.; Prescher, G.; Degenhardt, C.; Zwaans, B. The digital silicon photomultiplier—System architecture and performance evaluation. In Proceedings of the IEEE Nuclear Science Symposuim & Medical Imaging Conference, Knoxville, TN, USA, 30 October–6 November 2010; pp. 1722–1727. [Google Scholar]
- Degenhardt, C.; Rodrigues, P.; Trindade, A.; Zwaans, B.; Mulhens, O.; Dorscheid, R.; Thon, A.; Salomon, A.; Frach, T. Performance Evaluation of a Prototype Positron Emission Tomography Scanner Using Digital Photon Counters (DPC). In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), Anaheim, CA, USA, 27 October–3 November 2012; pp. 2820–2824. [Google Scholar]
- Seifert, S.; van der Lei, G.; van Dam, H.T.; Schaart, D.R. First Characterization of a Digital SiPM Based Time-of-Flight PET Detector With 1 mm Spatial Resolution. Phys. Med. Biol. 2013, 58, 3061–3074. [Google Scholar] [CrossRef]
- Schneider, F.R.; Shimazoe, K.; Somlai-Schweiger, I.; Ziegler, S.I. A PET Detector Prototype Based on Digital SiPMs and GAGG Scintillators. Phys. Med. Biol. 2015, 60, 1667–1679. [Google Scholar] [CrossRef]
- Streun, M.; Beer, S.; Daemen, J.; Dorscheid, R.; Erven, A.; Jokhovets, L.; Meessen, L.; Mekala, N.B.; Mulhens, O.; Noldgen, H.; et al. PhenoPET: A Dedicated PET Scanner for Plant Research Based on Digital SiPMs (DPCs). In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1–3. [Google Scholar]
- Streun, M.; Borggrewe, K.; Chlubek, A.; Dautzenberg, M.; Degenhardt, C.; Dorscheid, R.; Durini, D.; Erven, A.; Jokhovets, L.; Meessen, L.; et al. PhenoPET—Results from the Plant Scanner. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop (NSS/MIC/RTSD), Strasbourg, France, 29 October–6 November 2016; pp. 1–2. [Google Scholar]
- España, S.; Marcinkowski, R.; Keereman, V.; Vandenberghe, S.; Van Holen, R. DigiPET: Sub-millimeter Spatial Resolution Small-Animal PET Imaging Using Thin Monolithic Scintillators. Phys. Med. Biol. 2014, 59, 3405–3420. [Google Scholar] [CrossRef]
- Borghi, G.; Peet, B.J.; Tabacchini, V.; Schaart, D.R. A 32 mm × 32 mm × 22 mm Monolithic LYSO:Ce Detector with Dual-sided Digital Photon Counter Readout for Ultrahigh-performance TOF-PET and TOF-PET/MRI. Phys. Med. Biol. 2016, 61, 4929–4949. [Google Scholar] [CrossRef]
- Borghi, G.; Tabacchini, V.; Schaart, D.R. Towards Monolithic Scintillator Based TOF-PET Systems: Practical Methods for Detector Calibration and Operation. Phys. Med. Biol. 2016, 61, 4904–4928. [Google Scholar] [CrossRef] [PubMed]
- Borghi, G.; Tabacchini, V.; Bakker, R.; Schaart, D.R. Sub-3 mm, near-200 ps TOF/DOI-PET Imaging with Monolithic Scintillator Detectors in a 70 cm Diameter Tomographic Setup. Phys. Med. Biol. 2018, 63, 155006. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, R.; Mollet, P.; Van Holen, R.; Vandenberghe, S. Sub-millimetre DOI Detector Based on Monolithic LYSO and Digital SiPM for a Dedicated Small-animal PET System. Phys. Med. Biol. 2016, 61, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.T.; Borghi, G.; Seifert, S.; Schaart, D.R. Sub-200 ps CRT in Monolithic Scintillator PET Detectors Using Digital SiPM Arrays and Maximum likelihood Interaction Time Estimation. Phys. Med. Biol. 2013, 58, 3243–3257. [Google Scholar] [CrossRef]
- Schug, D.; Weissler, B.; Gebhardt, P.; Schulz, V. Crystal Delay and Time Walk Correction Methods for Coincidence Resolving Time Improvements of a Digital-Silicon-Photomultiplier-Based PET/MRI Insert. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 178–190. [Google Scholar] [CrossRef]
- Weissler, B.; Gebhardt, P.; Dueppenbecker, P.M.; Wehner, J.; Schug, D.; Lerche, C.W.; Goldschmidt, B.; Salomon, A.; Verel, I.; Heijman, E.; et al. A Digital Preclinical PET/MRI Insert and Initial Results. IEEE Trans. Med. Imaging 2015, 34, 2258–2270. [Google Scholar] [CrossRef]
- Hallen, P.; Schug, D.; Weissler, B.; Gebhardt, P.; Salomon, A.; Kiessling, F.; Schulz, V. PET performance evaluation of the small-animal Hyperion IID PET/MRI insert based on the NEMA NU-4 standard. Biomed. Phys. Eng. Express 2018, 4, 065027. [Google Scholar] [CrossRef]
- Müller, F.; Schug, D.; Hallen, P.; Grahe, J.; Schulz, V. Gradient Tree Boosting-Based Positioning Method for Monolithic Scintillator Crystals in Positron Emission Tomography. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 411–421. [Google Scholar] [CrossRef]
- Müller, F.; Schug, D.; Hallen, P.; Grahe, J.; Schulz, V. A Novel DOI Positioning Algorithm for Monolithic Scintillator Crystals in PET Based on Gradient Tree Boosting. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 465–474. [Google Scholar] [CrossRef]
- Mandai, S.; Jain, V.; Charbon, E. A 780 x 800 μm2 Multichannel Digital Silicon Photomultiplier With Column-Parallel Time-to-Digital Converter and Basic Characterization. IEEE Trans. Nucl. Sci. 2014, 61, 44–52. [Google Scholar] [CrossRef]
- Gersbach, M.; Maruyama, Y.; Trimananda, R.; Fishburn, M.W.; Stoppa, D.; Richardson, J.A.; Walker, R.; Henderson, R.; Charbon, E. A Time-Resolved, Low-Noise Single-Photon Image Sensor Fabricated in Deep-Submicron CMOS Technology. IEEE J. Solid-State Circuits 2012, 47, 1394–1407. [Google Scholar] [CrossRef]
- Field, R.M.; Realov, S.; Shepard, K.L. A 100 fps, Time-Correlated Single-Photon-Counting- Based Fluorescence-Lifetime Imager in 130 nm CMOS. IEEE J. Solid-State Circuits 2014, 49, 867–880. [Google Scholar] [CrossRef]
- Perenzoni, M.; Perenzoni, D.; Stoppa, D. A 64 X 64-Pixels Digital Silicon Photomultiplier Direct TOF Sensor With 100-MPhotons/s/pixel Background Rejection and Imaging/Altimeter Mode With 0.14% Precision Up To 6 km for Spacecraft Navigation and Landing. IEEE J. Solid-State Circuits 2017, 52, 151–160. [Google Scholar] [CrossRef]
- Tamborini, D.; Markovic, B.; Villa, F.; Tosi, A. 16-Channel Module Based on a Monolithic Array of Single-Photon Detectors and 10-ps Time-to-Digital Converters. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 218–225. [Google Scholar] [CrossRef]
- Fishburn, M.W.; Charbon, E. System Tradeoffs in Gamma-Ray Detection Utilizing SPAD Arrays and Scintillators. IEEE Trans. Nucl. Sci. 2010, 57, 2549–2557. [Google Scholar] [CrossRef]
- Verger, L.; Boitel, M.; Gentet, M.; Hamelin, R.; Mestais, C.; Mongellaz, F.; Rustique, J.; Sanchez, G. Characterization of CdTe and CdZnTe Detectors for Gamma-Ray Imaging Applications. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2001, 458, 297–309. [Google Scholar] [CrossRef]
- Levin, C.S. New imaging technologies to enhance the molecular sensitivity of positron emission tomography. Proc. IEEE 2008, 96, 439–467. [Google Scholar] [CrossRef]
- Barber, H.B. Applications of Semiconductor Detectors to Nuclear Medicine. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1999, 436, 102–110. [Google Scholar] [CrossRef]
- Butler, J.F.; Lingren, C.L.; Doty, F.P. Cd/sub 1-x/Zn/sub x/Te Gamma Ray Detectors. IEEE Trans. Nucl. Sci. 1992, 39, 605–609. [Google Scholar] [CrossRef]
- Toney, J.E.; Brunett, B.A.; Schlesinger, T.E.; Van Scyoc, J.M.; James, R.B.; Schieber, M.; Goorsky, M.; Yoon, H.; Eissler, E.; Johnson, C. Uniformity of Cd1–xZnxTe Grown by High-pressure Bridgman. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1996, 380, 132–135. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, Z.; Deen, M.J.; Peng, H. Improving the Spatial Resolution in CZT Detectors Using Charge Sharing Effect and Transient Signal Analysis: Simulation Study. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 808, 60–70. [Google Scholar] [CrossRef]
- Gu, Y.; Matteson, J.L.; Skelton, R.T.; Deal, A.C.; Stephan, E.A.; Duttweiler, F.; Gasaway, T.M.; Levin, C.S. Study of a High-resolution, 3D Positioning Cadmium Zinc Telluride Detector for PET. Phys. Med. Biol. 2011, 56, 1563–1584. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.J.; Jeong, M.; Kim, H.S.; Kim, S.Y.; Ha, J.H. Preliminary Research of CZT Based PET System Development in KAERI. J. Radiat. Prot. Res. 2016, 41, 81–86. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Sundaram, A.; Reed, M.; Eger, J.; Montémont, G.; Verger, L.; He, Z.; Hugg, J.W.; Abbaszadeh, S.; et al. Development of Large-volume high-performance Monolithic CZT Radiation Detector. In Proceedings of the Hard X-Ray, Gamma-Ray, and Neutron Detector Physics XX; Fiederle, M., Burger, A., James, R.B., Payne, S.A., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10762, p. 25. [Google Scholar]
- Komarov, S.; Yin, Y.; Wu, H.; Wen, J.; Krawczynski, H.; Meng, L.-J.; Tai, Y.-C. Investigation of the Limitations of the Highly Pixilated CdZnTe Detector for PET Applications. Phys. Med. Biol. 2012, 57, 7355–7380. [Google Scholar] [CrossRef] [PubMed]
- Drezet, A.; Monnet, O.; Montemont, G.; Rustique, J.; Sanchez, G.; Verger, L. CdZnTe Detectors for the Positron Emission Tomographic Imaging of Small Animals. In Proceedings of the IEEE Symposium Conference Record Nuclear Science, Rome, Italy, 16–22 October 2004; Volume 7, pp. 4564–4568. [Google Scholar]
- Peng, H.; Levin, C.S. Design Study of a High-Resolution Breast-dedicated PET System Built from Cadmium Zinc Telluride Detectors. Phys. Med. Biol. 2010, 55, 2761–2788. [Google Scholar] [CrossRef] [PubMed]
- Matteson, J.L.; Gu, Y.; Skelton, R.T.; Deal, A.C.; Stephan, E.A.; Duttweiler, F.; Huszar, G.L.; Gasaway, T.M.; Levin, C.S. Charge Collection Studies of a High Resolution CZT-based Detector for PET. In Proceedings of the 2008 IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 503–510. [Google Scholar]
- Abbaszadeh, S.; Gu, Y.; Reynolds, P.D.; Levin, C.S. Characterization of a Sub-Assembly of 3D Position Sensitive Cadmium Zinc Telluride Detectors and Electronics from a Sub-millimeter Resolution PET System. Phys. Med. Biol. 2016, 61, 6733–6753. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Levin, C.S. New-generation Small Animal Positron Emission Tomography System for Molecular Imaging. J. Med. Imaging (Bellingham Wash.) 2017, 4, 011008. [Google Scholar]
- Budtz-Jorgensen, C.; Kuvvetli, I. New Position Algorithms for the 3D CZT Drift Detector. IEEE Trans. Nucl. Sci. 2017, 1611–1618. [Google Scholar] [CrossRef]
- Keesing, D.B.; Mathews, A.; Komarov, S.; Wu, H.; Song, T.Y.; O’Sullivan, J.A.; Tai, Y.-C. Image Reconstruction and System Modeling Techniques for Virtual-pinhole PET Insert Systems. Phys. Med. Biol. 2012, 57, 2517–2538. [Google Scholar] [CrossRef]
- Drezet, A.; Monnet, O.; Mathy, F.; Montemont, G.; Verger, L. CdZnTe Detectors for Small Field of View Positron Emission Tomographic Imaging. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 571, 465–470. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Levin, C.S. 3-D Position Sensitive CZT PET System: Current Status. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop (NSS/MIC/RTSD), Strasbourg, France, 29 October–6 November 2016; pp. 1–2. [Google Scholar]
- Vereos Digital PET/CT. Available online: https://www.usa.philips.com/healthcare/product/HC882446/vereos-digital-petct-proven-accuracy-inspires-confidence (accessed on 20 March 2019).
- Philips Healthcare. Truly Digital PET Imaging: Philips Proprietary Digital Photon Counting Technology. Available online: https://philipsproductcontent.blob.core.windows.net/assets/20170523/ed2389b512114e209f2fa77c0145f267.pdf (accessed on 29 March 29ch 2019).
- Discovery, M.I. Available online: https://www.gehealthcare.com/en/products/molecular-imaging/discovery-mi (accessed on 20 March 2019).
- GE Healthcare. Lightburst Digital Detector: A White Paper. Available online: https://static1.squarespace.com/static/5a25ac3c914e6b61f6b983d8/t/5a31f8a8ec212deac346e90d/1513224366471/LDD_whitepaper_Final+JB50594XX.pdf (accessed on 20 March 2019).
- Biograph Vision. Available online: https://www.siemens-healthineers.com/molecular-imaging/pet-ct/biograph-vision (accessed on 20 March 2019).
- Van Sluis, J.J.; de Jong, J.; Schaar, J.; Noordzij, W.; van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance Characteristics of the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Reddin, J.S.; Scheuermann, J.S.; Bharkhada, D.; Smith, A.M.; Casey, M.E.; Conti, M.; Karp, J.S. Performance Evaluation of the SiPM-Based Siemens Biograph Vision PET/CT System. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, Australia, 10–17 November 2018; pp. 1–5. [Google Scholar]
- CelesteionTM PUREViSION Edition PET/CT. Available online: https://us.medical.canon/products/computed-tomography/celesteion/technology/ (accessed on 20 March 2019).
- Griesmer, J.; Futey, J.; Ojha, N.; Morich, M.; Demester, G. Philips Ingenuity TF PET/MR Calibration Overview. Available online: https://philipsproductcontent.blob.core.windows.net/assets/20170523/bdc837ad1a64431b9834a77c01545929.pdf (accessed on 20 March 2019).
- Ojha, N.; Hu, Z.; Shao, L.; Izquierdo, D.; Machac, J.; Ratib, O.; Zaidi, H.; Fuster, V.; Fayad, Z.A. Philips Ingenuity TF PET/MR Performance. Available online: https://philipsproductcontent.blob.core.windows.net/assets/20170523/6e7323e7323e4410b61fa77c01545b76.pdf (accessed on 20 March 2019).
- Qin, Y.; Howlader, M.M.R.; Deen, M.J.; Haddara, Y.M.; Selvaganapathy, P.R. Polymer Integration for Packaging of Implantable Sensors. Sens. Actuators B Chem. 2014, 202, 758–778. [Google Scholar] [CrossRef]
- Antolović, I.M.; Ulku, A.C.; Kizilkan, E.; Lindner, S.; Zanella, F.; Ferrini, R.; Schnieper, M.; Charbon, E.; Bruschini, C. Optical-stack Optimization for Improved SPAD Photon Detection Efficiency. In Proceedings of the Quantum Sensing and Nano Electronics and Photonics XVI; Razeghi, M., Lewis, J.S., Khodaparast, G.A., Tournié, E., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10926, p. 99. [Google Scholar]
- Jackson, C.; Wall, L.; O’Neill, K.; McGarvey, B.; Herbert, D. Through silicon via developments for silicon photomultiplier sensors. In Proceedings of the Optical Components and Materials XII, San Francisco, CA, USA, 7–12 February 2015; Volume 9359, p. 93591A. [Google Scholar]
- Arbat, A.; Comerma, A.; Trenado, J.; Gascon, D.; Vilà, A.; Garrido, L.; Dieguez, A. High voltage vs. High Integration: A Comparison Between CMOS Technologies for SPAD Cameras. In Proceedings of the Detectors and Imaging Devices: Infrared, Focal Plane, Single Photon, San Diego, CA, USA, 1–5 August 2010; Dereniak, E.L., Hartke, J.P., LeVan, P.D., Sood, A.K., Longshore, R.E., Razeghi, M., Eds.; 2010; p. 77801G. [Google Scholar]
- The 10 ps Challenge. Available online: https://the10ps-challenge.org/ (accessed on 10 October 2019).
- Rech, I.; Luo, G.; Ghioni, M.; Yang, H.; Xie, X.S.; Cova, S. Photon-Timing Detector Module for Single-Molecule Spectroscopy With 60-ps Resolution. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 788–795. [Google Scholar] [CrossRef]
- Acerbi, F.; Ferri, A.; Gola, A.; Cazzanelli, M.; Pavesi, L.; Zorzi, N.; Piemonte, C. Characterization of Single-Photon Time Resolution: From Single SPAD to Silicon Photomultiplier. IEEE Trans. Nucl. Sci. 2014, 61, 2678–2686. [Google Scholar] [CrossRef]
- Pratx, G.; Levin, C.S. Bayesian Reconstruction of Photon Interaction Sequences for High-resolution PET Detectors. Phys. Med. Biol. 2009, 54, 5073–5094. [Google Scholar] [CrossRef][Green Version]
- Tumer, T.O.; Cajipe, V.B.; Clajus, M.; Hayakawa, S.; Volkovskii, A. Multi-Channel Front-End Readout IC for Position Sensitive Solid-State Detectors. In Proceedings of the 2006 IEEE Nuclear Science Symposium Conference Record, San Diego, CA, USA, 29 October–1 November 2006; pp. 384–388. [Google Scholar]

| Reference, Year | Sensors | Crystal | Crystal Size (mm3) | CRT FWHM (ps) | Energy Resolution FWHM (%) a |
|---|---|---|---|---|---|
| [34], 2019 | Hamamatsu R1548 | LGSO | 2.45 × 5 × 15 (9 × 10 Array) | - | 13.1 |
| [31], 2013 [33], 2015 | - | LYSO | 2.35 × 2.35 × 15.2 (16 × 16 Array) | 473 | 11.2 |
| [30] b, 2013 | Hamamatsu R9800 | LYSO | 4 × 4 × 10(Single) | 200.5 | 11.1 |
| [30] b, 2013 | Hamamatsu R11194 | LYSO | 4 × 4 × 10 (Single) | 227.8 | 11.8 |
| [32], 2009 | Hamamatsu R9779 | LYSO | 4 × 4 × 20 (13 × 13 Array) | 432 | - |
| [32], 2009 | Photonis XP1912 | LYSO | 1.4 × 1.4 × 10 (13 × 13 Array) | 551 | - |
| [35] c, 2011 | Hamamatsu R9800 | LYSO | 4 × 4 × 10 (Single) | 198 254 | 10.6 10.8 |
| [29] d, 2008 | Hamamatsu PS-PMT R8400-00-M64 MOD | LYSO | 2.9 × 2.9 × 20 (16 × 16 Array) | 505 | 10.9 |
| Reference, Year | Sensors | Crystal | Crystal Size (mm3) | CRT FWHM (ns) | Energy Resolution @511 keV FWHM (%) | Spatial Resolution FWHM (mm) |
|---|---|---|---|---|---|---|
| [38], 2005 | Hamamatsu S8664-55 (2 × 2 Array) | LSO | 2 × 2 × 20 (9 × 9 Array) | 2.47 | 20.9 | 2 |
| [39], 2008 | Hamamatsu S8550 | LSO | 2 × 2 × 15 (Four Crystals) | 2.15 ± 0.11 | 12.8 ± 0.6 | - |
| [40], 2007 | LabPET detector | LYSO/LGSO phoswich pair | 2 × 2 × 10/12 (Single of Each) | 6.6 b 10.7 c | 24 ± 6 (LYSO) 25 ± 6 (LGSO) | 1.3–1.4 |
| [41], 2015 | LabPET II detector (two 4 × 8 arrays) | LYSO | 1.2 × 1.2 × 12 (8 × 8 Array) | 3.6 ± 0.3 | 20 ± 1 | 0.81 ± 0.04 |
| [42], 2013 | RMD PSAPD detector | LYSO | 0.9 × 0.9 × 1 (8 × 8 Array) | 15.7 ± 0.2 | 10.62 ± 0.04 | 0.84 ± 0.02 |
| [43], 2011 | Hamamatsu S8550-02 | LYSO:Ce | 21.4–23.5 × 18.5 × 10 (Monolithic) a | 27 | 13.2 | 2.12–2.64 |
| Reference, Year | Sensors | Crystal | Crystal Size (mm3) | Readout & DAQ Electronics | Sensitivity (%) CRT (ps)DOI FWHM (mm) | Energy Resolution FWHM (%) | Spatial Resolution FWHM (mm) | Applications |
|---|---|---|---|---|---|---|---|---|
| 2016, [113] a | SensL (MicroFB-30035-SMT) 12 × 12 Array | LYSO (Three-Layer Staggered) | 1.5 × 1.5 × 6 (35 × 35 Array, Top 36 × 36 Array, Middle 37 × 37 Array, Bottom) | Diode-Based Readout, Customized ADC Board | - - 6 | 11.8 @ 511 keV (Top) 9.6 @ 511 keV (Middle) 10.2 @ 511 keV (Bottom) (21 °C) | - | MINDView Brain PET Insert |
| SensL (MicroFC-30035-SMT) 12 × 12 Array | LYSO | 50 × 50 × 20 (Monolithic) | Resistive Readout, Customized ADC Board | - - 2 | 17 @ 511 keV (21 °C) | 1.5 | ||
| 2018, [114] b | SensL (MINDView-Series) 12 × 12 Array | LYSO | 50 × 50 × 20 (Monolithic) | Customized Readout and ADC Board | 7 c - 4 ± 1 | 17.5 ± 1.5 @ 511 keV (27 °C) d | 1.7 (CFOV) <2 (within the 120 mm Diameter of the Center) e | MINDView Brain PET Insert |
| 2017, [118] a | AdvanSiD (NUV-SiPMs) 4 × 8 Array | LYSO (Dual-Layer Staggered) | 3.3 × 3.3 × 8 (7 × 7 Array, Top) 3.3 × 3.3 × 12 (8 × 8 Array, Bottom) | TRIROC ASIC (64 Channels) | 7 - - | 16 @ 511 keV (Top) 18 @ 511 keV (Bottom) | - | PET/MRI/EEG TRIMAGE System |
| 2019, [119] a | AdvanSiD (NUV-SiPMs) 4 × 8 Array | LYSO:Ce (Dual-Layer Staggered) | 3.3 × 3.3 × 8 (7 × 7 Array, top) 3.3 × 3.3 × 12 (8 × 8 Array, Bottom) | TRIROC ASIC (64 Channels) | - 529 (Top)/501 (Bottom) - | 22 @ 511 keV (Top) 20 @ 511 keV (Bottom) | - | PET/MRI/EEG TRIMAGE System |
| 2018, [122] a | SensL (J-Series) 2 × 2 Array | LYSO | 2.76 × 2.76 × 18.1 (6 × 7 Array) | - | - 409 ± 39 - | 11.7 ± 1.5 @ 511 keV | - | Total-body Human EXPLORER: PET/CT System |
| 2016, [125] b | SensL (C-Series) 12 × 12 Array | LYSO (Pyramidal Shape) | Entrance Surface: 48 × 48 mm2 Exit Surface: 50 × 50 mm2 Thickness: 10 mm (Monolithic) | Customized ADC board (66 Channels) | 2.8 f - 2 | 15 ± 2 @ 511 keV (22 °C) ~14 @ 511 keV (15 °C) d | ~1 (whole FOV Range) g | Small-Animal PET Insert |
| Reference, Year | Sensors | Crystal | Crystal Size (mm3) | Energy Window (keV) | Trigger Scheme | CRT FWHM (ps) | Energy Resolution FWHM (%) | Spatial Resolution FWHM (mm) |
|---|---|---|---|---|---|---|---|---|
| [137], 2015 | DPC-3200-22-44 | LYSO | 2 × 2 × 6 (Single) | FWTM a | - | 171 | 12.6 | - |
| GAGG | 2 × 2 × 6 (Single) | FWTM a | - | 310 | 8.5 | - | ||
| GAGG | 3.2 × 3.8775 × 8 (8 × 8 Array) | FWTM a | - | 619 | 9.2 | - | ||
| [144], 2016 | DPC-3200-22-44 | LYSO | 32 × 32 × 5 (Monolithic) | 400–650 | 2 | 529 | 23 | 0.6 b |
| [141], 2016 | DPC-3200-22-44 | LYSO:Ce | 32 × 32 × 22 (Monolithic) | FWTM a | 1 | 147 | 10.2 | 1.1 c |
| [142], 2016 | DPC-3200-22-44 | LYSO:Ce | 32 × 32 × 22 (Monolithic) | FWTM a | 1 | 214 | 10.2 | 1.7 c |
| [136], 2013 | DPC-6400-44-22 | LSO:Ce,Ca | 24 × 24 × 10 (6 × 6 Array) | 450–570 | 2 | - | 12.8 | 0.97 d |
| Reference, Year | Sensors | Crystal | Crystal Size (mm3) | # of Detectors | Energy Window (keV) | Trigger Scheme | CRT FWHM (ps) | Energy Resolution FWHM (%) | Spatial Resolution FWHM (mm) |
|---|---|---|---|---|---|---|---|---|---|
| [135], 2012 | DPC-3200-22-44 | LYSO | 4 × 4 × 22 (8 × 8 Array) | 10 | 440–660 | - | 266 | 10.7 | ~2.4 a |
| [139], 2016 | DPC-3200-22-44 | LYSO | 1.85 × 1.85 × 10 (16 × 16 Array) | 12 | - | - | 298 | - | ~1.6 (CFOV) b |
| [140], 2014 | DPC-3200-22-44 | LYSO | 32 × 32 × 2 (Monolithic) | 4 c | 400–650 | 680 | 18 | 0.7 d | |
| [143], 2018 | DPC-3200-22-44 | LYSO:Ce | 32 × 32 × 22 (Monolithic) | 32 | FWTM | 1 | 212 | 10.2 | 2.9 (CFOV) e |
| [146], 2017 | DPC-3200-22 (4 × 4 array) | LYSO | 0.93 × 0.93 × 12 (30 × 30 Array) | 10 f | 250–625 500–520 | 2 1 | 436.1 240.4 | - | - |
| 4 × 4 × 10 (8 × 8 Array) | 250–625 500–520 | 2 1 | 289.4 208.4 | - | - | ||||
| [148], 2018 | DPC-3200-22 (4 × 4 array) | LYSO | 0.93 × 0.93 × 12 (30 × 30 Array) | 10 | 250–625 | 3 | 609 | 12.7 | 1.7 (CFOV) 2.5 (50 mm off the Center) g |
| Reference, Year | [152], 2012 | [151], 2014 | [19], 2014 | [153], 2014 | [155], 2014 | [154], 2017 c | |
|---|---|---|---|---|---|---|---|
| SPAD | Technology (CMOS) | 130 nm | 350 nm (HV) | 130 nm (CIS) | 130 nm | 350 nm | 150 nm |
| No. of SPADs (in One Pixel) | 1 | 16 × 26 | 24 × 30 | 1 | 1 | 2 × 2 | |
| Breakdown Voltage (V) | 14.4 | – | – | – | 28 | 18.8 | |
| Cell Pitch (µm2) | 50 × 50 | 30 × 30 | – | 48 × 48 | – | 60 × 60 | |
| Active Area (µm, diameter) | 8 (Circular) | 19.7 × 16.5 (Rect.) | 12.67 (Circular) | 5 (Octagonal) | 20 (Circular) | 14 (Circular) | |
| Fill Factor (%) | 2 | 21.2 | 35.7 b | 0.77 | – | 26.5 | |
| Peak PDP (%) | 25 @ 500 nm Vex = 1 V | 30 @ ~420 nm Vex = 4 V | 45 @ ~410 nm Vex = 1.5 V a | 30 @ 425 nm Vex = 1.5 V | 43 @ ~420 nm Vex = 5 V a | – | |
| Median DCR (Hz/µm2) @ R.T. | 2.0 @ Vex = 1 V | 250.0 @ Vex = 4 V a | 108.7 @ Vex = 1.5 V | 26.2 @ Vex = 2.5 V | 12.7 @ Vex = 5 V | 44.2 @ Vex = 3 V | |
| TDC | Clock Frequency (Hz) | 280 | 50 | 100 | – | – | – |
| No. of TDCs (in one pixel) | 1 | 48 | 2 | 1 | 1 | 0.5 | |
| Resolution LSB (ps) | 119 | 51.8 | 64.56 | 62.5 | 10 | 250 | |
| Dynamic Range (ns) | 100 | – | 261.59 | 64 | 160 | – | |
| DNL (LSB) | ±0.4 (Max) | 1.97 | –0.24 to +0.28 | <4 | – | –0.4 to +0.5 | |
| INL (LSB) | ±1.2 (Max) | 2.39 | –3.9 to +2.3 | <8 | – | –0.9 to +1.2 a | |
| Counting Rate (MHz) | 0.5 | – | – | – | 2.5 | - | |
| No. of bits | 10 | 19 | 12 | 10 | – | 16 | |
| TDC/SPAD | 1/1 | 3/26 | 2/720 | 1/1 | 1/1 | 1/8 | |
| System | Pixel Array | 32 × 32 | 1 × 1 | 8 × 16 | 64 × 64 | 16 × 1 | 64 × 64 |
| Application | FLIM | PET | PET | FLIM | – | SNL |
| Reference, Year | Electrode Pattern | Anode Structure | Cathode Structure | Size (mm3) | Biasing Voltage (V) | Energy Resolution (% FWHM) | Timing Resolution (ns) | Position Resolution (mm FWHM) | Application |
|---|---|---|---|---|---|---|---|---|---|
| [165], 2018 | Pixelated | 20 × 20 Anode Array | Common Cathode | 40 × 40 × 15 | - | <2.5 @ 662 keV | - | - | SPECT |
| Pixelated | 11 × 11 Anode Array | Common Cathode | 22 × 22 × 15 | - | <1 @ 662 keV a | - | - | SPECT | |
| [164], 2016 | Pixelated | 8 × 8 Anode Array | Common Cathode | 19.4 × 19.4 × 6 | 200–500 | 3.75 @ 511 keV 3.73 @ 662 keV | - | 3.07 | PET |
| [166], 2012 | Pixelated | 3 × 3 Anode Array | Common Cathode | 20 × 20 × 5 | 1000 | 7 @ 511 keV b 9 @ 511 keV c | 30 b 35 c | 0.35 (x, y) 0.4 (z) | PET |
| [172], 2017 | Cross-Strip | 0.15 mm Width, 0.4 mm Pitch | 1.9 mm Width, 2.0 mm Pitch | 20 × 20 × 5 | 350 | 1.14 @ 662 keV | - | 0.4 | - |
| [171], 2017 | Cross-Strip | 0.1 mm Width, 1 mm Pitch (39 Anodes) 0.4 mm Width, 1 mm Pitch (38 Steering Electrodes) | 4.9 mm Width, 5 mm Pitch (8 Cathodes) | 40 × 40 × 5 d | 500 | 7.43 ± 1.02 @ 511 keV e | 37 e | 0.76 | PET |
| [170], 2016 | Cross-Strip | 0.1 mm Width, 1 mm Pitch (39 Anodes) 0.4 mm Width, 1 mm Pitch (38 Steering Electrodes) | 4.9 mm Width, 5 mm Pitch (8 Cathodes) | 40 × 40 × 5 d | 500 | 7.35 ± 1.75 @ 511 keV e | 37 e | 0.76 | PET |
| [168], 2010 | Cross-Strip | 0.05 mm Width, 1 mm Pitch | 5 mm Pitch (Width Unknown) | 40 × 40 × 5 | - | 3 @ 511 keV | 8 | 1 | PET |
| [169], 2008 | Cross-Strip | 0.1 mm Width, 1 mm Pitch (39 Anodes) 0.2 mm Width, 1 mm Pitch (38 Steering Electrodes) | 4.9 mm Width, 5 mm Pitch (8 Cathodes) | 39 × 39 × 5 | 500–1500 | 2 @ 662 keV 2.2 @ 511 keV | - | 1 | PET |
| [167], 2004 | Cross-Strip | 0.9 mm Width, 1 mm Pitch (16 Anodes) | 3.9 mm Width, 4 mm Pitch (5 Cathodes) | 20 × 16 × 0.9 | 500 | - | 2.6 | 1 | - |
| [163], 2011 | Cross-Strip | 0.1 mm Width, 1 mm Pitch (38 Anodes) 0.2 mm Width, 1 mm Pitch (37 Steering Electrodes) | 5.4 mm Width, 5.5 mm Pitch (7 Cathodes) | 40 × 40 × 5 | - | 3.9 ± 0.19 @ 511 keV | - | 0.78 | PET |
| Reference | [11,12] | [13] | [14] |
|---|---|---|---|
| Manufacturer | Philips | GE | Siemens |
| Model Name | Philips-Ingenuity TF PET/MRI Rystem | SIGNA™ PET/MRI Rystem | Biograph mMR System |
| Scintillator Material | LYSO | LBS | LSO |
| Scintillator Size (mm3) | 4 × 4 × 22 | 4.0 × 5.3 × 25 | 4 × 4 × 20 |
| Sensor | PMT | SiPM | APD |
| Spatial Resolution (mm) | 4.7 | 4.57 | 4.6 |
| Timing Resolution (ps) | 550 | 385 | N/A |
| Energy Resolution (%) | 13 | 9.4 | - |
| Type | PMT | APD | Analog SiPM | dSiPM | CZT |
|---|---|---|---|---|---|
| Conversion Type | Indirect | Indirect | Indirect | Indirect | Direct |
| Magnetic Field Compatibility | No | Yes | Yes | Yes | Yes |
| ToF Capability | Limited | No | Yes | Yes | No |
| Signal Readout | Analog | Analog | Analog | Digital | Analog |
| Operating Voltage | High | Low | Low | Low | High |
| Compactness | Low | Medium | Medium | High | Medium |
| Readout Electronics | Complex | Complex | Complex | Simple | Very complex |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Chalich, Y.; Deen, M.J. Sensors for Positron Emission Tomography Applications. Sensors 2019, 19, 5019. https://doi.org/10.3390/s19225019
Jiang W, Chalich Y, Deen MJ. Sensors for Positron Emission Tomography Applications. Sensors. 2019; 19(22):5019. https://doi.org/10.3390/s19225019
Chicago/Turabian StyleJiang, Wei, Yamn Chalich, and M. Jamal Deen. 2019. "Sensors for Positron Emission Tomography Applications" Sensors 19, no. 22: 5019. https://doi.org/10.3390/s19225019
APA StyleJiang, W., Chalich, Y., & Deen, M. J. (2019). Sensors for Positron Emission Tomography Applications. Sensors, 19(22), 5019. https://doi.org/10.3390/s19225019






